Abstract
Background and aims
Large areas of paddy fields have been contaminated by cadmium (Cd) in both China and worldwide, resulting in excessive Cd accumulation in rice grains. Here, we investigated the effects of cultivars, water regimes, and growth stages on Cd accumulation in rice with different radial oxygen loss (ROL).
Methods
Two groups of experiments were conducted: pot trials with soil-added Cd and solution-added agar using 20 rice cultivars and a rhizobag trial with Cd-contaminated soil and pot trial with solution-added agar under flooded and non-flooded water regimes with three growth stages and two cultivars.
Results
Different rice cultivars exhibited different porosity, Cd tolerance, and Cd accumulation in grains, which were significantly correlated with ROL. Cd concentration in shoots was significantly lower under the flooded (0.13–1.01, mean 0.56 mg kg−1) than non-flooded regime (0.68–1.39, mean 0.97 mg kg−1). The low Cd-accumulating cultivar showed higher rates of ROL, higher Cd combined with Fe plaque formation, and lower Cd bioavailability in the rhizosphere soil than the high-Cd accumulating cultivar.
Conclusions
Rice cultivars grown under flooded regimes effectively reduced Cd accumulation in edible parts, and the later stage was crucial for reducing Cd accumulation. Low Cd-accumulating cultivars generally exhibited a higher ability to reduce Cd bioavailability in the rhizosphere soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is a hazardous heavy metal and chronic carcinogen, which poses considerable risks to human health (Mehmood et al. 2019; Xue et al. 2017; Yang et al. 2018). In China, large areas of agricultural land have been contaminated by toxic trace elements, especially Cd (Chen et al. 2018a; Hu et al. 2016; Wang et al. 2019). Elevated Cd in farmland can be easily taken up by crops, leading to an exceedance of the limits allowed in agricultural products, such as rice (Alloway 2013; Chen et al. 2018b; Jiang et al. 2018), thus resulting in increased health hazards to human populations in China and other rice-reliant countries (Chen et al. 2018a, b; Tian et al. 2019; Wuana and Okieimen 2011; Zhao et al. 2015). Therefore, developing guidelines and remediation technologies to mitigate Cd in paddy fields are crucial for the sustainable production and quality of rice and other food crops.
There are significant differences in Cd accumulation in grains among different types of rice (e.g., hybrid, conventional, glutinous, and red rice) (Wang et al. 2011). Understanding the differences among conventional rice varieties would be of interest to rice breeders. To date, excellent rice varieties have been bred through hybridization with low Cd-accumulating and high ROL conventional rice cultivars. Thus, screening appropriate rice cultivars, especially conventional rice genotypes, and minimizing the measures required to obtain low Cd accumulation are important steps (Honma et al. 2016; Wang et al. 2011). In the last ten years, many agronomic measures have been reported to reduce Cd accumulation in rice grain, including the selection of low Cd-accumulating rice cultivars, reduction of Cd bioavailability in soil, reduction of Cd uptake/translocation to the grain (e.g., transgenic accumulation of Cd in root cell vacuoles to limit transport from root to shoot), and application of various water regimes (Chen et al. 2018c; Pan et al. 2016; Rehman et al. 2015; Ueno et al. 2010; Yu et al. 2014; Zhao and Wang 2019). Ueno et al. (2010) demonstrated that the low Cd-accumulating gene OsHMA3 is critical for restricting Cd translocation to shoots. Several studies have suggested that water regimes can change the redox potential (Eh) and pH of soil, subsequently affecting Cd solubility and availability and its accumulation in rice (Honma et al. 2016; Mei et al. 2012; Zhao and Wang 2019). Cd bioavailability is mainly affected by soil pH and Eh (Honma et al. 2016; Tian et al. 2019). However, a detailed understanding of their roles/effects in Cd accumulation and bioavailability is needed, which may provide useful information for selecting appropriate genotypes and field management practices to reduce Cd accumulation in rice grown in Cd-contaminated soils.
Wetland plants grown in waterlogged areas possess strategies to cope with anaerobic environments. Oxygen supplied via the aerenchyma to roots in anaerobic substrates can diffuse into the rhizosphere, a process termed radial oxygen loss (ROL) (Armstrong 1979), which varies considerably among rice cultivars (Mei et al. 2009). Rhizosphere oxidation and ROL can help wetland plants tolerate floods (McDonald et al. 2001) and salinity (Malik et al. 2009), with higher oxidation ability resulting in higher tolerance. The tolerance of wetland plants for heavy metals or metalloids such as arsenic (As) is also correlated with ROL (Mei et al. 2012). However, the exact roles of ROL on Cd tolerance in rice remain unclear.
