Powders are obtained containing metal particles during chemical dispersion of Al–Mg (20 wt.%)-alloy. A method of high-temperature self-propagating synthesis is used in order to prepare cermet. Reaction sintering in a filtering combustion regime for a powder workpiece is implemented by heating in air at 550 – 600°C followed by initiation of metal exothermic reaction combustion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Development of cermets with different phase composition and structure types makes it possible to obtain new composite materials with a set of properties promising for their use in various areas of technology. Interest in cermet of the MgO–Al composition is due to the fact that it is possible to achieve a combination within it of high strength and refractoriness, inherent for magnesium oxide, and plasticity and thermal conductivity, typical for aluminum. This cermet, by having relatively low density, may exhibit quite good crack resistance, impact strength, and fatigue failure resistance. It should be noted that a potential favorable property for a cermet may be good thermal shock resistance due to presence of a very heat conducting metal component. In addition, it may also be promising for use as some elements of high-temperature technology structures, operating in an air atmosphere under thermal stress conditions.
Cermets are currently prepared by various methods. The main methods are quite complex, and require special expensive equipment. Judging from individual publications [1], a promising and very economic for cermet preparation of the MgO–Al composition may be reaction sintering of workpieces of chemically dispersed aluminum Al–Mg (20 wt.%)-alloy. However, in view of a lack of information required for practical implementation of this method, it is important to study and develop a production process for preparing cermet of MgO–Al composition by reaction sintering.
Results are given in this article for studying reaction sintering of powder prepared by chemical dispersion of Al–Mg (20 wt.%)-alloy and development on the basis of this effective technology for preparing cermet of the MgO–Al composition, characterized by a combination of high strength and thermal shock resistance with low density.
PROCEDURE FOR Al–Mg (20 wt.%)-ALLOY POWDER SYNTHESIS FOR CERMET PREPARATION
Chemical dispersion of Al–Mg-alloy was carried out by treating it with 20% aqueous caustic soda solution [2] in a vessel made of thermally stable and chemically resistant glass with continuous heat removal and mixing. The residue prepared from mother liquor was filtered and washed repeatedly with distilled water by filtration under vacuum. The volume of water expended for each washing cycle was 1 liter.
The change in medium pH index (residue in the form of a suspension) after each washing cycle was determined using an HI 98108 pH meter (Hannah Instruments). pH indices of the medium after n-fold residue washing by decanting are provided below:

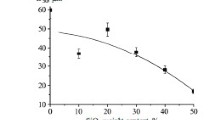
A distinguishing feature of chemical dispersion of Al–Mg alloy is a significant overall yield of coarse powder particles, relating to fractions from <50 to 315/200 μm, obtained using wet screen analysis. The distribution of the oxide particle weight fraction, an alloy chemical dispersion product, is given in Fig. 1. The faction 315/200 μm (Fig. 2) was selected for study. This is coarse-grained particles, among which it is clearly possible to separate particles with a grey shade and white color. X-ray phase analysis was performed in a DRN-3 instrument by a standard procedure [3]. Recording of diffraction patterns was carried out in Cu K α-filtered radiation (nickel filter) with a tube voltage of 30 kV and current strength 20 mA. ASTM card index data were used in order to identify phases.
The phase composition of powder fraction 315/200 _m is gibbsite Al(OH)3 (18.3 ± 2 wt.%) with a monoclinic crystal lattice; bayerite Al(OH)3 (2.9 ± 1 wt.%) with a hexagonal crystal lattice; Mg2Al(OH)6(CO3)0.5H2O (59.0 ± 4 wt.%); intermetallic Al3Mg2 (19.8 ± 4 wt.%) with a cubic crystal lattice.
Self-propagating high-temperature synthesis (SHS) was used in order to prepare compact cermet specimens implemented in so-called filtration systems [4].
From the position of classical ideas about SHS processes for accomplishing filtering combustion (FC) it is necessary to carry out ignition of a porous workpiece surface, which spatially consists of open communicating and gas-filled pores. After this phenomenologically direct flame combustion front propagation is observed as a result of occurrence of exothermic reaction in a gas–solid system.
Porous workpieces were used in order to carry out FC, prepared from metal powders. Metal alloys, boron, and silicon. Gas atmospheres used were oxygen, nitrogen, hydrogen, CO, and CO2. Then gas–solid reaction products are oxides, nitrides, hydrides, and carbides.
REACTION SINTERING OF POWDER SPECIMENS PREPARED BY CHEMICAL DISPERSION OF Al–Mg (20 wt.%)-ALLOY IN AIR IN A FC REGIME
Specimens for sintering were prepared by pressing powder fraction 315/200 μm under a pressure of 100 – 700 MPa. Specimen open porosity was from 49 to 53% (determined according to GOST 2409). In order to perform reaction sintering in an FC regime powder workpieces were installed on a support of corundum foam and placed in a muffle furnace.
As a result of heating by a prescribed regime (Fig. 3) at a certain temperature due to heat absorption from the furnace there was specimen surface ignition: at 500°C for specimen 1 at a pressure of 100 MPa, point a, and at 600°C for specimen 2, pressed at a pressure of 700 MPa, point b.
Powder workpiece (specimen) reaction sintering regime in air in a FC regime: 1 ) change in furnace space temperature (T f), measured by thermocouple; 2, 3 ) change in brightness temperature (T b) of sintered workpiece, measured by pyrometer; point a is temperature of the start of specimen “ignition”, pressed from powder fraction 315/200 μm under pressure of 100 MPa; point b is temperature for the start of “ignition” of specimen pressed from powder fraction 315/200 μm under pressure of 700 MPa; points c and d are specimen “extinction” temperature (T b = T f); point k is completion of isothermal soaking.
