Abstract—
We have studied the sintering of ceramic samples based on magnesium oxide, alumina, and magnesium aluminate spinel in order to assess the conceptual feasibility of producing ceramic foam materials in the Al2O3–MgO system and examined the effect of sintering aids on the sintering process. The range of firing temperatures has been found that ensures the preparation of materials with an open cellular pore structure: the optimal temperatures are from 1600 to 1700°C. The porosity of the synthesized materials is up to 85% and their compressive strength reaches 1.4 MPa.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Highly porous ceramic materials are currently of interest to research and development engineers because such materials are demanded in a variety of industrial applications. Owing to their low density, small thermal expansion coefficient, high thermal shock resistance, good chemical stability, refractoriness, and excellent mechanical properties, such materials are used as catalyst supports, high-temperature heat- and sound-insulation materials, high-temperature filters for molten melts and hot liquids and gases, and a basic component of ablating composites [1–3].
In recent years, the impregnation of polyurethane foam (PUF) skeletons with a slip suspension, followed by high-temperature heat treatment (firing), has been used increasingly in the fabrication of highly porous ceramic foams. Firing leads to the sintering of powder particles in the composition of the slip, removal of the PUF skeleton, and, as a consequence, the formation of a highly porous ceramic material similar in shape to the parent PUF structure [4–8]. To obtain highly porous structures containing high-strength, low-porosity walls between macropores, it is necessary to thoroughly choose components of ceramics and firing process parameters.
According to their chemical composition, ceramic foam materials are largely oxide ceramics (as a rule, based on Al2O3, ZrO2, or TiO2) and carbide ceramics (based on silicon carbide).
Among refractory-oxide-based ceramic foam materials, alumina- and mullite-based ceramics have the widest application. Such ceramics offer high thermal stability and chemical resistance, especially to oxidation. Moreover, the starting components for the preparation of alumina ceramics are inexpensive and readily available. Their long-term stability at high temperatures allows porous alumina ceramics to be successfully used in filtration boxes or furnace preheaters for filtering large volumes of liquid metals. Necessary conditions for such an application are open porosity at a level from 80 to 90%, dominated by connected cellular pores, and compressive strength of at least 0.5 MPa.
At the same time, ceramics based on magnesium oxide (magnesia usta) and magnesium aluminate spinel are promising materials for catalyst supports and foam filters. Such ceramics also offer high chemical and thermal stability. Natural spinels range widely in composition. Moreover, natural spinels always contain an amount of impurities. Pure spinels can be prepared by synthesis from appropriate oxides. Among pure compounds of this class, MgO ⋅ Al2O3 spinel, having a cubic lattice, is most frequently used. It is rather stable to the effect of slags and fluxes. Studies of spinel-based ceramic materials can provide the key to resolving many problems pertaining to high-temperature slagging in steelmaking and production of aluminum castings.
Small amounts of magnesium aluminate spinel can be prepared by melting mixtures of magnesium oxide and alumina in electric arc furnaces. Sintered mixtures of MgO and Al2O3 are used to a limited extent, even though the sintering of these oxides is not very commercially feasible for the manufacture of dense, low-porosity ceramics [9, 10]. The sintering of MgO with Al2O3 is accompanied by considerable volume changes, so spinel formation between grains being sintered increases the thickness of the intergranular layer. Such expansion cannot be prevented because the density of spinel is considerably lower than that of its constituent oxides. At the same time, it is worth noting that the expansion that occurs during the sintering of MgO with Al2O3 is useful in the manufacture of porous ceramic materials, for example, for fettling of steel-melting furnaces. Nevertheless, the manufacture of technical refractories from pure magnesium aluminate spinel is very limited because large expenditures are needed to manufacture high-purity products capable of competing with other refractories.
As a rule, the preparation of coarse-grained ceramics by sintering pure magnesium oxide and alumina powders involves solid-state reactions through ion interdiffusion between powder particles at temperatures on the order of 1500–1950°C for several hours. The firing temperature can be lowered by adding sintering aids to the composition of reaction mixtures. It is worth noting that magnesium oxide and alumina are sintering aids for each other; that is, both the addition of magnesium oxide to the composition of corundum ceramics and the addition of alumina to the composition of magnesium oxide ceramics help accelerate the sintering process and, accordingly, allow the firing temperature of ceramic products to be lowered. A previous report [11] described the synthesis of high-temperature mullite-based ceramic foam materials by firing appropriate fine powders based on a combustible foam and demonstrated the conceptual feasibility of producing such materials.
