Abstract
Background and aims
Water spinach is a common leafy vegetable in Asia, with a strong ability to accumulate cadmium (Cd) in its edible parts. The aims of this study were to investigate the effects of cultivar variation and water management on Cd accumulation in this plant.
Methods
Three experiments were conducted: a soil pot trial with 32 cultivars, a rhizobox trial with 4 cultivars under flooded and non-flooded conditions and an uptake kinetics trial with 2 cultivars.
Results
There were significant differences in Cd accumulation between the different cultivars, and Cd concentrations in shoots were significantly lower in the flooded (0.25–1.4, mean 0.90 mg kg−1 DW) than in the non-flooded (1.9–4.7, 3.2 mg kg−1) treatments. Cultivars with a low Cd accumulation had a lower Cd bioavailability and mobility in the rhizosphere soil, higher Cd combined with Fe plaque on roots, lower Cd uptake capacity by roots, and lower Cd transfer factors than those with a high Cd accumulation.
Conclusions
Water spinach grown under anaerobic conditions effectively reduces Cd accumulation in edible parts. Low Cd-accumulating cultivars tend to possess a high ability to reduce Cd bioavailability in rhizosphere soil, as well as decrease Cd uptake, and translocation from root to shoot.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd), one of the most hazardous heavy metals, has long been recognized as a major human health threat. Human exposure to Cd in terms of food-chain transfer should be given top priority (Satarug et al. 2003; Clemens et al. 2013) because Cd is readily taken up and translocated to different parts of the plant (Florijn and Van Beusichem 1993). Large areas of agricultural fields have been contaminated by heavy metals, especially Cd, in China (Huang et al. 2007; Hang et al. 2009; Williams et al. 2009). Cadmium contamination in main food crops and leafy vegetables grown in Cd-contaminated fields has aroused considerable attention in recent years (Arao et al. 2003; Zhuang et al. 2009; Liu et al. 2010).
It has been demonstrated that genetic variation contributes to changes in Cd concentrations in crops (Grant et al. 2008; Clemens et al. 2013). Wide variations in the concentration of Cd between cultivars have been documented in many crops, including, but not restricted to, rice (Oryza sativa) (Arao and Ishikawa 2006; Liu et al. 2007; Wang et al. 2011), barley (Hordeum vulgare) (Chang et al. 1982; Chen et al. 2007), carrot (Daucus carrota) (Harrison 1986), maize (Zea mays) (Hinesly et al. 1978), common wheat (Triticum aestivum L.) (Stolt et al. 2006), lettuce (Lactuca indica L.) (Thomas and Harrison 1991), pea (Pisum sativum) (Rivera-Becerril et al. 2002) and potato (Solanum tuberosum) (Dunbar et al. 2003). There has been a great advance in the genetic and molecular understanding of the mechanisms underlying genotypic variation in Cd accumulation in rice (Ueno et al. 2010; Miyadate et al. 2011) and Arabidopsis thaliana (Chao et al. 2012). This variation is mostly explained by variation in HMA3 transporter genes. In rice, different OsHMA3 alleles are responsible for the efficiency of Cd translocation to the shoot from the root (Ueno et al. 2010; Ishikawa et al. 2011; Miyadate et al. 2011; Uraguchi and Fujiwara 2013). OsNramp5 has recently been reported as a major transporter in rice responsible for the transport of Cd and Mn from the external solution to root cells (Sasaki et al. 2012), and the defective transporter protein encoded by the mutant OsNramp5 greatly decreases Cd uptake by roots, resulting in decreased Cd in the straw and grain (Ishikawa et al. 2012).
Apart from the need to study Cd uptake at a molecular level to characterize the Cd transporter(s), the role of the rhizosphere as the root/soil interface where roots access trace elements is easily underestimated (Wei and Twardowska 2013). Agricultural practices (e.g., water management) also play an important role in heavy metal (e.g., As, Cd, Hg) uptake and accumulation in rice (Xu et al. 2008; Arao et al. 2009; Wang et al. 2014). Arao et al. (2009) reported that flooding decreases the Cd concentration in rice grains. Flooding for 3 weeks before and after heading proved to be the most effective method in reducing grain Cd, but this treatment increases As concentration in grains. This suggests that it is possible to reduce Cd accumulation in crops through alteration of the growth environment by appropriate water management.
It has been reported that Cd uptake and accumulation by a plant are affected by the uptake kinetics by its roots (Zhao et al. 2006; Van der Vliet et al. 2007). In addition, for wetland plants such as rice grown in anaerobic environments, radial oxygen loss (ROL) from roots plays a key role in iron (Fe) plaque formation on root surfaces and in the rhizosphere, and then impacts on the conditions (redox potential, pH, activity and mobility of toxic elements) in the rhizosphere (María-Cervantes et al. 2010; Mei et al. 2012; Yang et al. 2012). This process affects Cd availability in rhizosphere soil and Cd translocation to the above-ground parts of plants (Fitz and Wenzel 2002; Cheng et al. 2014). Furthermore, other biological factors (e.g., the activity of iron oxidizing bacteria and root exudates) will also affect Cd availability in the rhizosphere (Macfie and Crowder 1987; Fitz and Wenzel 2002; Greger and Landberg 2008). These results suggest that the characteristics of roots and their rhizosphere may have important roles in Cd uptake and accumulation in plants.
Water spinach (Ipomoea aquatica Forsk) is a common vegetable grown and consumed in Southeast Asia, notably China, Thailand and Vietnam (Göthberg et al. 2002). This plant possesses a strong ability to accumulate Cd in its edible parts which may impose health risks (Wang et al. 2007; Hseu et al. 2013). For example, Zhuang et al. (2009) reported that this vegetable accumulates the highest Cd concentration [0.65 mg kg−1, on a fresh weight (FW) basis] in its edible parts among the selected vegetables grown in a field contaminated by Cd (total Cd and DTPA-extractable Cd concentrations: 1.6 and 0.29 mg kg−1 in the soil, respectively), exceeding the proposed maximum concentration (0.2 mg kg−1 FW) set by the FAO/WHO Food Standards Programme Codex Alimentarius Committee (CAC) (Codex Standard 248–2005). There is thus an urgency to find measures to reduce Cd accumulation in this leafy vegetable. Water spinach is a wetland plant (Marcussen et al. 2008; Vymazal and Švehla 2013) as well as a xerophytic plant, and can be cultured under both flooded and non-flooded conditions. There are a substantial number of cultivars of this vegetable which may have different abilities in Cd accumulation. It is suggested that Cd accumulation in edible parts of this important vegetable could be reduced by screening cultivars with a low Cd accumulation and introducing appropriate water management. However, little information is available on the effects of cultivars and water management regimes on Cd accumulation in edible parts of this plant.
We hypothesize that (1) there is a great variation between cultivars in Cd accumulation in the edible parts of water spinach; low Cd-accumulating cultivars may possess higher abilities in regulating their rhizosphere conditions (pH, Eh, Fe3+/Fe2+) and thereby reducing Cd bioavailability in the rhizosphere through their root activities (ROL, oxidation, Fe plaque formation); low Cd-accumulating cultivars may also have weaker root Cd uptake kinetics and lower translocation efficiencies from root to shoot, and (2) flooded treatments may have a greater ability in reducing Cd bioavailability in rhizosphere soil, leading to a reduction of Cd accumulation in plants, compared to non-flooded treatments.
