Abstract
In order to clarify how cadmium (Cd) chemical forms in planta relate to the genotype difference in Cd accumulation of spinach (Spinacia oleracea L.), two low-Cd and two high-Cd cultivars were compared under a hydroponic experiment with two concentrations of Cd (8.98 or 44.71 μmol Cd L−1). The concentrations of phosphorus in the hydroponic system were also adjusted to two levels (0.5 and 1.0 mmol L−1) to investigate the influence of phosphorus on the forms and accumulation of Cd in the tested cultivars. Average Cd concentrations in shoots were 8.50−10.06 mg kg−1 for high-Cd cultivars and 6.11–6.64 mg kg−1 for low-Cd cultivars a under lower Cd treatment and were as high as 24.41–31.35 mg kg−1 and 19.65–25.76 mg kg−1, respectively, under a higher treatment. Phosphorus significantly decreased Cd accumulation in the tested cultivars, and the effect had superiority over the cultivar alternation under higher Cd stress. Cadmium in the NaCl-extractable fraction of the plant tissues showed the greatest relationship to genotype difference of Cd accumulation. The difference in the capacity to binding Cd into F HAc, F HCl, or F Residue was another important mechanism involving in the genotype difference in Cd accumulation of spinach. Among them, average proportion of Cd in F HAc in low-Cd cultivars was higher than that in high-Cd cultivars in association with the effect of phosphorus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Contamination of agricultural soil by heavy metals such as Cu, Zn, Cd, and Pb is a substantial problem globally, especially in China (Murtaza et al. 2008; Nicholson et al. 2003). These heavy metals present a threat to human health when they enter the food chain (Satarug et al. 2003). The contamination is mainly caused by pollutant discharges from industrial and mining processes as well as a result of overuse or improper use of pesticides, insecticides and chemical fertilizers in agriculture. In China, millions of acres of agricultural lands and over 12 million tons of grain are contaminated by heavy metals. Ten percent of rice in China contains excessive cadmium, a heavy metal known to cause cancer, osteoporosis, cardiovascular disease, and renal dysfunction (Nawrot et al. 2010; Wu and Zhu 2014). Various soil cleanup techniques have been proposed and proven effective (Mulligan et al. 2001). However, it is a challenge to employ these techniques in many developing countries because of their high costs (Ebbs et al. 1997; Salt et al. 1995). Furthermore, in China, farmers cannot afford to leave agricultural soils long-term fallow for the remediation process due to the high demand for food products.
One of the alternative strategies for reducing the entrance of Cd into the human food chain is to select cultivars that accumulate low levels of Cd in their edible parts (Grant et al. 2008; Huang et al. 2015; McLaughlin et al. 1994; Wang et al. 2009; Xin et al. 2013; Yu et al. 2006; Zhu et al. 2007). This cultivar selection strategy is feasible because, for a number of agronomic plant species, significant differences exist among cultivars in Cd uptake and accumulation (Grant et al. 2008). A wide variation in Cd accumulation among current cultivars has been reported for some staple crops (Clarke et al. 2002; Dai et al. 2010; Liu et al. 2010; McLaughlin et al. 1994; Yu et al. 2006) and leafy vegetables (Dai et al. 2012; Huang et al. 2014; Liu et al. 2010; Qiu et al. 2011a; Wang et al. 2007, 2009; Xue et al. 2014; Zhang et al. 2013a, b; Zhou et al. 2013; Zhu et al. 2007).
There has been considerable research seeking to understand the underlying genetic, molecular, biochemical, and physiological processes that contribute to the low Cd accumulation phenotype and to lower the risk of Cd entering the food chain (Clarke et al. 1997; Grant et al. 2008; Huang et al. 2009; Ishikawa et al. 2012; Ishimaru et al. 2012; Li et al. 2007; Penner et al. 1995; Tanhuanpää et al. 2007). For example, Grant et al. (2008) has succeeded in breeding of a low-Cd durum wheat cultivar named AC Napoleon in Canada. Xin et al. (2010) has reported a new cultivar of water spinach (Ipomoea aquatica Forsk.) with high shoot biomass and low shoot Cd and Pb concentrations.
Phosphorus (P) is a macronutrient that accounts for ∼0.2 % of plant dry weight and when limiting can reduce plant growth and yield. This element is essential for the synthesis of nucleic acids, phospholipids, and ATP. It has also been reported that addition of P-based materials to soils can influence the bio-availability of heavy metals such as Pb, Cd, and Zn. The amendment of P to soils reduced the accumulation of Cd in both low-Cd and high-Cd cultivars of Chinese flowering cabbage (Brassica parachinensis L.) (Qiu et al. 2011b).