In addition, ROL can cause significant changes in rhizosphere soil chemistry, such as pH, Eh, and metal availability, and microbial population abundance (Fitz and Wenzel 2002). In roots, ROL plays a key role in iron (Fe) plaque formation on root surfaces and mobility of toxic elements in the rhizospheric soil (María-Cervantes et al. 2010; Mei et al. 2012, 2014). Recently, Tian et al. (2019) reported significant differences in Cd accumulation and bioavailability in paddy soil under different water regimes and different rice growth stages and found that soil Eh is related to Cd uptake in rice. Intermittent irrigation is effective at decreasing Cd accumulation in rice grains and the heading stage is a crucial period for reducing Cd uptake (Honma et al. 2016; Sun et al. 2007). Previous studies have also shown that flooding can enhance root porosity, leading to greater ROL from rice roots (Jackson and Armstrong 1999). These results suggest that the characteristics of roots and their rhizosphere may play important roles in Cd uptake and accumulation in plants. However, few studies have systematically examined the influence of cultivars, water regimes, and growth stages on Cd accumulation in rice with different ROL.
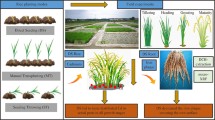
To fill this knowledge gap, we conducted pot trials with soil-added Cd and solution-added agar using 20 conventional rice cultivars as well as a rhizobag trial with Cd-contaminated soil and pot trial with solution-added agar comparing two cultivars, ‘low Cd-accumulating Suyunuo’ and ‘high Cd-accumulating Guangluai’, under flooded and non-flooded regimes during three growth stages (i.e., seedling, tillering, and booting). The present study aimed to investigate the (1) relationships among ROL and Cd tolerance and accumulation in rice grains, (2) effects of ROL on pH, Eh, Fe plaque formation, and Cd in rice rhizosphere soil solution, and (3) effects of water regimes and growth stages on biomass, ROL, and Cd in rice tissues.
Materials and methods
Experiment 1 - soil pot trial with different levels of Cd
Cultivars tested Twenty conventional rice (O. sativa L.) cultivars, i.e., Basmati 370, C 039, Fenghuazhan, Guangluai, IR 56, Jingxian 89, Molixinzhan, Qihuangzhan, Qiguizao, Qishanzhan, Sanerai, Sanhuangzhan, Sanluzhan 7, Suyunuo, Texianzhan 13, Yuefengzhan, Zhenguiai, Zhong 4188, Zhongerruanzhan, and Zhonghua 11, were obtained from the Rice Research Institute in Guangdong Province and Professor Guiquan Zhang, Academy of Agriculture, South China Agricultural University.
Soil preparation Control soil collected from a bamboo field (0–20 cm depth) at Sun Yat-Sen University was thoroughly mixed with Cd [0 and 100 mg Cd kg−1 as Cd(NO3)2·4H2O], and then allowed to equilibrate for three weeks. The control soil used for the pot experiment contained 11.2 g kg−1 organic matter (OM), 5.22 g kg−1 organic C, 16.1 g kg−1 total Fe, and 30.6 cmol kg−1 soil cation exchange capacity (CEC), with a pH value of 5.98. Total and available Cd contents were 0.18 mg kg−1 and 0.02 mg kg−1, respectively (Table 1). The soil was air-dried and then passed through a 2-mm sieve. Soil pH values were determined in water (solid:water ratio of 1:2.5) (Qi et al. 2014). Total organic carbon (TOC) was measured with a total organic carbon analyzer (TOC-VCPH, Shimadzu, Japan) (Li et al. 2012). The OM content in soil samples was measured calorimetrically by oxidation with potassium dichromate (Yang et al. 2016), and the CEC values were determined using the ammonium acetate method after washing with alcohol (Kahr and Madsen 1995). Total Fe was determined by ICP-OES (Perkin Elmer, USA) (Li et al. 2012). Available Cd was extracted by 0.01 mol L−1 CaCl2 (Houben et al. 2013) and total Cd in soil was determined by acid-digestion with aqua regia and perchloric acid (Zhou et al. 2015).