Three trials were conducted in order to confirm the hypotheses. The first trial investigated variations in Cd accumulation and biomass of 32 cultivars growing in Cd-contaminated soil, under both flooded and non-flooded conditions. The second soil rhizobox trial investigated the differences in the ecophysiological features of roots [root porosity, ROL, Fe plaque formation on roots] and rhizosphere features (pH, Eh, Fe3+/Fe2+, Cd speciation and mobility), using two low Cd-accumulating cultivars and two high Cd-accumulating cultivars, growing in Cd-contaminated soil under both flooded and non-flooded conditions. An uptake kinetics trial was also conducted under hydroponic conditions using one low and one high Cd-accumulating cultivar. The major aims of this study were thus to investigate (1) the variation of Cd accumulation in different cultivars of water spinach, (2) the effects of water management on Cd accumulation in this plant, and (3) the potential interaction between cultivars and water management.
Materials and methods
Experiment 1 - soil pot trial under flooded and non-flooded conditions
Cultivars tested
Seeds of 32 common water spinach cultivars (Table 1) were selected and acquired from local seed companies in Guangdong, Hubei, Hunan, Jiangxi and Jiangsu Provinces, P R China.
Soil preparation
A ‘clean’ soil was collected from a paddy field (0–20 cm depth) on the campus of South China Agricultural University, Guangdong Province, China. The soil contained 10 % organic matter (OM) [Total organic carbon (TOC) measured with a total organic carbon analyzer (TOC-VCPH, Shimadzu, Japan) (Li et al. 2012), and OM content calculated as a portion of TOC (Pribyl 2010)], 0.63 g kg−1 total K (determined by ICP-OES, Perkin Elmer, USA) (Li et al. 2012), 1.3 g kg−1 total N and 1.1 g kg−1 total P (using a Smartchem discrete auto analyzer, Smartchem200, AMS Westco, Italy) (Li et al. 2012). The soil pH (1:2 soil/water, suspensions) (Gleason et al. 2003) was 6.28 and the total Cd concentration (determined by AAS, Z-5300, Hitachi) of the soil was 0.09 mg kg−1. The soil was air-dried, sieved to < 8 mm. After thoroughly being homogenized, PVC pots (18 cm in upper diameter and 14 cm in height) were filled with 1.2 kg of the prepared soil. The soils were spiked with 1 mg kg−1 Cd in the form of 2.0 mg CdCl2 dissolved in 300 ml pot−1 deionized water. Two weeks after the Cd addition, 2.0 g pot−1 of a compound basal fertilizer [N:P:K = 26:6:13, in the form of (NH2)2CO, KH2PO4 and K2SO4] were added to the bulk soil and mixed thoroughly. The soil in each pot was kept submerged using deionized water for a month for equilibration in a glasshouse with the following conditions: temperature day/night 28/18 °C, day/night relatively humidity 60/80 %, and 16 h of light with a > 350 μmol m−2 s−1 photon flux density. After soil equilibration, the final total Cd and diethylene triamine pentaacetic acid (DTPA)-extractable Cd concentrations were 1.06 and 0.32 mg kg−1, respectively.
Experimental design
Seeds of water spinach were sterilized in 30 % H2O2 (w/v) solution for 15 min, followed by thorough washing with deionized water and then 10 seeds of each cultivar were sown into each prepared pot on 2 April 2012. The experiment was arranged in a randomized complete block design with four replicates; a total of 256 pots were used. The experimental plants were grown in a glasshouse at a temperature of 18–28 °C and watered daily with deionized water to maintain 70 % of water-holding capacity. On the 10th day after germination, seedlings were thinned to four per pot. One week later, two water treatments were conducted by adjusting the water regime for each cultivar: flooded or non-flooded for 60 days. Deionized water was added daily to raise the water status to full saturation capacity with c. 2 cm of standing water for the flooded treatment and to 70 % of the soil water-holding capacity for the non-flooded treatment. All plants of the cultivars were harvested on 19 June 2012. Biomass of shoot and root and concentrations of Cd in shoot and root tissue dry matter were subsequently determined. The DTPA-extractable Cd concentrations in soils collected from the root zone of the 11 cultivars (selected randomly) were also determined.
Experiment 2 - rhizobox trial and observation of root anatomy
Cultivars tested
Four water spinach cultivars [cv. Baigengliuye (BGLY) and cv. Qianyouqing 298 (QYQ 298) possessing higher Cd accumulation, designated as ‘high Cd-accumulating cultivars’; cv. Chunqingliuye (CQLY) and cv. Zixinshuiweng (ZXSW) with lower Cd accumulation, ‘low Cd-accumulating cultivars’] were selected and used in this rhizobox trial according to the results of Experiment 1. Sterilized seeds were germinated in the moist ‘clean’ soil. After 10 days, uniform seedlings (at the 3 leaf stage) were selected.
Rhizobox and soil preparation
A rhizobox was designed according to a modified version described by Youssef and Chino (1989) and Mei et al. (2012). It was constructed of a Plexiglass box (15 × 15 × 12 cm high) with an open top. Each box was separated into four sections (zones) by nylon netting (50 μm mesh) [a central zone or rhizosphere soil zone, 8 mm (4-0-4 mm) in length, and left and right near-rhizosphere soil zones (4–8 mm), a near bulk soil zone (8–40 mm) and a bulk soil zone (>40 mm)]. Each section was designated as S1, S2, S3, S4 respectively (Fig. S1).
The soil used in the rhizobox trial was collected from a paddy field (0–20 cm depth) in the Fankou mining area, Guangdong, China, in February 2013. Most paddy soil in this area has been contaminated by irrigation water polluted by mine drainage (Li et al. 2012). The soil was air-dried and then passed through a 2-mm sieve. The basic chemical properties of the soil were: 9.8 % organic matter, 1.3 g kg−1 total N, 1.5 g kg−1 total P, 4.3 g kg−1 total K, a pH of 5.98, 11 mg kg−1 Cd (analytical methods for all these elements were the same as that described in Experiment 1), 32 g kg−1 Fe and 227 mg kg−1 Mn (determined by ICP-OES, Perkin Elmer, USA) (Li et al. 2012).
Experimental design
In order to reveal the effects of water management on their rhizosphere, two seedlings of the same cultivar were planted in the centre of each rhizobox (with 3.0 kg soil), growing under flooded (with 2 cm of water above soil surface) and non-flooded (70 % of the soil water-holding capacity) conditions and harvested on day 75 (15 November 2013), respectively. In total, 24 rhizoboxes were used in this trial (4 cultivars × 2 water management regimes × 3 replicates). The rhizoboxes were placed in a glasshouse and arranged in a randomized complete block design for the growth period.