This study is concerned with sintering processes and optimization of firing conditions of ceramic materials based on fine magnesium oxide and alumina powders with the use of sintering aids in order to obtain ceramic foam materials having a connected cellular porosity above 80% and compressive strength above 0.5 MPa.
EXPERIMENTAL
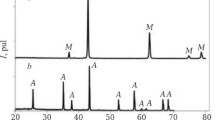
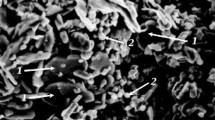
The starting materials for the preparation of slip suspensions were M5 synthetic corundum powder (Russian Federation State Standard GOST 3647-80) with an average particle size of 5 μm and magnesium oxide (periclase) powder (Russian Federation State Standard GOST 13 236-83) with an average particle size of 1 μm (Figs. 1, 2).
Slip suspensions were prepared using aqueous solutions of aluminum oxychloride and magnesium chloride. These compounds act as technological binders in producing green compacts and as sintering aids during firing.
Green compacts were produced by impregnating the slip suspensions into PUF blocks with an open cellular pore structure formed by cellular pores 0.5 to 2.0 mm in size, following which the excess slip was squeezed out. The samples were fired in a Nabertherm HT 16/18 electric furnace in the temperature range 1000–1700°C under nonisothermal conditions: heating of the samples in the furnace, holding at a predetermined temperature, and furnace-cooling.
The phase composition of the samples was determined by X-ray diffraction on a DRON-3M diffractometer (Cu radiation, angular range 2θ = 20°–80°, step scan mode with a step size of 0.05° and a counting time per data point of 2 s). To identify the phases present, X-ray diffraction patterns were compared to PDF2 data. Optical micrographs were obtained in transmitted and reflected light on an Olympus BX-51 microscope. Scanning electron microscopy (SEM) images were obtained in a Hitachi S-405A electron microscope operated at an accelerating voltage of 25 kV. The compressive strength of the materials was determined on an Instron 5965 testing machine.
EXPERIMENTAL RESULTS
According to the X-ray diffraction data, the starting powders consisted of corundum (α-Al2O3) and cubic magnesium oxide (MgO), without X-ray diffraction detectable impurities (Fig. 1). The absence of foreign inclusions in the powders was also confirmed by optical and electron microscopy results (Fig. 2).
To obtain sedimentation-resistant slip suspensions, we varied the composition of the temporary technological binder (the liquid phase of the suspensions). According to our results, the use of distilled water as a temporary technological binder leads to rapid sedimentation of the magnesium oxide and alumina powders present in the composition of the slips. This causes phase separation of the green compacts during the impregnation of PUF skeletons with the slips and, as a consequence, leads to deformation and cracking of the materials during firing. Using aqueous aluminum oxychloride and magnesium chloride solutions instead of water, we were able to obtain strain-free ceramic materials having porosity at a level from 80 to 85% and compressive strength in the range 0.5–0.8 MPa. In air at temperatures above 900°C, these components irreversibly convert into the corresponding oxides—alumina and magnesium oxide—without changing the phase composition of the ceramic materials. In view of this, in subsequent syntheses of ceramic foams we used only aqueous aluminum oxychloride and magnesium chloride solutions.
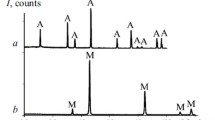
The influence of firing temperature and time on the properties of the ceramics was studied using three groups of materials, with Al2O3 : MgO weight ratios of 10 : 90, 72 : 28 (spinel ratio), and 95 : 5. The results demonstrate that firing at temperatures from 1000 to 1400°C for 1 h leads to the formation of trace levels of magnesium aluminate spinel. At firing temperatures from 1500 to 1600°C, we observed active spinel formation, but the starting alumina and magnesium oxide persisted in the structure of the materials. An essentially full conversion of the starting oxides into spinel was achieved at a temperature of 1700°C.
At firing temperatures below 1300°C, we observed only sintering of powder particles with each other. As a result, the ceramic materials had low strength. Active sintering of particles, accompanied by a reduction in micropore size in the cell walls and an increase in the strength of the materials, occurred at firing temperatures above 1600°C and firing times more than 30 min. In particular, the ceramic material prepared by firing at a temperature of 1700°C for 1 h had dense cell walls and cellular micropores reproducing the shape of the starting PUF skeletons.