Spinach (Spinacia oleracea L.) is an important leafy vegetable that is cultivated and consumed all over the world, particularly in Southeast Asia during the majority of the year. Spinach has been described as a Cd-accumulating species (Alexander et al. 2006; Chunilall et al. 2004; Kuboi et al. 1986). A strong influence of cultivar on shoot Cu, Zn, and Cd concentrations was observed in a previous study in Germany with 11 spinach cultivars (Römer and Keller 2002). However, a similar study carried out in England found no significant variations in Cd, Cu, Pb, or Zn concentrations among five spinach cultivars grown on metal-spiked soil (Alexander et al. 2006). There is little available information about the mechanisms affecting the genotype differences of Cd uptake, translocation, and accumulation in spinach. In our previous study, the maximum difference in shoot Cd concentration varied by 7.2-fold among 29 spinach cultivars (unpublished data). We identified two low-Cd accumulation cultivars (low-Cd group) and two high-Cd accumulation cultivars (high-Cd group) in the study. These four spinach cultivars allow for a further investigation of the mechanisms associated with the genotype differences. In the present study, the chemical forms of Cd in plant tissues between the low-Cd cultivars and the high-Cd cultivars were compared in order to provide insight into the relevant biochemical mechanisms. Due to the previous researches reporting the effects of soil phosphorus on Cd accumulation of spinach (Dheri et al. 2007; Keller and Römer 2001; Römer and Keller 2002), phosphorus concentration was also altered to investigate how the interaction between Cd and P contributes to the genotype difference. We hypothesize that the genotype-dependent Cd accumulation of spinach is related to chemical forms of Cd, and phosphorus is a crucial factor that interacts with Cd to influence the chemical form of Cd within the plant tissues and, therefore, the extent of Cd accumulation.
Material and methods
Spinach cultivars
The four tested cultivars of spinach used in the present study were DMMNKS and CY (low Cd-accumulating cultivars) and CJQNDH and CJQLDY (high Cd-accumulating cultivars). Prior study (unpublished data) established the characteristics of these lines. Shoot Cd concentrations of DMMNKS, CY, CJQNDH, and CJQLDY grown in Cd-contaminated soil (Cd concentration up to 0.79 mg kg−1) were 0.49, 0.44, 1.72, and 1.40 mg kg−1. The high-Cd group had tissue concentrations generally 3.4-fold higher than that of the low-Cd group.
Preparation of plant samples and experimental treatments
Seeds of the tested cultivars were sterilized by 2 % (v/v) H2O2 for 10 min and then sown into a cuboid pot (60 × 40 × 8 cm) filled with vermiculite at a rate of 80 seeds pot−1. Hoagland’s nutrient solution was applied every day to maintain the moisture content of the culture media and provide the necessary nutrients. The Hoagland solution containing 5 mmol L−1 Ca(NO3)2·2H2O, 5 mmol L−1 KNO3, 2 mmol L−1 MgSO4·7H2O, 1 mmol L−1 KH2PO4, 0.1 mmol L−1 EDTA-Fe, 47 μmol L−1 H3BO3, 1 μmol L−1 MnCl2·4H2O, 1 μmol L−1 ZnSO4·7H2O, 0.01 μmol L−1 H2MoO4, and 0.25 μmol L−1 CuSO4·5H2O. The pots were placed in a greenhouse at the Guangdong University of Petrochemical Technology (Maoming City, China) with a light intensity of 500–800 μmol m−2 s−1, day length of about 11 h, day/night temperatures of 30 °C/25 °C, and relative humility of 40–45 %.
A separate hydroponic experiment using 500-mL containers was conducted to test the genotype differences in Cd chemical forms in the plant tissues. Each container was filled with 400 mL Hoagland solution with different concentrations of Cd and P as treatments. Control (with no Cd added, designated as Cd0) and two Cd treatments (designated as Cd1 and Cd2) were conducted. The Cd1 and Cd2 treatments were carried out by adding Cd in the form of Cd(NO3)2 for 8.93 and 44.64 μmol L−1, respectively, into the Hoagland solution, and the final Cd concentrations were 8.98 and 44.71 μmol L−1, respectively. The P treatments were established by reducing the concentration of KH2PO4 to half of the typical Hoagland solution concentration (P1) or left at the typical solution concentration (P2). Overall, there were six Cd-P levels, which were assigned as Cd0P1, Cd0P2, Cd1P1, Cd1P2, Cd2P1, and Cd2P2. Concentrations of free Cd2+ in the solutions calculated using Geochem-EZ program (Shaff et al. 2010) for Cd1P1, Cd1P2, Cd2P1, and Cd2P2 were 1.1, 1.0, 23.0, and 21.5 μg L−1, respectively.
On Jan. 4, 2012, the 20th day after the seeding of spinach, four seedlings with uniform size and with four leaves were identified. The seedlings were transplanted to each 500-mL plastic container covered by a cap that allowed four plants to be established in each container. The seedlings were passed through a hole (15-mm diameter) in the cap and held in place with sterile cottons. The experiment used a completely randomized design with three replicates per treatment. Thus, there are a total of 72 containers (4 cultivars × 6 Cd-P levels × 3 replicates) in the hydroponic experiment.
Sampling of both shoots and roots was carried out on Jan. 19, 2012, after a 15-day growth period. All shoot samples were thoroughly rinsed with deionized water, and roots were with a 0.5 mM CaCl2 solution for 30 min and then rinsed with deionized water. Each tissue sample was weighed, frozen in liquid nitrogen, and stored at −80 °C until use.