Half of the plant samples per rhizobox was then used to measure the root porosity and rate of ROL from roots; the other half was used to measure concentrations of Cd in shoot and root tissues, and Cd, Fe and Mn in Fe plaque on root surfaces. In addition, the following were also determined in this experiment: soil Eh, pH, Fe3+, Fe2+, concentrations of Cd, Fe and Mn, and concentrations of extracted Cd fractions in rhizosphere soil collected from S1 zone and in non-rhizosphere soil from S4 zone.
In addition, in order to observe variation in the root aerenchyma between cultivars with different capacities in accumulating Cd, seedlings of two cultivars [one high Cd-accumulating (cv. QYQ 298) and one low (cv. ZXSW)] were cultured simultaneously in 25 % Hoagland’s solution (1938) with 0.1 % agar (Wiengweera et al. 1997). Fresh cross-sections of roots of 30-d-old plants were cut by hand with a sharp razor at a distance of 7.0 cm from the root tip. These specimens were examined and photographed using a scanning electron microscope (SEM) (S520; Hitachi, Japan) (Deng et al. 2009; Cheng et al. 2012).
Experiment 3 – uptake kinetics trial
Cultivars tested
Two water spinach cultivars [one high Cd-accumulating (cv. QYQ 298) and one low-Cd accumulating (cv. ZXSW)] were selected and used in this experiment according to the results obtained from Experiments 1 and 2.
Experimental design
Sterilized seeds were germinated in acid-washed quartz sand for 10 d. Twenty-four seedlings of each cultivar were then transferred to plastic containers (12 L) and grown in 25 % Hoagland’s nutrient solution for 10 d. Uniform seedlings (20-d-old) were washed in deionized water and excised at the basal node. The excised roots were incubated in aerated test solution containing 0.5 mM CaCl2 and 2 mM MES (adjusted to pH 6.0 using KOH) and 0, 5, 10, 20, 35, 50 μM Cd for 30 min at room temperature (25 °C) (four replicates per treatment). After incubation, roots were rinsed in a fresh ice-cold solution containing 5 m M CaCl2 and 5 mM MES at pH 6.0 for 2 min and incubated in a fresh ice-cold nutrient solution of the same composition for 15 min to remove Cd adsorbed onto the root surfaces and from the root free space, following the method described by Esteban et al. (2008). Fresh roots were weighed, oven-dried at 70 °C for 2 days, and then ground to a powder for determination of Cd concentrations as described below.
Measurements of root porosity, rates of ROL, and extraction of Fe plaque
Root porosity (% gas volume/root volume) of plants was measured by the pycnometer method (Jensen et al. 1969; Kludze et al. 1993). The rates of ROL from roots were determined according to the Ti3+-citrate method described by Kludze et al. (1993) and Mei et al. (2009). Cadmium, Fe and Mn in Fe plaque on root surfaces were extracted by dithionite-citrate-bicarbonate (DCB) method (Otte et al. 1989).
Chemical analyses of soil and plant samples
Soil samples were collected from the S1 zone (rhizosphere soil) and S4 zone (bulk soil) of the rhizoboxes in Experiment 2. At harvest, all the rhizoboxes were transported to a N2-filled box in the laboratory. The soil samples taken from S1 and S4 zones (left and right) were mixed separately, and then each soil sample was placed and stored in a vacuum tube for further analysis under N2 conditions (Keon et al. 2001). Soil pH and Eh were measured with a pH/Eh meter (TM-39, Germany). Concentrations of Fe2+ and Fe3+ in soil samples were measured using the method described by Begg et al. (1994). For the analysis of Fe2+ and Fe3+, the soil was sampled as approximately 0.8–1 g from each tube and immediately immersed in 5 cm3 deoxygenated 5 mM CaCl2 to prevent oxidation. The sampling and immersion procedures taking < 20 s was to ensure that very little oxidation could take place (Begg et al. 1994). For total concentrations of Cd, Fe and Mn in the soil, subsamples (0.5 g dry wt) were digested in a mixture HNO3-HCl-HClO4 (1:3:1, v/v).
Sequential Cd extraction was conducted following the method of Tessier et al. (1979). Soil Cd was then specified as fraction F1: exchangeable Cd; fraction F2: Cd bound to carbonates; fraction F3: Cd bound to Fe-Mn oxides; fraction F4: Cd bound to organic matter; fraction F5: residual Cd. Oven-dried plant tissues were digested in a mixture HNO3-HClO4 (3:1, v/v). Concentrations of Cd in the digests of the soil and plant samples, Fe plaque extracts for total Cd, soil extracts (for Cd fractions) were determined by AAS. Concentrations of Fe and Mn in soil digests and in Fe plaque extracts from root surfaces were determined by inductively-coupled plasma optical emission spectrometry (ICP-OES; Optima 2000 DV, Perkin Elmer, USA). Blanks and standard plant (tea, GBW-08303) and soil (GBW-07435) reference materials (China Standard Materials Research Center, Beijing, P.R. China) were used to meet QA/QC requirements. Cadmium, Fe, and Mn recovery rates were 90 ± 10 %.
Statistical analyses
To estimate Cd translocation from root to shoot, the transfer factor (TF) was calculated as follows (Hart et al. 1998):
Cadmium mobility factors (MF), a modified version of that proposed by Kabala and Singh (2001), were calculated using the formula:
Data were analyzed using the SPSS 18.0 statistical software package and summarized as means ± standard errors (SE). A statistical comparison of treatment means was examined by one-way ANOVA followed by Tukey-HSD tests. Coefficients of determination (R2) and significance probabilities (P) were computed for linear regression fits.
Results
Experiment 1 - soil pot trial
Biomass and Cd accumulation in water spinach and DTPA-extractable Cd in soils
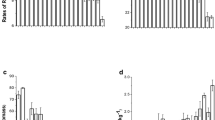
Summarized data for shoot biomass, Cd concentrations in shoots and roots, and Cd content of shoots of the 32 cultivars of water spinach in the flooded and non-flooded treatments are presented in Fig. 1. Shoot biomass varied significantly among the cultivars; shoot dry weights (DW) in the flooded and non-flooded treatments ranged from 532 to 2731 mg DW shoot−1 (mean 1330 mg DW shoot−1), and 451 to 1326 mg DW shoot−1 (mean 866 mg DW shoot−1), respectively. Half of the selected cultivars had significantly higher shoot biomass in the flooded treatment than in the non-flooded one (P < 0.05) (Fig. 1a). There were also large differences in Cd concentrations in shoot and root tissues and Cd content per shoot between the cultivars in the flooded and non-flooded treatments (P < 0.05) (Figs. 1 b, c and d). Cadmium concentrations in shoot (Fig. 1b) and root tissues (Fig. 1c) in the flooded treatment were significantly lower than those in the non-flooded treatment for all the 32 cultivars (P < 0.01). Cadmium concentrations ranged from 0.25 to 1.4 mg kg−1 (mean 0.90 mg kg−1 DW) and 1.9 to 4.7 mg kg−1 (mean 3.2 mg kg−1 DW) in shoots and from 0.82 to 4.3 mg kg−1 (mean 2.0 mg kg−1 DW), and 3.4 to 7.5 mg kg−1 (mean 5.5 mg kg−1 DW) in roots in the flooded and the non-flooded treatments, respectively. Cadmium contents per shoot (Fig. 1d) in the flooded treatment (ranged from 0.43 to 2.0 μg shoot−1; mean 1.2 μg shoot−1) were significantly lower than those in the non-flooded treatment (1.2 to 5.9 μg shoot−1; mean 2.8 μg shoot−1) for 25 of the cultivars (P < 0.05).