The physicomechanical characteristics of the samples synthesized in the range 1600–1700°C (Fig. 3) demonstrate that their porosity correlates with their strength characteristics, independent of their chemical composition: with increasing porosity, the strength of the materials decreases. The highest compressive strength, 1.3–1.4 MPa, was offered by the materials with the lowest alumina content: 10 wt %. They were characterized by porosity at a level of 70 vol %. The materials with the spinel MgO : Al2O3 ratio had the lowest strength: from 0.2 to 1.2 MPa. Accordingly, they had the highest porosity: 75–86 vol %.
The present results demonstrate that, in the temperature range 1600–1700°C, magnesium oxide and alumina irreversibly and fully react with each other to form magnesium aluminate spinel (Fig. 4). The phase composition of the materials is determined by the MgO : Al2O3 ratio in the starting powder. The oxide present in a substoichiometric amount fully reacts with the oxide present in excess to form spinel, and some of the oxide present in excess persists. The ceramic materials prepared by reacting a stoichiometric (spinel) ratio of magnesium oxide and alumina powders consisted of only magnesium aluminate spinel (with trace levels of unreacted magnesium oxide due to a slight deviation from stoichiometry related to errors of the equipment used).
The materials were found to have an open cellular pore structure, reproducing the pore structure of the parent PUF sponges (Fig. 5). The cellular macropores range in size from 0.5 to 2 mm, which also corresponds to the macropore size in the PUF sponges. The pore walls do not contain any through holes, which can be formed in materials as a result of PUF burnout during firing. This suggests the possibility of producing ceramic foam materials by the method used in this work. In all of the samples, the pore walls consist of sintered grains 1–10 μm in size and contain micropores 1–20 μm in size, due to the presence of voids between the sintered particles. At the same time, the structure of the pore walls depends on the MgO : Al2O3 ratio in the materials. The materials prepared from alumina-enriched starting mixtures or at the spinel alumina : magnesium oxide ratio consist of sintered particles 3–10 μm in size, with the corresponding composition. The materials prepared from magnesium oxide-enriched starting mixtures consist of spinel particles 3–10 μm in size, surrounded by magnesium oxide particles on the order of 1 μm in size, which corresponds to the particle size of the starting magnesium oxide powder.
DISCUSSION
The purpose of this work was to examine the feasibility of preparing MgO–Al2O3 ceramic materials having an open cellular pore structure with a volume fraction of connected pores in the range 85–90% and compressive strength in the range 0.5–1 MPa. Moreover, it is desirable that the heat treatment temperature of green compacts not exceed 1600–1700°C. To meet these requirements, we developed a process for the preparation of materials by sintering magnesium oxide and alumina powders at various MgO : Al2O3 ratios and a small particle size in order to ensure a high rate of solid-state sintering. To produce ceramics with a tailored pore structure, we impregnated PUF skeletons with slips, and to ensure sedimentation stability of slips and reduce the sintering temperature we added sintering aids to the composition of the materials. Previous results [12–18] demonstrate high effectiveness of additives that accelerate solid-state sintering of corundum and zirconia ceramics at firing temperatures on the order of 1550–1600°C.
The present results demonstrate effectiveness of using aluminum oxychloride- and magnesium chloride-based additives: basic to the mechanism underlying their effect is acceleration of diffusion processes on the surface of corundum and magnesium oxide particles. The effectiveness of such additives during firing is due to the fact that they act as gluing components and produce intimate contacts between particles being sintered, with a large contact area [19–22]. Moreover, the use of magnesium chloride and aluminum oxychloride ensures an increase in sintering rate owing to the formation of additional alumina, magnesium oxide, and spinel on grain boundaries of the powders being sintered, due to chemical reactions between the chlorides and atmospheric air. As a consequence, the sintering process improves the initial bonding between particles, leading to an increase in the strength of the final ceramic body [19].
Without sintering aids in the slips, firing in the temperature range chosen, 1600–1700°C, ensured no increase in the strength of the materials. On heating, sintering aids convert into small magnesium oxide and alumina particles, which contain a high density of structural defects. In the absence of crystals containing numerous defects, during firing the diffusion of ions between particles being sintered is hindered. Previously reported data [15, 16] indicate that, in the absence of sintering aids in green compacts, solid-state sintering conditions are only ensured by firing dense ceramics produced by pressing dry or wetted powders. For this reason, when water was used as a technological binder, there were no contacts between the particles being sintered, which prevented ceramics bodies from gaining strength during the firing process.