Extraction of Cd in different chemical forms
Cadmium associated with various chemical forms in the plant tissues was determined by successively extracting tissues with the following sequence of solutions (Wu et al. 2005):
-
(1)
80 % ethanol (F E), extracting inorganic Cd associated with nitrate, chloride, or aminophenol Cd;
-
(2)
distilled water (F D), extracting water-soluble Cd associated with organic acids or as Cd(H2PO4)2;
-
(3)
1 M NaCl (FNaCl), extracting pectate- and protein-associated Cd;
-
(4)
2 % acetic acid (HAc, F HAc), extracting insoluble CdHPO4, Cd3(PO4)2, and other Cd-phosphate complexes;
-
(5)
0.6 M HCl (FHCl), extracting Cd in oxalate;
-
(6)
Cd in residues (F residue).
Frozen plant materials were cut into small pieces of 1–2 mm2, mixed with 37.5 mL of the appropriate extraction solution and incubated at 30 °C for 18 h. The extraction solution was then separated, and the residual material was re-extracted an additional volume of the same extraction solution (37.5 mL) under the same conditions for another 6 h. The two extracts were combined. This double extraction procedure was repeated a second time for the plant tissue. The residual plant material was extracted with the next extraction solution in the sequence, using the same procedure described above. All of the extracts (150 mL for each) were evaporated to constant mass and digested in a microwave digester (WX-8000, Shanghai Xinyi) with an oxidizing mixture of acids (HNO3–HClO4, 5:1, v/v). The digests were used for the analysis of Cd concentration.
Analysis for Cd
Cadmium concentrations in the digests were determined by FAAS (Hitachi Z-5300, Japan). The precision of the analytical procedures for plant material was assessed using a Certified Reference Material (CRM) (GBW-07603) provided by the National Research Center for CRM, China. Total Cd concentrations in shoot and root samples were determined with the same method following acid digestion with HNO3–HClO4 (5:1, v/v). The Cd concentrations were based on the fresh weights of samples before separation or extraction.
Data statistics
Total Cd concentration of each tissue was obtained by summing Cd concentrations in the 6 fractions for shoots or roots. The least significant difference (LSD) test followed by a three-way ANOVA (full model and reduced models) was performed based on the results of BF test and OBRIEN test by SAS 9.3 (Cary, NC).
Results
Total Cd concentration
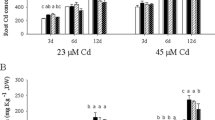
Although growth of the tested spinach plants were depressed by the Cd treatments and the average biomasses under Cd1P2 and Cd2P2 decreased by 23.6 and 36.1 %, respectively, when comparing with control (unpublished data), the plants grew well and no chlorosis was observed under all treatments. Total shoot and root Cd concentrations in the tested cultivars of spinach under different Cd-P treatments are shown in Table 1. The control was undetectable; hence, the data are not shown. Results from three-way ANOVA for the data of shoot Cd concentrations (Table 2) indicated that the effect of cultivar, Cd concentration, and P concentration were all significant (P < 0.05). Significance was also observed in Cd × P interaction (P < 0.05), but no significance was determined in cultivar × Cd, cultivar × P, and cultivar × Cd × P interactions (P > 0.05).
Low-Cd cultivars (DMMNKS and CY) generally had significantly lower shoot Cd concentrations (P < 0.05) than the high-Cd cultivars (CJQNDH and CJQLDY) except for the Cd1P2 treatment (Table 1). Average shoot Cd concentrations in the low-Cd cultivars were only 66.0 % (P1, 0.5 mmol L−1 of KH2PO4) and 71.0 % (P2, 1.0 mmol L−1 of KH2PO4) of those in the high-Cd cultivars under Cd1, while under Cd2, the differences were higher at 82.1 % (P1) and 80.52 % (P2). This indicated that higher Cd exposure would lead to a decrease of genotype difference in shoot Cd accumulation. Considering effects of both cultivar × P and cultivar × Cd × P interactions were not significant, level of phosphorus in cultivating solution might be less related to the genotype difference in shoot Cd accumulation in spinach, although higher level of phosphorus, compared to lower level, declined shoot Cd accumulation in both high-Cd and low-Cd cultivars.
The mean reduction in shoot Cd concentration as a function of cultivars (−39.0 to −51.5 %, (average shoot Cd concentration in low-Cd cultivars − average shoot Cd concentration in high-Cd cultivars) / average shoot Cd concentration in high-Cd cultivars × 100) were greater than those by P supplement (−8.7 to −18.4 %) (average shoot Cd concentration under P2 treatments − average shoot Cd concentration under P1 treatments) / average shoot Cd concentration under P2 treatments × 100) under Cd1. Under Cd2, however, those mean reductions from P treatment (−28.4 to −31.1 %) were greater than those from the different cultivar (−21.7 to −24.2 %). These results illustrate why there was a significant variation in Cd × P interaction according to the three-way ANOVA.
For total root Cd concentrations, it was found that the effect of cultivar was not significant (P > 0.05) according to three-way ANOVA (Table 2), although the concentrations of low-Cd cultivars were all lower than those of high-Cd cultivars. The effects of Cd and P concentrations were each significant (P < 0.05). Similar to shoots, variation in root Cd concentrations derived from Cd × P interaction were significant (P < 0.05), but insignificant for those from cultivar × Cd, cultivar × P, and cultivar × Cd × P interactions (P > 0.05).