Shoot biomass (a), Cd concentrations (mg kg−1 DW) in shoots (b) and roots (c), and Cd content in shoots (d) of the 32 cultivars of water spinach grown in soil amended with 1 mg Cd kg−1 (as CdCl2) under flooded and non-flooded conditions (Mean ± SE, n = 4). * and ** indicate that the differences between the flooded and non-flooded treatments were significant at the P < 0.05 and P < 0.01 level, respectively
Cadmium concentrations in the shoots of 28 of the 32 cultivars in the flooded treatment were lower than 0.2 mg kg−1 FW [CAC Standard (Codex Standard 248-2005) and Maximum Levels of Contaminants in Foods of China (MLCF, GB 2762-2012)], but Cd concentrations in shoots of all 32 cultivars exceeded this standard in the non-flooded treatment (fresh weight basis) (Fig. S2).
DTPA-extractable Cd concentrations in soils collected from the root zone of the 11 cultivars selected under the flooded condition (range: 0.10 to 0.47; mean 0.25 mg Cd kg−1) were significantly lower than those under the non-flooded condition (0.37 to 0.69; mean 0.54 mg kg−1) (P < 0.001) (Fig. 2a).
DTPA-extractable Cd concentrations in soil of the selected 11 cultivars (a) and Cd transfer factors of 32 cultivars (b) of water spinach grown in soil amended with 1 mg Cd kg−1 (as CdCl2) under flooded and non-flooded conditions (Mean ± SE, n = 4). * and ** indicate that the differences between the flooded and non-flooded treatments were significant at the P < 0.05 and P < 0.01 level, respectively
Correlations between Cd in shoots, roots, Cd transfer factors and DTPA-extractable Cd in soils
Transfer factors of Cd from root to shoot varied significantly between the cultivars grown under both the flooded (ranged from 0.18 to 0.79; mean 0.51) and the non-flooded (0.29 to 0.89; mean 0.60) conditions (P < 0.05), but the transfer factors within the same cultivar were not significantly different between the two water conditions for most of the cultivars (Fig. 2b). Cadmium concentrations in shoots were significantly and positively correlated with Cd concentrations in root tissues (P < 0.001) (Fig. 3a) and the DTPA-extractable Cd concentrations in soils collected from the root zone (P < 0.001) (Fig. 3b). Significant correlations were also found between Cd concentrations in shoots and Cd transfer factors both under the flooded (P < 0.001) and non-flooded (P < 0.01) conditions (Fig. 3c).
Correlations between Cd concentrations (mg kg−1 DW) in shoots and roots (a) and transfer factors (c) (n = 256) of the 32 cultivars of water spinach, and correlations between Cd concentrations in shoots and DTPA-extractable Cd concentrations in soil (b) (n = 88) of the selected 11 cultivars of water spinach grown in soil amended with 1 mg Cd kg−1 (as CdCl2) under flooded and non-flooded conditions
Experiment 2 - rhizobox trial and observation of root anatomy
Root porosity and rates of ROL of four cultivars in the rhizobox trial
Dry weight of shoots and roots, root porosity and rates of ROL from roots in the four cultivars in the flooded treatment were significantly higher than those in the non-flooded treatment (Table 2). Differences in the dry weight of shoots and roots between the low Cd-accumulating cultivars (cvs. ZXSW and CQLY) and the high Cd-accumulating cultivars (cvs. BGLY and QYQ 298) were not obvious in both the flooded and the non-flooded treatments. Higher porosity and rates of ROL were recorded in the low Cd-accumulating cultivars (cvs. ZXSW and CQLY), whereas they were lower in the high Cd-accumulating cultivars (cvs. BGLY and QYQ 298) in both the flooded and the non-flooded treatments.
Cadmium in shoot and root tissues and Cd, Fe and Mn in Fe plaque
Concentrations of Cd in shoot and root tissues and Cd, Fe and Mn in Fe plaque on root surfaces varied between the four cultivars grown in the rhizoboxes filled with Cd-polluted soil with about 11 mg Cd kg−1, and also changed under flooded and non-flooded conditions (Table 3). Concentrations of Cd ranged from 1.5 to 5.5 mg kg−1 (DW) in shoot tissues, 1.9 to 4.4 mg kg−1 (DW) in root tissues, 1.1 to 2.0 mg kg−1 (DW) in Fe plaque on root surfaces under the flooded condition, and ranged from 3.2 to 8.9 mg kg−1 (DW) in shoot tissues, 4.0 to 4.4 mg kg−1 (DW) in root tissues, and were not detectable on root surfaces under non-flooded conditions. The cultivars with lower Cd accumulations in Experiment 1 (cvs. CQLY and ZXSW) also accumulated lower Cd in their shoot and root tissues, and higher Cd in Fe plaque on root surfaces compared to cvs. BGLY and QYQ 298 with higher Cd accumulations in this rhizobox trial (Table 3). Cultivars accumulated much higher Fe and Mn on root surfaces under flooded than under non-flooded conditions. In the flooded treatment, cvs. CQLY and ZXSW accumulated higher Fe and Mn on root surfaces than did cvs. BGLY and QYQ 298 (Table 3).
Redox, pH, Fe3+/Fe2+ quotients, Cd, Fe and Mn in rhizosphere and bulk soils
Results for soil Eh and pH values, Fe3+/Fe2+ quotients, and concentrations of Cd, Fe and Mn in the rhizosphere and bulk soils of water spinach grown under the flooded and non-flooded conditions are shown in Fig. 4. Under flooded conditions, all Eh values in S1 (ranged from 162 to 192 mV) were significantly higher than those in S4 (12–27 mV), whereas pH values in the S1 were significantly lower than those in S4 of all four cultivars studied. The Fe3+/Fe2+ quotients generally decreased from S1 (1.3-2.0) to S4 (1.1-1.3). Concentrations of Cd, Fe and Mn in soils mostly decreased from S1 (rhizosphere soil) to S4 (bulk soil) for the four cultivars studied. Similar situations were also found under the non-flooded conditions. pH values and concentrations of Cd, Fe and Mn in the rhizosphere soil (S1) under the flooded condition were significantly higher than those under the non-flooded condition for the same cultivar; the opposite was also true for Eh values and Fe3+/Fe2+ quotients (Fig. 4).
Chemical changes in rhizosphere (S1: 4-0-4 mm) and bulk (S4: >40 mm) soil zones of rhizoboxes of four water spinach cultivars (cvs. BGLY, QYQ 298, CQLY, ZXSW) grown in the soil contaminated with Cd under flooded and non-flooded conditions (Mean ± SE, n = 3), Eh (a), pH (b), Fe3+/Fe2+(c), Cd (d), Fe (e) and Mn (f). Different letters within the same soil zone indicate significant differences between the cultivars (P < 0.05) (BGLY and QYQ 298: high Cd-accumulating cultivars, CQLY and ZXSW: low Cd-accumulating cultivars)
Under the flooded condition, the cultivars with low Cd accumulation (cvs. CQLY and ZXSW) tended to have higher Eh, Fe3+/Fe2+ quotients and concentrations of Cd, Fe and Mn but lower pH values in S1 than the cultivars with higher Cd accumulation (cvs. BGLY and QYQ 298). Under the non-flooded conditions, variations of these elements between the cultivars studied were not significantly different.