The lower ignition temperature for specimen 1 compared with that for igniting specimen 2 is connected with the more significant proportion of pore space in specimen 1 and active surface participating in exothermic gas–solid reaction (Fig. 4, frame 2). For this reason T 1b(max) > T 2b(max) .
Cine pictures of specimen filtering combustion, pressed from powder fraction 315/200 μm under pressure of 100 MPa: 1 ) pressed specimen on foam corundum support; 2 ) specimen ignition, point a in Fig. 3; 3 ) specimen combustion, T 1b(max) in Fig. 3; 4, 5 ) specimen extinction; 6 ) end of specimen filtering combustion, point c in Fig. 3.
Then combustion front propagation proceeds for a specimen surface into its volume and achievement of a maximum brightness temperature T 1b(max) is observed for specimen 1 at 1200°C (Fig. 4, frame 3) and T 2b(max) at 900°C. Measurement of brightness temperature was performed with a CEM DT-9862 type pyrometer.
Maximum values of brightness temperature exceed the aluminum melting temperature for specimen 1 by a factor of 1.8, and for specimen 2 by a factor of 1.4. However, discharge outwards of melt from the volume of specimens was not observed. It may be suggested that this is connected with the high strength of surface oxide films at the surface of Al–Mg particles, exceeding stresses arising with development of heated molten metal. During CF heated molten metal fills the pore space of a sintered powder workpiece. As a result of this its permeability for a gaseous reaction component decreases continuously. As a result of this a gradual reduction is observed in brightness temperature for specimens until its total extinction (see Fig. 3, points c and d, T b = T f, ∆P = 0, for specimen 1, frames 4 – 6 in Fig. 4).
During subsequent isothermal soaking (see Fig. 3, section dk) there is specimen shrinkage as a result of sintering of very fine reaction products. The amount of shrinkage varies from 21 to 25%. Relative linear shrinkage is evaluated from the change in prismatic specimen height with a size of 8 × 9 × 50 mm after FC. The cermet specimens obtained as a result of FC had open porosity of 59 – 64%, apparent density 1.2 – 1.25 g/cm3 (determined according to GOST 2409), and ultimate strength in compression 120 – 160 MPa (determined according to GOST 23775). Specimen microstructure is shown in Fig. 5.
Cermet fracture surface microstructure, sintered from powder fraction 315/200 μm: 1 ) diffusion-bonded particles (grains); 2 ) slit-like pores; 3 ) cavities formed due to grain explosion; 4 ) smooth grain fracture surface as a result of quasibrittle fracture; 5 ) grain contact area, within which “liquid-phase consolidation” is realized with specific compaction pressure of 100 (a) and 700 MPa (b ).
Further study of cermet preparation by the FC method showed that the final sintered cermet phase composition (ratio of metal – metal oxide phases) is determined mainly by the amount of original powder workpiece porosity and the reaction sintering regime selected. By varying these factors it is possible to provide retention within the cermet composition of a significant proportion of metal or its total oxidation.
CONCLUSION
It has been established that sintering in a combustion filtering regime for a powder workpiece is implemented by heating in air up to 500 – 600°C followed by initiation of an exothermic metal combustion reaction “ignition” stage), which is maintained for a certain time due to sucking air into the pore space of a sintered workpiece as a result of a drop in air oxygen partial pressure contained within pores and washing over a workpiece.
The brightness temperature of a sintered workpiece is 900 – 1200°C. Under these conditions the strength of surface oxide films at the surface of Al-Mg-alloy particles exceeds stresses arising with development of an overheated metal melt, and therefore its discharge throughout the volume of a sintered workpiece does not proceed.
During reaction sintering in a filtering combustion scheme metal phase oxidation proceeds due to diffusion of atomic oxygen towards molten metal through surface oxide films.
References
A. Zeerleder, “Über Sintern von Aluminiumlågierungen,” Z. Metallkunde, 41(8), 228 – 233 (1950).
A. Yu. Omarov, F. Z. Badaev, and Yu. G. Trifonov, “Production scheme for sintering nanodispersed powders prepared by chemical dispersion,” Novye Ogneupory, No. 10, 32 – 35 (2012).
E. S. Lukin and N. T. Andrianov, Ceramic Production Technical Analysis and Monitoring [in Russian], Stroiizdat, Moscow (1986).
E. A. Levashov, A. S. Rogachev, V. I. Yukhvid, et al., Physicochemical and Production Bases of Self-Propagating High-Temperature Synthesis [in Russian], BINOM, Moscow (1999).
The work was carried out with financial support of the Russian Ministry of Education and Science within the framework of research work “Technology, structure, and properties of new aluminum oxide ceramics from chemical dispersion of aluminum alloys with zirconium, vanadium, and molybdenum” according state assignment No. 11.425.2014/K on equipment of the Collective Use Center “High-tech in engineering.”
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Novye Ogneupory, No. 6, pp. 62 – 65, June 2015.
Rights and permissions
About this article
Cite this article
Vasin, A.A., Tarasovskii, V.P., Omarov, A.Y. et al. Study of Cermet Synthesis from Powders Prepared by Chemical Dispersion of Al–Mg (20 wt.%)-Alloy. Refract Ind Ceram 56, 310–313 (2015). https://doi.org/10.1007/s11148-015-9836-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11148-015-9836-y