More intense differences in Cd concentration in roots in response to the P treatment were observed. The mean decreases in response to the P treatment (−66.3 to −71.5 % under Cd1 and −30.0 to −51.6 % under Cd2) were generally greater than for the cultivar effect (−12 to −51.6 % under Cd1 and −13.8 to −32.8 % under Cd2). Different from the shoot Cd concentrations, the decrease in root Cd concentration in response to the P treatment was smaller under the Cd2 treatment than the Cd1 treatment. Consistent to that in shoot, level of phosphorus in cultivating solution seemed no significant influence on genotype difference in root Cd accumulation.
Cd concentrations in different chemical forms
Shoot and root Cd concentrations in different chemical forms of the tested cultivars as well as results of two-way ANOVA are shown in Tables 3 and 4. For the shoots, the most obvious genotype-associated responses were observed in F NaCl, and the differences of shoot Cd in the fraction between low-Cd and high-Cd cultivars were significant under Cd1P1, Cd2P1, and Cd2P2 (P < 0.05). The Cd in F NaCl, F HAc, and F HCl revealed a consistent change pattern that P2 treatment significantly decreased their concentrations unrelated to cultivar under Cd1P1, Cd2P1, and Cd2P2. For the roots, there was no any Cd fraction that exhibited a significant variation derived from cultivar under all of the Cd-P treatments. However, P2 treatment significantly increased Cd concentrations in F D under Cd2 treatment and significantly lowered Cd concentrations in F NaCl and F HCl under both Cd treatments (P < 0.05). These results indicated that the P treatment affected Cd speciation in spinach more effectively than the cultivar alternation did, which is consistent with those observed in the total Cd accumulation.
Proportions of Cd in different chemical forms
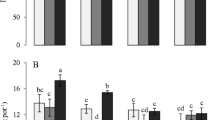
Proportions of Cd in different chemical forms in shoots and roots are shown in Figs. 1 and 2. In both shoots and roots, the proportions exhibited a general trend of F NaCl > F HAc > F HCl > F D > F E > F Residue. This result indicated that Cd in F NaCl, which accounted for more than 50 % of total Cd in both shoots and roots, played the most important role in Cd accumulation and detoxification in spinach. Differences in the proportions of Cd in F NaCl between low-Cd and high-Cd groups were not obvious, and the proportions for the low-Cd group were generally lower than or similar to those of the high-Cd group in both shoots and roots. Cd-P treatments did not consistently influence the proportion of Cd in F NaCl in both shoots and roots, but Cd2 treatments increased the proportion in roots when compared to Cd1 treatments.
Proportions of Cd in different chemical forms in shoots of spinach. F E , Cd in ethylalcohol extracted form; F D , Cd in water-soluble form; F NaCl , Cd in NaCl extracted form; F HAc , Cd in acetic acid extracted form; F HCl , Cd in hydrochloric acid extracted form; F residue , Cd in residue. Cd concentrations in Cd1 and Cd2 were 8.98 and 44.71 μmol L−1, respectively; P concentrations in P1 and P2 were 0.5 and 1.0 mmol L−1, respectively
Proportions of Cd in different chemical forms in roots of spinach. F E , Cd in ethylalcohol extracted form; F D , Cd in water-soluble form; F NaCl , Cd in NaCl extracted form; F HAc , Cd in acetic acid extracted form; F HCl , Cd in hydrochloric acid extracted form; F residue , Cd in residue. Cd concentrations in Cd1 and Cd2 were 8.98 and 44.71 μmol L−1, respectively; P concentrations in P1 and P2 were 0.5 and 1.0 mmol L−1, respectively
The sums of proportions of Cd in F HAc, F HCl, and F Residue, which were presumed to be forms with lower mobility within the plant, were 24–36 % in shoots and 24–40 % in roots and were generally higher in low-Cd cultivars than in high-Cd cultivars especially for those under Cd2. The average proportions of Cd in F HAc in shoots were 19.88 % (P1) and 16.81 % (P2) for low-Cd cultivars, higher than those of high-Cd cultivars (17.94 % under P1 and 15.09 % under P2). The average proportions in roots were 17.40 % (P1) and 23.64 % (P2) for low-Cd cultivars and also higher than those of high-Cd cultivars (14.16 % under P1 and 21.80 % under P2). The average proportions displayed a higher value under P2 than under P1 for both low-Cd and high-Cd groups, indicating that a higher level of phosphorus can enhance the formation of Cd-phosphates.
Under Cd2, the total proportions of Cd in F HAc, F HCl, and F Residue greatly decreased in both shoots and roots compared to those under Cd1, indicating that the capacity to chemically deactivate Cd in vivo was restrained when Cd stress increased from Cd1 to Cd2. The sums of the proportions generally decreased in shoots but increased in roots with the P concentration was increased from P1 to P2, implying different effects of P on Cd chemical forms between the shoots and roots.