Fractions and mobility factors of Cd in rhizosphere and bulk soils
Concentrations and proportion of extractable Cd fractions (F1-F5) in the rhizosphere soil (S1) and in the bulk soil (S4) are presented in Tables 4 and S1. Concentrations of fractions F1, F2 and F5 in the rhizosphere soil under the flooded condition were significantly lower than those under the non-flooded conditions for all the cultivars tested; the opposite was also true for fractions F3 and F4. Cadmium mobility factors (MF%) in the rhizosphere soils of the four cultivars tested ranged from 6.8 to 12 % (mean 9.6 %) in the flooded treatment (Table 4) and were much lower than the value (mean 24 %) in the non-flooded treatment (Table S1).
Under the flooded condition, Cd concentrations of fraction F1 (exchangeable Cd), F2 (Cd bound to carbonates) and F5 (residual Cd) tended to increase from the rhizospere soil (S1) to the bulk soil (S4), whilst concentrations of fractions F3 (Cd bound to Fe-Mn oxides) and F4 (Cd bound to organic matter) decreased from rhizospere soil to bulk soil for most cultivars. The cultivars with low Cd accumulation (cvs. CQLY and ZXSW) had lower fractions F1 and F2, but higher fractions F3 to F5 in the rhizosphere soil (S1) than the cultivars with higher Cd accumulation (cvs. BGLY and QYQ 298). Regarding mobility factors, those for cvs. CQLY (7.4 %) and ZXSW (6.8 %) were lower in S1 than those for cvs. BGLY (12 %) and QYQ 298 (12 %). The differences in concentrations and proportion of extracted Cd were not significant between the cultivars under non-flooded conditions (Table S1).
Root anatomy in water spinach
The typical structure of aerenchyma was different between the cultivars studied (Fig. 5). Compared with the transverse section of cv. QYQ 298, cv. ZXSW possessed more extensive aerenchyma and a larger proportion of central cylinder in the root cross-section; cv. ZXSW has more developed aerenchyma extending radially from the endodermis to the exodermis in comparison to cv. QYQ 298.
Cross-sectional scanning electron micrographs of the root (7 cm behind root tip, root length 8–9 cm) showing aerenchyma in two water spinach cultivars (a: cv. ZXSW, b: cv. QYQ 298) grown in 25 % Hoagland’s nutrient solution containing 0.1 % (w/v) agar (30-d-old). Abbreviations: ex exodermis, ae aerenchyma, en endodermis, cc central cylinder, arrows indicate radial extending of aerenchyma. Scale bars: 1 mm
Experiment 3 - uptake kinetics trial
Cadmium influx in cvs. ZXSW and QYQ 298 showed a hyperbolic increase with increasing concentrations of Cd (Fig. 6). The concentration-dependent influx data could be fitted better to Michaelis–Menten functions using non-linear curve fitting than to simple linear regressions. In cv. ZXSW, the average V max (maximum influx rate) (6.30 μmol g−1 FW h−1) for Cd uptake by roots was lower than for cv. QYQ 298 (31.10 μmol g−1 FW h−1), and the corresponding K m (Michaelis constant) value in roots of cv. ZXSW (37 μM) was also lower than that of cv. QYQ 298 (160 μM).
Discussion
Variations between cultivars in Cd accumulation
The results presented showed that Cd concentrations in the shoot (edible part) and root tissues varied considerably among the cultivars of water spinach (Fig. 1). Cadmium concentrations varied in shoots and roots of the cultivars by about 5.7- and 5.2-fold in the flooded treatment, and 2.5- and 2.2-fold in the non-flooded treatment, respectively. Variations in Cd accumulation between different cultivars may be related to intrinsic internal factors, such as the abilities for Cd uptake and accumulation in roots (Lux et al. 2011) and transfer factors of Cd from root to shoot (Clemens et al. 2002; Uraguchi et al. 2009), and also to environmental factors, especially properties of the rhizosphere (Arao et al. 2009; Zheng and Zhang 2011).
Uptake of Cd by roots has been considered a key process in overall plant Cd accumulation; active uptake of Cd into roots has been demonstrated in various plants (Hart et al. 1998; Chan and Hale 2004). The present study shows that Cd in the shoots of water spinach had a positive correlation with Cd in root tissues (Fig. 3a). The V max and K m value for cv. QYQ 298 (higher Cd-accumulating cultivar) was significantly higher than for cv. ZXSW (lower Cd-accumulating cultivar) (Fig. 6). Similar results have been reported in Cd uptake by different ecotypes of Noccaea caerulescens that both V max and K m values in the higher Cd-accumulating ecotype were more than five times higher than those in the lower one (Redjala et al. 2009). It suggested that the uptake of Cd into root cells was reduced in this special cv. ZXSW possibly by lowering the activity of membrane transporters. Our results also show that Cd concentration in shoots of different water spinach cultivars is significantly and positively correlated with Cd transfer factors from root to shoot (Fig. 3c), suggesting that the xylem-mediated root-to-shoot Cd translocation is another key process in shoot Cd accumulation in water spinach cultivars.
In the rhizosphere of wetland plants, oxygen [O2] concentration, pH and redox potential (Eh) are all important physico-chemical parameters of biogeochemical processes in the substratum (Reddy and Patrick 1977; Fitz and Wenzel 2002; Colmer 2003). Our results showed that pH values were significantly decreased, whilst Eh and Fe3+/Fe2+ quotients were markedly increased in the rhizosphere soil (S1), compared with those of the bulk soil (S4) in all the cultivars tested (Fig. 4). Kirk and Bajita (1995) indicated that the acidification of rhizosphere soil of rice was the result of H+ ions being released from the roots to balance intake of excess cations over anions, and H+ generated in oxidation of Fe2+ by root-released O2. The data from the rhizobox trial (Tables 2, 3 and Fig. 4) and observation of root cross-sections (Fig. 5) suggested that the cultivars with lower Cd accumulation have higher root porosity and rates of ROL, have higher degrees of Fe plaque formation on their root surfaces, and possess a highly-developed aerenchyma and have larger effects on the rhizosphere pH, Eh and Fe3+/Fe2+ balance than the cultivars with higher Cd accumulation. These data indicate that the low Cd-accumulating cultivars may have a greater ability in Fe-plaque formation and in modifying their rhizosphere properties, partly due to higher ROL capacities than the high Cd-accumulating cultivars. Previous studies reported that wetland plants (Yang et al. 2012) and rice cultivars (Mei et al. 2012) with higher root porosity and ROLs possess a higher ability to modify their rhizosphere, therefore reducing the accumulation of toxic elements (Pb and As) in their above-ground tissues. The present study also showed that the concentrations of Cd, Fe and Mn in the rhizosphere soil (S1) of cvs. CQLY and ZXSW having higher rates of ROL were significantly higher than those of cvs. BGLY and QYQ 298 with lower rates of ROL (Fig. 4). Among the biotic factors, the oxidizing capacity of plant roots is the most important one controlling Fe plaque formation, since the Fe plaque can act as a barrier to heavy metal (e.g., Cd) or metalloid (e.g., As) sequestration and uptake (Tripathi et al. 2014). Thus, the increased [O2] present in the rhizosphere will induce more Fe2+ and Mn2+ to be oxidized, more Fe plaque formation on root surfaces and in the rhizosphere and therefore more Cd, Fe and Mn to be fixed on root surfaces and in the rhizosphere.