Firing at temperatures on the order of 1600–1700°C for more than 1 h leads to spinel formation as a result of chemical interaction between corundum and magnesium oxide particles. Thus, the pore walls forming the ceramic skeleton consist of sintered grains of the starting component present in excess and spinel grains 3–10 μm in size, which corresponds to the grain size of the parent corundum in the green compact. During heat treatment of the materials, the micron-sized magnesium oxide grains and large corundum grains react to form spinel. Interdiffusion of the two oxides contributes to the sintering of the particles and the formation of a ceramic skeleton. The pore walls in the materials contain micropores because they are formed on account of the voids between the alumina, magnesium oxide, and spinel particles being sintered. Note that, after firing, the material contains not only closed but also open micropores.
Our results demonstrate that the materials prepared by firing at temperatures from 1650 to 1700°C for 2 h have the optimal strength, at a level of 0.7–1.4 MPa, and a total porosity of 85%. Note that the highest strength, 1.1–1.4 MPa, is offered by the materials enriched in one of the oxides (MgO or Al2O3). The second oxide in such materials acts as a sintering aid, facilitating strength gain. If alumina and magnesium oxide in the composition of a green compact are in the spinel ratio, firing leads to the formation of magnesium aluminate spinel, and the material does not contain any sintering aid with a different phase composition, which otherwise might contribute to strength gain. For this reason, such materials have lower strength: 0.7–1.0 MPa.
CONCLUSIONS
The present results demonstrate the possibility of producing alumina- and magnesium oxide-based ceramic foam filters having a cellular pore structure with an open porosity of up to 85% and compressive strength in the range 0.7–1.4 MPa. The optimal conditions for the preparation of such materials are firing in the range 1650–1700°C for 2 h, with the use of magnesium chloride and aluminum oxychloride additions in the starting slip. The possibility of using fine alumina and magnesium oxide powders as a spinel source allows the cost of ceramic foam articles to be reduced. Moreover, using such raw materials, one can use pulverized powders, without mechanical activation via premilling, which will ensure high porosity in the macropore walls in foam filters owing to voids between the sintered particles and allow the material to be free of unreacted starting components.
To maximize the strength of the material and reduce the firing temperature, further optimization of the ceramic composition is needed: by selecting sintering aids, varying the particle size composition of the preceramic powders, and optimizing the composition of slip suspensions via selection of electrolytes capable of increasing the volume fraction of the solid phase in the slip in order to increase the number of contacts between particles during firing.
REFERENCES
Kablov, E.N., Innovative projects at the All-Russia Research Institute of Aviation Materials Russian Federation State Scientific Center (Federal State Unitary Enterprise) for implementing “Strategic directions in materials and materials processing technologies development over a period up to 2030,” Aviatsionnye Mater. Tekhnol., 2015, no. 1, pp. 3–33. https://doi.org/10.18577/2071-9140-2015-0-1-3-33
Kablov, E.N., At the intersection of science, education, and industry, Ekspert, 2015 no. 15 (941), pp. 49–53.
Taslicukur, Z., Balaban, C., and Kuskonmaz, N., Production of ceramic foam filters for molten metal filtration using expanded polystyrene, J. Eur. Ceram. Soc., 2007, no. 27, pp. 637–640.
RF Patent 2 377 224, Byull. Izobret., 2009, no. 9.
RF Patent 2 580 959, Byull. Izobret., 2016, no. 10.
US Patent 4 664 858, 1987.
Kablov, E.N., Shchetanov, B.V., Ivakhnenko, Yu.A., and Balinova, Yu.A., Promising high-temperature reinforcing fibers for metallic and ceramic composite materials, Tr. Vseross. Inst. Aviatsionnykh Mater. Elektron. Nauchno-Tekh. Zh., 2013, no. 2, paper 5. http://www.viam-works.ru. Cited March 4, 2019.
Buchilin, N.V., Prager, E.P., and Ivakhnenko, Yu.A., Effect of plasticizing additives on rheological characteristics of slips for the preparation of alumina-based porous ceramic materials, Tr. Vseross. Inst. Aviatsionnykh Mater. Elektron. Nauchno-Tekh. Zh., 2016, no. 8, paper 06. http://www.viam-works.ru. Cited March 7, 2019.https://doi.org/10.18577/2307-6046-2016-0-8-6-6
Senina, M.O., Lemeshev, D.O., Kolesnikov, V.A., Methods of synthesizing alumomagnesium spinel powders for obtaining transparent ceramic (review), Steklo Keram., 2017, no. 10, pp. 19–25.
Gorshkov, V.A., Miloserdov, P.A., Yukhvid, V.I., Sachkova, N.V., and Kovalev, I.D., Preparation of magnesium aluminate spinel by self-propagating high-temperature synthesis metallurgy methods, Inorg. Mater., 2017, vol. 53, no. 10, pp. 1046–1052.