For the proportions of Cd in F E and F D, the fractions with higher activity, the sums were 11–20 % in shoots and 6–11 % in roots and were not consistently different between the low-Cd and the high-Cd cultivars for either shoots or roots. This demonstrated that these two fractions did not differ as a function of cultivar. The sums under Cd2 were generally higher than those under Cd1 in shoots, but were reversed in roots, indicating perhaps that roots of spinach could more effectively deactivate Cd under higher Cd exposure than shoots. The sums of proportions in both tissues of all the tested cultivars (except cv. CJQNDH) were higher under P2 than under P1.
Discussion
Genotype-dependent Cd accumulation in spinach
In the present study, differences in total Cd concentrations in shoots and roots between low-Cd and high-Cd cultivars of spinach under hydroponic condition were consistent with the results obtained under soil culture condition in our previous unpublished study. Hence, the specific genotype differences in Cd accumulation are stable, reproducible traits and not specifically dependent on the growth conditions. Similar results have been obtained in many crops such as rice (Oryza sativa L.) (Yu et al. 2006), asparagus bean (Vigna unguiculata subsp. Sesquipedalis L.) (Zhu et al. 2007), hot pepper (Capsicum annuum L.) (Xin et al. 2014), water spinach (Wang et al. 2009), Chinese flowering cabbage (Qiu et al. 2011a), small Chinese cabbage or pakchoi (Brassica chinensis L.) (Xue et al. 2014), Chinese leaf mustard (Brassica juncea L. Czern. et cross. var. juncea) (Dai et al. 2012), and amaranth (Amaranthus spp.) (Zhou et al. 2013). Some researchers have investigated the genetic mechanisms regulating Cd accumulation and detoxification, and special attention has been given to phytochelatins (PCs), a type of Cd-induced metal-binding proteins (peptides) in plants. Phytochelatins are a class of glutathione-derived peptides which can help to transport Cd into vacuole in the form of a Cd-PC complex (Clemens 2006). RNAi-mediated silencing of OsPCS1 had been attempted and resulted in the reduction of Cd accumulation in the RNAi rice seeds approximately by half (Li et al. 2007). It was found that Cd-sensitive barley genotype had less Cd integrated with proteins/pectates as compared with Cd-resistant genotypes (Wu et al. 2005). Beside Cd tolerance, Cd accumulation was also found to be associated with proteins/pectate-bound Cd in certain vegetable crops such as Chinese flowering cabbage (Qiu et al. 2011a) and amaranth (Zhou et al. 2013). These results established the relationship between PC-Cd complexes and certain Cd chemical form, i.e., the NaCl-extractable fraction.
Much high Cd accumulations were found in the tested cultivars of spinach in both the previous and the present study. According to our previous study, the maximum shoot Cd concentration among the 29 tested cultivars was 145.4 mg kg−1 (dry weight basis) in soil containing 14.1 mg kg−1 Cd (unpublished data). According to the water content in shoots (about 90 %) of spinach under soil culture conditions in the previous study, shoot Cd concentration of the high-Cd cultivars under Cd1 (1 mg L−1) in the present study would be >100 mg kg−1 (dry weight basis), exceeding the critical level for Cd hyperaccumulator (Baker and Brooks 1989), and it would be >300 mg kg−1 under Cd2 treatment (5 mg L−1). Hence, spinach is a crop with high Cd pollution risk once cultivated under Cd-contaminated soils, and identification and popularization of low-Cd cultivars are crucial ways for ensuring food safety in spinach production. Based on the genotype-dependent Cd accumulation of spinach verified in the present study, breeding of low-Cd cultivars of the species should be considered.
Chemical mechanisms related to genotype difference in Cd accumulation of spinach
The profile of Cd chemical forms in shoots and roots of spinach was characterized by a high proportion of F NaCl. It was found that the greatest amount of Cd was extracted by 1 M NaCl and this accounted for >50 % of the Cd in both shoots and roots. This result has been observed in several vegetable crops. Qiu et al. (2011b) found that proportions of Cd in F NaCl in shoots and roots of Chinese flowering cabbage grown under Cd-contaminated soils were close to or exceeded 50 %. Dai et al. (2012) reported that proportions of Cd in F NaCl in shoots of Chinese leaf mustard were >40 %. For vegetable amaranth, proportions of Cd in F NaCl in stems and roots were also predominated (40–60 %) when plants were grown in Cd-contaminated soils (Zhou et al. 2013).
Similar to studies mentioned above, the proportion of Cd in the F NaCl fraction of shoots from spinach was generally lower in low-Cd cultivars than in high-Cd cultivars. Under Cd1 treatment, significant genotype differences of shoot Cd were only appeared in the F NaCl according to two-way ANOVA. This may be related to the higher capacity in the high-Cd cultivars to resist the toxic effects involving in phytochelatins (PCs). As has been mentioned above, the majority of Cd in F NaCl is integrated with proteins/pectates, including Cd bound to PCs (Wu et al. 2005). The PC-Cd complex could pass through a vacuole membrane and the Cd could precipitate within the vacuole as insoluble phosphates. This has been recognized as a major Cd detoxification mechanism of plants (Clemens 2006). In roots of spinach, however, proportions of Cd in F NaCl were similar between low-Cd and high-Cd cultivars, which implied that the Cd in F NaCl might be less related to the genotype difference in Cd detoxification and translocation of spinach.