Cadmium mobility factors in the rhizosphere soil of all four cultivars tested were lower than those in the bulk soil, especially for the special cultivars with lower Cd-accumulating and higher rates of ROL (cvs. CQLY and ZXSW) (Table 4). These cultivars consistently with higher degrees of Fe plaque on roots have a greater ability to reduce Cd mobility and bioavailability in their rhizosphere, in turn reducing Cd accumulation in their shoots. These observations suggest that the changes in fractions and mobility of Cd in the rhizosphere may be directly or indirectly correlated with ability in ROL and Fe plaque formation; these changes have direct effects on Cd accumulation in plants. It has been reported that ROL-induced Fe plaque promotes Cd deposition on to root surfaces, leading to a limitation of Cd transfer and distribution in rice (Cheng et al. 2014).
Effects of water management on biomass and Cd accumulation
Our study demonstrates that Cd concentrations in shoots of water spinach grown under flooded conditions were markedly lower than those under non-flooded conditions (Fig. 1 and Table 3). In the Cd-amended soil, the mean Cd concentration in shoots of the 32 cultivars in the flooded treatment was 0.90 mg Cd kg−1, compared to 3.2 mg Cd kg−1 in the non-flooded treatment (Fig. 1b). The same dramatic effect of water treatments on shoot Cd accumulation was further validated in the rhizobox trial using naturally Cd-contaminated soil (Table 3). In a previous study, Arao et al. (2009) also reported that flooding decreases Cd concentration in rice grains.
A decrease of Cd in shoots of plants under flooded conditions may be related to several factors, such as Cd bioavailability in the rhizosphere (Liu et al. 2008) and the dilution effect of any increases in biomass (Wang et al. 2014). Data presented show that the low Cd accumulation in shoots under flooded conditions (Fig. 1 and Table 3) may be mainly due to the fact that the flooded treatment has greater effects on the properties of rhizosphere soil (e.g., pH, Eh) (Fig. 4) and has a great ability in reducing Cd bioavailability and mobility in rhizosphere soil (Fig. 2, Table 4, Table S1) when compared to the non-flooded treatment. Concentrations of exchangeable Cd (F1 fraction) and the mobility factors (MF) for Cd in the rhizosphere soils of the four cultivars under flooded conditions were significantly lower than those under non-flooded conditions (Table 4, Table S1). Soil Cd in F3 (bound to Fe-Mn oxides) in the flooded treatment was significantly higher than that in non-flooded one, suggesting that with flooding more Cd was adsorbed and stabilized in Fe-Mn oxides during the process of rhizosphere oxidation to sequester Cd in the rhizosphere soil, and therefore Cd mobility was slowed down. These results agree with the aforementioned hypothesis. In addition, Arao et al. (2009) also reported that Cd in soils is consistently lower after flooding than that in aerobic conditions, when soil Eh decreases below −200 mV after flooding.
The reductions of Cd concentration in shoots in the flooded treatment may be partly due to a dilution effect, because shoot biomass of most cultivars studied was higher under flooded conditions than that under non-flooded conditions (Fig. 1a). The content of Cd in shoots was much lower in the flooded treatment than that in the non-flooded one, which is likely due to the diminished concentrations of Cd in shoots (Fig. 1). More importantly, Cd concentrations in shoots of 28 of the 32 cultivars studied under flooded conditions were < 0.2 mg Cd kg−1 FW [the maximum allowable Cd concentration in leafy vegetables recommended for China (GB 2762-2012) and the CAC (Codex Standard 248-2005)] (Fig. S2). This result suggests that an appropriate water management could help to obtain safe edible parts of water spinach grown in Cd-contaminated soil together with a satisfactory crop yield.
Conclusions
This study investigated the cultivar variations and effects of water management on Cd accumulation in water spinach. Our results demonstrated that there were large variations in Cd accumulation in the 32 cultivars studied under both flooded and non-flooded conditions. Flooding markedly increased shoot biomass and decreased shoot Cd accumulation. The root and rhizosphere characteristics of the cultivars have important roles in Cd uptake and accumulation in this important crop plant. The cultivars which showed low Cd accumulation in edible parts possess higher ability in reducing Cd mobility and bioavailability in their rhizosphere soils. They also have low Cd translocation from root to shoot. The fact that the flooded treatment markedly reduced Cd accumulation may be mainly due to a reduction in Cd mobility and bioavailability in the rhizosphere and an increase in shoot biomass. Our study suggests that we can significantly reduce Cd accumulation in edible parts by selecting appropriate cultivars and adopting a suitable water management regime when water spinach is grown in Cd-contaminated soils.