Buchilin, N.V., Lyulyukina, G.Yu., and Varrik, N.M., Effect of firing conditions on the structure and properties of highly porous mullite-based ceramic materials, Tr. Vseross. Inst. Aviatsionnykh Mater. Elektron. Nauchno-Tekh. Zh., 2017, no. 5 (53), paper 04, pp. 32–41. http://www.viam-works.ru. Cited March 7, 2019. https://doi.org/10.18577/2307-6046-2017-0-5-4-4
Kryuchkov, Yu.N., Determination of the average capillary radius in porous materials, Steklo Keram., 2018, no. 4, pp. 16–21.
Slyusar’, O.A. and Uvarov, V.M., Effect of complex additives on ceramic slip mobility, Steklo Keram., 2017, no. 2, pp. 44–46.
Kichkailo, O.V. and Levitskii, I.A., Rheological characteristics of slips in making heat-resistant lithium-aluminum-silicate ceramic, Steklo Keram., 2017, no. 7, pp. 37–44.
Evteev, A.A., Some aspects of optimizing the firing schedule of ceramic composites containing eutectic additives, Tr. Vseross. Inst. Aviatsionnykh Mater. Elektron. Nauchno-Tekh. Zh., 2016, no. 2 (38), pp. 101–106. http://www.viam-works.ru. Cited March 7, 2019. https://doi.org/10.18577/2307-6046-2016-0-2-12-12
Shchegoleva, N.E., Chainikova, A.S., and Orlova, L.A., Sintering process in the preparation of glass-ceramics based on strontium aluminosilicate glass by semidry pressing, Aviatsionnye Mater. Tekhnol., 2018, no. 4 (53), pp. 55–62. https://doi.org/10.18577/2071-9140-2018-0-4-55-62
Safronova, T.V., Putlyaev, V.I., Filippov, Ya.Yu., Shatalova, T.B., and Fatin, D.S., Ceramics based on brushite powder synthesized from calcium nitrate and disodium and dipotassium hydrogen phosphates, Inorg. Mater., 2018, vol. 54, no. 2, pp. 195–207.
Savinykh, D.O., Khainakov, S.A., Boldin, M.S., Orlova, A.I., Aleksandrov, A.A., Lantsev, E.A., Sakharov, N.V., Murashov, A.A., Garcia-Granda, S., Nokhrin, A.V., and Chuvil’deev, V.N., Preparation of NZP-type Ca0.75 + 0.5xZr1.5Fe0.5(PO4)3 –x(SiO4)x powders and ceramic, thermal expansion behavior, Inorg. Mater., 2018, vol. 54, no. 12, pp. 1267–1273.
Khimicheskaya tekhnologiya keramiki: Uch. posobie dlya vuzov (Chemical Technology of Ceramics: A Learning Guide for Higher Education Institutions), Guzman, I.Ya., Ed., Moscow: OOO RIF Stroimaterialy, 2003.
Zhitnyuk, S.V., Oxygen-free ceramic materials for aerospace engineering (a review), Tr. Vseross. Inst. Aviatsionnykh Mater. Elektron. Nauchno-Tekh. Zh., 2018, no. 8 (68), pp. 81–88. http://www.viam-works.ru. Cited March 7, 2019.https://doi.org/10.18577/2307-6046-2018-0-8-81-88
Kashin, D.S., Dergacheva, P.E., and Stekhov, P.A., Refractory coatings produced by slip casting (a review), Tr. Vseross. Inst. Aviatsionnykh Mater. Elektron. Nauchno-Tekh. Zh., 2018, no. 5 (65), pp. 64–75. http://www.viam-works.ru. Cited March 7, 2019. https://doi.org/10.18577/2307-6046-2018-0-5-64-75
Kovtunov, A.I., Khokhlov, Yu.Yu., and Myamin, S.V., Fabrication technology and properties of composite foams, Aviatsionnye Mater. Tekhnol., 2015, no. 3 (36), pp. 64–68. https://doi.org/10.18577/2071-9140-2015-0-3-64-68
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Tsarev
Rights and permissions
About this article
Cite this article
Buchilin, N.V., Basargin, O.V., Varrik, N.M. et al. Sintering Behavior of Highly Porous Ceramic Materials Based on the Al2O3–MgO System. Inorg Mater 56, 417–424 (2020). https://doi.org/10.1134/S0020168520040032
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168520040032