Total proportions of Cd in the insoluble fractions (F HAc, F HCl, and F Residue) became generally higher in low-Cd cultivars than in high-Cd cultivars. This could be considered as one of the mechanisms involved in the genotype difference in shoot Cd accumulation of spinach. In some crops such as pakchoi (Xue et al. 2014) and watercress (Wang et al. 2015), the proportion of Cd in F HAc was the greatest for both shoots and roots when the plants were grown under Cd stresses. As a mechanism associated with Cd accumulation and detoxification, it relies on the formation of insoluble CdHPO4, Cd3(PO4)2, and other Cd-phosphates within plant tissues (Clemens 2006). For spinach, average proportions of Cd in F HAc were also higher in low-Cd than in high-Cd cultivars, indicating that the mechanism of Cd detoxification involving F HAc was relevant to the genotype difference in Cd accumulation of spinach.
Effect of phosphorus on Cd accumulation of spinach
The change in the P concentration in the culture solution resulted generally in a significant decrease of total Cd concentrations in both shoots and roots of spinach. Similar results were obtained by Keller and Römer (2001), Römer and Keller (2002), and Dheri et al. (2007). It was worth noting that the effect of P concentration on the reduction of shoot Cd accumulation was more significant than the cultivar effect under higher Cd stress. These results were consistent with those obtained by Qiu et al. (2011b) in Chinese flowering cabbage. The effect of phosphorus corresponds to the variation in Cd in F HAc, which is mainly composed of CdHPO4, Cd3(PO4)2, and other Cd-phosphate complexes. Qiu et al. (2011b) reported that the proportions of Cd in F HAc of the tested cultivars of Chinese flowering cabbage increased with soil P level, in consistency with an investigation by Jiang et al. (2007), who found that increased P in soil caused substantial precipitation of P-Cd complexes in cell wall and vacuoles in corn. A similar finding was reported in strawberry (Fragaia ananassa D.) by Nuzahath et al. (2013). In this study here, the results obtained for spinach were similar to the above-mentioned studies. Considering that increased P concentration decreased the proportions of Cd in F NaCl and F HCl in both shoots and roots, the lowered Cd accumulation under higher P might be attributed to the elevated Cd precipitation as insoluble Cd-P complexes.
As to the decrease of proportion of Cd in F NaCl and F HCl in spinach caused by P supply, similar results were also reported in strawberry (Nuzahath et al. 2013). Since studies on the relationship between P behavior and Cd chemical forms are, thus far, insufficient, no reasonable explanation for the phenomenon could be currently given and further investigations are required.
Conclusion
Verification of genotype dependence in Cd accumulation of spinach is provided by comparing the results from our previous and the present study. Spinach has a prominent ability to accumulate Cd and shall thus receive more attention in the identification and breeding of its low-Cd-accumulating genotypes. The obvious differences in the concentrations of different chemical forms of Cd between low-Cd and high-Cd cultivars indicated that the hypothesis in the present study is partly acceptable. That is, there is a genotype-dependent effect on Cd accumulation, translocation, and detoxification that is likely related to distribution of Cd across the various chemical forms. An increased supply of phosphorus decreased significantly Cd accumulations in both high-Cd and low-Cd cultivars without a significant difference between the high-Cd and low-Cd cultivars. Therefore, the external concentration of phosphorus influenced Cd accumulation of spinach, but might not be a crucial factor that affects genotype difference in Cd accumulation of the species.
References
Alexander PD, Alloway BJ, Dourado AM (2006) Genotypic variations in the accumulation of Cd, Cu, Pb and Zn exhibited by six commonly grown vegetables. Environ Pollut 144:736–745
Baker AJM, Brooks RR (1989) Terrestrial higher plants which hyperaccumulate metallic elements. A review of their distribution, ecology and phytochemistry. Biorecovery 1:81–126
Chunilall V, Kindness A, Jonnalagadda SB (2004) Heavy metal uptake by Spinach leaves grown on contaminated soils with lead, mercury, cadmium, and nickel. J Environ Sci Heal B 39:473–481
Clarke JM, Leisle D, Kopytko GL (1997) Inheritance of cadmium concentration in five durum wheat crosses. Crop Sci 37:1722–1726
Clarke JM, Norvell WA, Clarke FR, Buckley WT (2002) Concentration of cadmium and other elements in the grain of near-isogenic durum lines. Can J Plant Sci 82:27–33
Clemens S (2006) Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 88:1707–1719
Dai Q, Huang B, Yang Z, Yuan J, Yang J (2010) Identification of cadmium-induced genes in maize seedlings by suppression subtractive hybridization. Front Environ Sci Eng in China 4:449–458
Dai H, Yang Z, Xin J (2012) Genotype variation in Cd accumulation and chemical forms and histochemical distribution of Cd in low-and high-Cd cultivars of Chinese leaf mustard. Fresen Environ Bull 21:2746–2757
Dheri GS, Brar MS, Malhi SS (2007) Influence of phosphorus application on growth and cadmium uptake of spinach in two cadmium-contaminated soils. J Plant Nutr Soil Sci 170:495–499
Ebbs SD, Lasat MM, Brady DJ, Cornish J, Gordon R, Kochian LV (1997) Phytoextraction of cadmium and zinc from a contaminated soil. J Environ Qual 26:1424–1430
Grant CA, Clarke JM, Duguid S, Chaney RL (2008) Selection and breeding of plant cultivars to minimize cadmium accumulation. Sci Total Environ 90:301–310
Huang B, Xin J, Yang Z, Zhou Y, Yuan J, Gong Y (2009) Suppression subtractive hybridization (SSH)-based method for estimating Cd-induced differences in gene expression at cultivar level and identification of genes induced by Cd in two water spinach cultivars. J Agric Food Chem 57:8950–8962
Huang B, Xin J, Dai H, Liu A, Zhou W, Liao K (2014) Translocation analysis and safety assessment in two water spinach cultivars with distinctive shoot Cd and Pb concentrations. Environ Sci Pollut Res 21:11565–11571
Huang B, Xin J, Dai H, Zhou W, Peng L (2015) Identification of low-Cd cultivars of sweet potato (Ipomoea batatas (L.) Lam.) after growing on Cd-contaminated soil: uptake and partitioning to the edible roots. Environ Sci Pollut Res. doi: 10.1007/s11356-015-4449-z.