References
Arao T, Ishikawa S (2006) Genotypic differences in cadmium concentration and distribution of soybeans and rice. Jarq-Japn Agric Res Q 40:21–30
Arao T, Ae N, Sugiyama M, Takahashi M (2003) Genotypic differences in cadmium uptake and distribution in soybeans. Plant Soil 251:247–253
Arao T, Kawasaki A, Baba K, Mori S, Matsumoto S (2009) Effects of water management on cadmium and arsenic accumulation and dimethylarsinic acid concentrations in Japanese rice. Environ Sci Technol 43:9361–9367
Begg MCB, Kirk GJD, Mackenzie AF, Neue HU (1994) Root-induced iron oxidation and pH changes in the lowland rice rhizosphere. New Phytol 128:469–477
Chan DY, Hale BA (2004) Differential accumulation of Cd in durum wheat cultivars: uptake and retranslocation as sources of variation. J Exp Bot 55:2571–2579
Chang AC, Page AL, Foster KW, Jones TE (1982) A comparison of cadmium and zinc accumulation by four cultivars of barley grown in sludge-amended soils. J Environ Qual 11:409–412
Chao DY, Silva A, Baxter I, Huang YS, Nordborg M, Danku J, Lahner B, Yakubova E, Salt DE (2012) Genome-wide association studies identify Heavy Metal ATPase3 as the primary determinant of natural variation in leaf cadmium in Arabidopsis thaliana. Plos Genet 8(9):e1002923
Chen F, Dong J, Wang F, Wu F, Zhang G, Li G, Chen Z, Chen J, Wei K (2007) Identification of barley genotypes with low grain Cd accumulation and its interaction with four microelements. Chemosphere 67:2082–2088
Cheng H, Chen DT, Tam NFY, Chen GZ, Li SY, Ye ZH (2012) Interactions among Fe2+, S2−, and Zn2+ tolerance, root anatomy, and radial oxygen loss in mangrove plants. J Exp Bot 63(7):2619–2630
Cheng H, Wang MY, Wong MH, Ye ZH (2014) Does radial oxygen loss and iron plaque formation on roots alter Cd and Pb uptake and distribution in rice plant tissues? Plant Soil 375:137–148
Clemens S, Palmgren MG, Kramer U (2002) A long way ahead: understanding and engineering plant metal accumulation. Trends Plant Sci 7:309–315
Clemens S, Aarts MGM, Thomine S, Verbruggen N (2013) Plant science: the key to preventing slow cadmium poisoning. Trends Plant Sci 18(2):92–99
Colmer TD (2003) Aerenchyma and an inducible barrier to radial oxygen loss facilitate root aeration in upland, paddy and deepwater rice (Oryza sativa L.). Ann Bot 91:301–309
Deng H, Ye ZH, Wong MH (2009) Lead, zinc and iron (Fe2+) tolerance in wetland plants and relation to root anatomy and spatial pattern of ROL. Environ Exp Bot 65:353–362
Dunbar KR, McLaughlin MJ, Reid RJ (2003) The uptake and partitioning of cadmium in two cultivars of potato (Solanum tuberosum L.). J Exp Bot 54:349–354
Esteban E, Moreno E, Peñalosa J, Cabrero JI, Millán R, Zornoza P (2008) Short and long-term uptake of Hg in white lupin plants: kinetics and stress indicators. Environ Exp Bot 62:316–322
Fitz WJ, Wenzel WW (2002) Arsenic transformation in the soil-rhizosphere-plant system: fundamentals and potential application to phytoremediation. J Biotechnol 99:259–278
Florijn PJ, Van Beusichem ML (1993) Cadmium distribution in maize inbred lines: effects of pH and level of Cd supply. Plant Soil 153:79–84
Gleason SM, Ewel KC, Hue N (2003) Soil redox conditions and plant-soil relationships in a Micronesian mangrove forest. Estuar Coast Shelf Sci 56:1065–1074
Göthberg A, Greger M, Bengtsson BE (2002) Accumulation of heavy metals in water spinach (Ipomoea aquatica) cultivated in the Bangkok region, Thailand. Environ Toxicol Chem 21(9):1934–1939
Grant CA, Clarke JM, Duguid S, Chaney RL (2008) Selection and breeding of plant cultivars to minimize cadmium accumulation. Sci Total Environ 390:301–310
Greger M, Landberg T (2008) Role of rhizosphere mechanisms in Cd uptake by various wheat cultivars. Plant Soil 312:195–205
Hang XS, Wang HY, Zhou JM, Ma CL, Du CW, Chen XQ (2009) Risk assessment of potentially toxic element pollution in soils and rice (Oryza sativa) in a typical area of the Yangtze River Delta. Environ Pollut 157:2542–2549
Harrison HA (1986) Carrot response to sludge application and bed type. J Am Soc Hortic Sci 11:211–215
Hart JJ, Welch RM, Norvell WA, Sullivan LA, Kochian LV (1998) Characterization of cadmium binding, uptake, and translocation in intact seedlings of bread and durum wheat cultivars. Plant Physiol 116:1413–1420
Hinesly TD, Alexander DE, Ziegler EL, Barrett GL (1978) Zinc and Cd accumulation by corn inbreds grown on sludge amended soil. Agron J 70:425–428
Hseu ZY, Jien SH, Wang SH, Deng HW (2013) Using EDDS and NTA for enhanced phytoextraction of Cd by water spinach. J Environ Manag 117:58–64
Huang SS, Liao QL, Hua M, Wu XM, Bi KS, Yan CY, Chen B, Zhang XY (2007) Survey of heavy metal pollution and assessment of agricultural soil in Yang zhong district, Jiangsu Province, China. Chemosphere 67:2148–2155
Ishikawa S, Suzui N, Ito-Tanabata S, Ishii S, Igura M, Abe T, Kuramata M, Kawachi N, Fujimaki S (2011) Real-time imaging and analysis of differences in cadmium dynamics in rice cultivars (Oryza sativa) using positron-emitting 107Cd tracer. BMC Plant Biol 11:172
Ishikawa S, Ishimaru Y, Igura M, Kuramata M, Abe T, Senoura T, Hase Y, Arao T, Nishizawa NK, Nakanishi H (2012) Ion-beam irradiation, gene identification, and marker-assisted breeding in the development of low-cadmium rice. Proc Natl Acad Sci U S A 109(47):19166–19171
Jensen CR, Luxmoore RJ, Van-Gundy SD, Stolzy LH (1969) Root air space measurements by a pycnometer method. Agron J 61:474–475
Kabala C, Singh BR (2001) Fractionation and mobility of copper, lead and zinc in soil profiles in the vicinity of a copper smelter. J Environ Qual 30:485–495
Keon NE, Swartz CH, Brabander DJ, Harvey C, Hemond HF (2001) Validation of an arsenic sequential extraction method for evaluating mobility in sediments. Environ Sci Technol 35:2778–2784
Kirk GJD, Bajita JB (1995) Root-induced oxidation, pH changes and zinc solubilization in the rhizosphere of lowland rice. New Phytol 131:129–137
Kludze HK, Delaune RD, Patrick WH (1993) Aerenchyma formation and methane and oxygen exchange in rice. Soil Sci Soc Am J 51:368–391
Li B, Wang X, Qi XL, Huang L, Ye ZH (2012) Identification of rice cultivars with low brown rice mixed cadmium and lead contents and their interactions with the micronutrients iron, zinc, nickel and manganese. J Environ Sci 24:1790–1798
Liu J, Qian M, Cai G, Yang J, Zhu Q (2007) Uptake and translocation of Cd in different rice cultivars and the relation with Cd accumulation in rice grain. J Hazard Mater 143:443–447
Liu JC, Yan CL, Zhang RF, Lu HL, Qin GQ (2008) Speciation changes of cadmium in mangrove (Kandelia candel (L.)) rhizosphere sediments. Bull Environ Contam Toxicol 80:231–236
Liu JG, Cao CX, Wong MH, Zhang ZJ, Chai YH (2010) Variations between rice cultivars in iron and manganese plaque on roots and the relation with plant cadmium uptake. J Environ Sci 22:1067–1072