Ishikawa S, Ishimaru Y, Igura M, Kuramata M, Abe T, Senoura T, Hase Y, Arao T, Nishizawa NK, Nakanishi H (2012) Ion-beam irradiation, gene identification, and marker-assisted breeding in the development of low-cadmium rice. PNS 109:19166–19171
Ishimaru Y, Takahashi R, Bashir K, Shimo H, Senoura T, Sugimoto K, Ono K, Yano M, Ishikawa S, Arao T, Nakanishi H, Nishizawa NK (2012) Characterizing the role of rice NRAMP5 in manganese, iron and cadmium transport. Sci Rep-UK 2:286
Jiang HM, Yang JC, Zhang JF (2007) Effects of external phosphorus on the cell ultrastructure and the chlorophyll content of maize under cadmium and zinc stress. Environ Pollut 147:750–756
Keller H, Römer W (2001) Cu, Zn, and Cd acquisition by two spinach cultivars depending on P nutrition and root exudation. J Plant Nutrit Soil Sc 164:335–342
Kuboi T, Noguchi A, Yazaki J (1986) family-dependent cadmium accumulation characteristics in higher plants. Plant and Soil 92:405–415
Li JC, Guo JB, Xu WZ, Ma M (2007) RNA interference-mediated silencing of phytochelatin synthase gene reduce cadmium accumulation in rice seeds. J Integr Plant Biol 49:1032–1037
Liu WT, Zhou QX, An J, Sun YB, Liu R (2010) Variations in cadmium accumulation among Chinese cabbage cultivars and screening for Cd-safe cultivars. J Hazard Mater 173:737–743
McLaughlin MJ, Williams CMJ, McKay A, Kirkham R, Gunton J, Jackson J, Thompson R, Dowling B, Partington D, Smart MK, Tiller KG (1994) Effect of cultivar on uptake of cadmium by potato tubers. Aust J Ag Res 45:1483–1495
Mulligan CN, Yong RN, Gibbs BF (2001) Remediation technologies for metal-contaminated soils and groundwater: an evaluation. Eng Geol 60:193–207
Murtaza G, Ghafoor A, Qadir M (2008) Accumulation and implications of cadmium, cobalt and manganese in soils and vegetables irrigated with city effluent. J Sci Food Agric 88:100–107
Nawrot TS, Staessen JA, Roels HA, Munters E, Cuypers A, Richart T, Ruttens A, Smeets K, Clijsters H, Vangronsveld J (2010) Cadmium exposure in the population: from health risks to strategies of prevention. Biometals 23:769–782
Nicholson FA, Smith SR, Alloway BJ, Carlton-Smith C, Chambers BJ (2003) An inventory of heavy metals inputs to agricultural soils in England and Wales. Sci Total Environ 311:205–219
Nuzahath A, Abdukadir A, Dilnur M (2013) Effect of phosphorus on chemical forms and physiological properties of cadmium in Fragaia ananassa D. Chin J Soil Sci 44:1460–1464 (in Chinese)
Penner GA, Clarke J, Bezte LJ, Leisle D (1995) Identification of RAPD markers linked to a gene governing cadmium uptake in durum wheat. Genome 38:543–547
Qiu Q, Wang Y, Yang Z, Xin J, Yuan J, Wang J, Xin G (2011a) Responses of different Chinese flowering cabbage (Brassica parachinensis L.) cultivars to cadmium and lead exposure: screening for Cd + Pb pollution-safe cultivars. Clean: Soil, Air, Water 39:925–932
Qiu Q, Wang Y, Yang Z, Yuan J (2011b) Effects of phosphorus supplied in soil on subcellular distribution and chemical forms of cadmium in two Chinese flowering cabbage (Brassica parachinensis L.) cultivars differing in cadmium accumulation. Food Chem Toxicol 49:260–2267
Römer W, Keller H (2002) Variability of Cu, Zn and Cd content of spinach cultivars depending on P nutrition. Gartenbauwissenschaft 67:255–264
Salt DE, Blaylock M, Kumar NPBA, Dushenkov V, Ensley BD, Chet I, Raskin I (1995) Phytoremediation: a novel strategy for the removal of toxic metals from the environment using plants. Nat Biotechnol 13:468–474
Satarug S, Baker JR, Urbenjapol S, Haswell-Elkins M, Reilly PEB, Williams DJ, Moore MR (2003) A global perspective on cadmium pollution and toxicity in non-occupationally exposed population. Toxicol Lett 137:65–83