Lux A, Martinka M, Vaculík M, White PJ (2011) Root responses to cadmium in the rhizosphere: a review. J Exp Bot 62(1):21–37
Macfie SM, Crowder AA (1987) Soil factors influencing ferric hydroxide plaque formation on roots of Typha latifolia L. Plant Soil 102:177–184
Marcussen H, Joergensen K, Holm PE, Brocca D, Simmons RW, Dalsgaard A (2008) Element contents and food safety of water spinach (Ipomoea aquatica Forssk.) cultivated with wastewater in Hanoi, Vietnam. Environ Monit Assess 139:77–91
María-Cervantes A, Conesa HM, González-Alcaraz MN, Álvarez-Rogel J (2010) Rhizosphere and flooding regime as key factors for the mobilisation of arsenic and potentially harmful metals in basic, mining-polluted salt marsh soils. Appl Geochem 25:1722–1733
Mei XQ, Ye ZH, Wong MH (2009) The relationship of root porosity and radial oxygen loss on arsenic tolerance and uptake in rice grains and straw. Environ Pollut 157:2550–2557
Mei XQ, Wong MH, Yang Y, Dong HY, Qiu RL, Ye ZH (2012) The effects of radial oxygen loss on arsenic tolerance and uptake in rice and on its rhizosphere. Environ Pollut 165:109–117
Miyadate H, Adachi S, Hiraizumi A, Tezuka K, Nakazawa N, Kawamoto T, Katou K, Kodama I, Sakurai K, Takahashi H, Satoh-Nagasawa N, Watanabe A, Fujimura T, Akagi H (2011) OsHMA3, a P1B-type of ATPase affects root-to-shoot cadmium translocation in rice by mediating efflux into vacuoles. New Phytol 189:190–199
Otte ML, Rozema J, Koster L, Haarsma MS, Broekman RA (1989) Iron plaque on roots of Aster tripolium L.: interaction with zinc uptake. New Phytol 111:309–317
Pribyl DW (2010) A critical review of the conventional SOC to SOM conversion factor. Geoderma 156:75–83
Reddy CN, Patrick WH Jr (1977) Effect of redox potential and pH on the uptake of Cd and Pb by rice plants. J Environ Qual 6:259–262
Redjala T, Sterckeman T, Morel JL (2009) Cadmium uptake by roots: Contribution of apoplast and of high- and low-affinity membrane transport systems. Environ Exp Bot 67:235–242
Rivera-Becerril F, Calantzis C, Turnau K, Caussanel J-P, Belimov AA, Gianinazzi S, Strasser RJ, Gianinazzi-Pearson V (2002) Cadmium accumulation and buffering of cadmium-induced stress by arbuscular mycorrhiza in three Pisum sativum L. genotypes. J Exp Bot 53:1177–1185
Sasaki A, Yamaji N, Yokosho K, Ma JF (2012) Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Plant Cell 24(5):2155–2167
Satarug S, Baker JR, Urbenjapol S, Haswell-Elkins M, Reilly PEB, Willams DJ (2003) A global perspective on cadmium pollution and toxicity in non-occupationally exposed population. Toxicol Lett 137:65–83
Stolt P, Asp H, Hultin S (2006) Genetic variation in wheat cadmium accumulation on soils with different cadmium concentrations. J Agron Crop Sci 192:201–208
Tessier A, Campbell P, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:851–884
Thomas GM, Harrison HC (1991) Genetic line effects on parameters influencing cadmium concentration in lettuce (Lactuca sativa L.). J Plant Nutr 14:953–962
Tripathi R, Tripathi P, Dwivedi S, Kumar A, Mishra A, Chauhan PS, Norton GJ, Nautiyal CS (2014) Roles for root iron plaque in sequestration and uptake of heavy metals and metalloids in aquatic and wetland plants. Metallomics 6:1789–1800
Ueno D, Yamaji N, Kono I, Huang CF, Ando T, Yano M, Ma JF (2010) Gene limiting cadmium accumulation in rice. Proc Natl Acad Sci U S A 107(38):16500–16505
Uraguchi S, Fujiwara T (2013) Rice breaks ground for cadmium-free cereals. Curr Opin Plant Biol 16(3):328–334
Uraguchi S, Mori S, Kuramata M, Kawasaki A, Arao T, Ishikawa S (2009) Root-to-shoot Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. J Exp Bot 60:2677–2688
Van der Vliet L, Peterson C, Hale B (2007) Cd accumulation in roots and shoots of durum wheat: the roles of transpiration rate and apoplastic bypass. J Exp Bot 58:2939–2947
Vymazal J, Švehla J (2013) Iron and manganese in sediments of constructed wetlands with horizontal subsurface flow treating municipal sewage. Ecol Eng 50:69–75
Wang JL, Wei F, Yang ZY (2007) Inter-and Intra-specific variations of Cd accumulation of 13 leafy vegetable species grown in Cd contaminated soils. J Agric Food Chem 34:1154–1158
Wang MY, Chen AK, Wong MH, Qiu RL, Cheng H, Ye ZH (2011) Cadmium accumulation in and tolerance of rice (Oryza sativa L.) varieties with different rates of radial oxygen loss. Environ Pollut 159:1730–1736
Wang X, Ye ZH, Li B, Huang LN, Meng M, Shi JB, Jiang GB (2014) Growing rice aerobically markedly decreases mercury accumulation by reducing both Hg bioavailability and the production of MeHg. Environ Sci Technol 48:1878–1885
Wei S, Twardowska I (2013) Main rhizosphere characteristics of the Cd hyperaccumulator Rorippa globosa (Turcz.) Thell. Plant Soil 372:669–681
Wiengweera A, Greenway H, Thomson CJ (1997) The use of agar nutrient solution to simulate lack of convection in waterlogged soils. Ann Bot 80:115–123
Williams PN, Lei M, Sun GX, Huang Q, Lu Y, Deacon C, Meharg AA, Zhu YG (2009) Occurrence and partitioning of cadmium, arsenic and lead in mine impacted paddy rice: Hunan, China. Sci Total Environ 43:637–642
Xu XY, McGrath SP, Meharg AA, Zhao FJ (2008) Growing rice aerobically markedly decreases arsenic accumulation. Environ Sci Technol 42:5574–5579
Yang JX, Liu Y, Ye ZH (2012) Root-induced changes (pH, Eh, Fe2+ and speciation of Pb and Zn) in rhizosphere soils of four wetland plants with different ROL. Pedosphere 22:518–527
Youssef RA, Chino M (1989) Root-induced changes in the rhizosphere of plants II. Distribution of heavy metal across the rhizosphere in soil. Soil Sci Plant Nutr 35:609–621
Zhao FJ, Jiang RF, Dunham SJ, McGrath SP (2006) Cadmium uptake, translocation and tolerance in the hyperaccumulator Arabidopsis halleri. New Phytol 172:646–654
Zheng SN, Zhang MK (2011) Effect of moisture regime on the redistribution of heavy metals in paddy soil. J Environ Sci 23:434–443
Zhuang P, Zou B, Li NY, Li ZA (2009) Heavy metal contamination in soils and food crops around Dabaoshan Mine in Guangdong, China: implication for human health. Environ Geochem Health 31:707–715
Acknowledgments
This work was funded by the National Natural Science Foundation of China (30770417), a Start-up Research Grant for Newly Recruited Professors/(Research) Chair Professors, The Hong Kong Institute of Education (RG24/13-14R) and National ‘863’ projects of China (2012AA061510). We thank Prof. A.J.M. Baker (The Universities of Melbourne and Queensland, Australia) for help in the initial preparation and improvement of this paper and the anonymous reviewers for their helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Juan Barcelo.
Rights and permissions
About this article
Cite this article
Xiao, Q., Wong, M.H., Huang, L. et al. Effects of cultivars and water management on cadmium accumulation in water spinach (Ipomoea aquatica Forsk.). Plant Soil 391, 33–49 (2015). https://doi.org/10.1007/s11104-015-2409-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2409-5