Shaff JE, Schultz BA, Craft EJ, Clark RT, Kochian LV (2010) GEOCHEM-EZ: a chemical speciation program with greater power and flexibility. Plant Soil 330:207–214
Tanhuanpää P, Kalendar R, Schulman AH, Kiviharju E (2007) A major gene for grain cadmium accumulation in oat (Avena sativa L.). Genome 50:588–594
Wang J, Fang W, Yang Z, Yuan J, Zhu Y, Yu H (2007) Inter- and intraspecific variations of cadmium accumulation of 13 leafy vegetable species in a greenhouse experiment. J Agric Food Chem 55:9118–9123
Wang J, Su L, Yang J, Yuan J, Yin A, Qiu Q, Zhang K, Yang Z (2015) Comparisons of cadmium subcellular distribution and chemical forms between low-Cd and high-Cd accumulation genotypes of watercress (Nasturtium officinale L. R. Br.). Plant Soil. doi: 10.1007/s11104-015-2580-8
Wang J, Yuan J, Yang Z, Huang B, Zhou Y, Xin J, Gong Y, Yu H (2009) Variation in cadmium accumulation among 30 cultivars and cadmium subcellular distribution in 2 selected cultivars of water spinach (Ipomoea aquatica Forsk.). J Agric Food Chem 57:8942–8949
Wu L, Zhu D (2014) Food safety in China: a comprehensive review. CRC Press, Boca Raton, p 55–183
Wu F, Dong J, Qian QQ, Zhang GP (2005) Subcellular distribution and chemical form of Cd and Cd−Zn interaction in different barley genotypes. Chemosphere 60:1437–1446
Xin J, Huang B, Yang Z, Yuan J, Dai H, Qiu Q (2010) Responses of different water spinach cultivars and their hybrid to Cd, Pb and Cd-Pb exposures. J Hazard Mater 175:468–476
Xin J, Huang B, Liu A, Zhou W, Liao K (2013) Identification of hot pepper cultivars containing low Cd levels after growing on contaminated soil: uptake and redistribution to the edible plant parts. Plant Soil 373:415–425
Xin J, Huang B, Dai H, Liu A, Zhou W, Liao K (2014) Characterization of cadmium uptake, translocation and distribution in young seedlings of two hot pepper cultivars that differ in fruit cadmium concentration. Environ Sci Pollut Res 21:7449–7456
Xue M, Zhou Y, Yang Z, Lin B, Yuan J, Wu S (2014) Comparisons in subcellular and biochemical behaviors of cadmium between low-Cd and high-Cd accumulation cultivars of pakchoi (Brassica chinensis L.). Front Environ Sci Eng 8:226–238
Yu H, Wang J, Fang W, Yuan J, Yang Z (2006) Cadmium accumulation in different rice cultivars and screening for pollution-safe cultivars of rice. Sci Total Environ 370:302–309
Zhang K, Wang J, Yang Z, Xin G, Yuan J, Xin J, Huang C (2013a) Genotype variations in accumulation of cadmium and lead in celery (Apium graveolens L.) and screening for low Cd and Pb accumulative cultivars. Front Environ Sci Eng 7:85–96
Zhang K, Yuan J, Kong W, Yang Z (2013b) Genotype variations in cadmium and lead accumulations of leafy lettuce (Lactuca sativa L.) and screening for pollution-safe cultivars for food safety. Environ Sci: Processes Impacts 15:1245–1255
Zhou Y, Xue M, Yang Z, Gong Y, Yuan J, Zhou C (2013) High cadmium pollution risk on vegetable amaranth and a selection for pollution-safe cultivars to lower the risk. Front Environ Sci Eng 7:219–230
Zhu Y, Yu H, Wang J, Yang Z (2007) Heavy metal accumulations of 24 asparagus bean cultivars grown in soil contaminated with Cd alone and with multiple metals (Cd, Pb and Zn). J Agric Food Chem 55:1045–1052
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos. 20877104 and 21277178) and Guangdong Natural Science Foundation (Grant No. s2011010004936).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
The authors declare that they have no competing interests.
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Yin, A., Yang, Z., Ebbs, S. et al. Effects of phosphorus on chemical forms of Cd in plants of four spinach (Spinacia oleracea L.) cultivars differing in Cd accumulation. Environ Sci Pollut Res 23, 5753–5762 (2016). https://doi.org/10.1007/s11356-015-5813-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5813-8