Experimental design Seeds were sterilized in 30% H2O2 (w/v) solution for 15 min, thoroughly washed with deionized water, and then germinated in moist perlite. After three weeks, uniform three-leaved seedlings were selected and transplanted into plastic pots. The black-painted plastic pots (7.5 cm diameter and 14 cm high) contained 1 kg of soil per pot and had no drainage holes. A total of 160 pots were prepared, with four replicates for each treatment per cultivar. The pots were placed in a greenhouse and arranged in a randomized completed block design during rice-growing stages (from early March to mid-July). The soil was maintained under flooded conditions (with 2 cm of water above the soil surface) during the whole growth period of 95–135 d, which varied among different cultivars. To ensure normal growth and development of rice plants, potassium chloride (KCl) solution (in distilled water) was applied after transplantation of seedlings to provide 28.6 mg K kg−1 in soil, and nitrogen was supplied as a solution of urea [CO (HN2)2] (in distilled water) in four equal parts for a total of 76.3 mg N kg−1 in soil during the growth period. Rice plants were harvested at maturity (cut 4 cm above the soil) and then separated into straw and grain. The samples were washed thoroughly with tap water and deionized water, and then oven-dried at 70 °C to a constant weight.
The tolerance index (TI) of the rice cultivars to Cd was quantified based on Mei et al. (2009) as follows:
A parallel agar-solution trial was designed to observe changes in ROL. Seeds from the 20 rice cultivars were used in this deoxygenated nutrient experiment. Uniform seedlings were selected and transplanted into blackened plastic pots (7.5 cm diameter and 14 cm high, one seedling per pot) filled with deoxygenated 50% strength Hoagland’s nutrient solution (Hoagland and Arnon 1950) containing 0.1% (w/v) agar. Some agar powder was added to pure water (w/v, 1:1000) and stirred by a magnetic stirrer at 100 °C until all agar dissolved into the boiled water. The obtained 0.1% agar solution was cooled to room temperature for further experiments. In the cooled agar solution and nutrient mixture, the agar gel and rice roots were suspended in culture medium. As stated in Wiengweera et al. (1997), dilute agar prevents convective movements in solution and better mimics the changes in gas composition found in waterlogged soil (i.e., decreased O2 and increased ethylene) compared with other methods used to impose root-zone O2 deficiency in solution. The agar was added to simulate changes in the composition of dissolved O2 and CO2, and their movements in the rhizosphere of roots under waterlogged soil conditions (Wiengweera et al. 1997). The pots were placed in a greenhouse and arranged in a completely randomized design, and the solutions were renewed once every 6 days. After 30 days, the plants were used for measurement of ROL rates and porosity of rice roots. The rates of ROL of rice seedlings were determined according to the Ti3+-citrate method described in Experiment 2. Root porosity (% gas volume/root volume) was measured by a pycnometer method (Jensen et al. 1969; Kludze et al. 1993).
Experiment 2 - Rhizobag trial with Cd-contaminated soil
Cultivars tested Two rice cultivars [cv. Guangluai with higher Cd accumulation, designated as ‘high Cd-accumulating Guangluai’; cv. Suyunuo with lower Cd accumulation, designated as ‘low Cd-accumulating Suyunuo’] were selected for a rhizobag trial and parallel agar-solution trial according to the results of Experiment 1 to reveal the effects of ROL on rice plant rhizospheres.
Soil preparation Soil used in the rhizobag trial was collected from a Cd-contaminated paddy field (0–20 cm depth) in Shaoguan, Guangdong Province, China. The soil was air-dried and then passed through a 2-mm sieve. The basic physicochemical properties of the paddy soil were pH of 6.38, soil OM content of 6.12 g kg−1, total Fe of 34.8 g kg−1, and soil cation exchange capacity of 20.9 cmol kg−1. Total and available Cd contents were 2.18 mg kg−1 and 0.86 mg kg−1, respectively (Table 1).
Experimental design The rhizobags were designed according to Liu et al. (2006), with some modification. The bags were constructed from nylon (4 cm diameter and 14 cm high) with an open top. The bags were filled with 0.5 kg of soil and placed into a pot (12 cm diameter and 17 cm high) pre-filled with soil, with a total weight of 2.5 kg pot−1. Seeds of two rice cultivars (cv. Suyunuo and Guangluai) were geminated after 2 weeks, one seedling (three leaves) of each cultivar was planted in the center of the nylon bag, which separated the soil into rhizosphere and non-rhizosphere. The rhizobag trial was designed to investigate the effects of water regimes on low-Cd and high-Cd accumulating rice cultivars grown in Cd-contaminated soil at different growth stages. The soil pot trial consisted of two water treatments, i.e., flooded (2 cm of water on soil surface) and non-flooded (aerated, 70% of soil water-holding capacity). In total, 48 rhizobags were used in the soil pot trial (two cultivars × two water regimes × three stages × four replicates). The rhizobags were placed in a greenhouse and arranged in a randomized complete block design for the growth period. The plants in the rhizobag trial were harvested on day 30 (seedling stage), day 60 (tillering stage), and day 90 (booting stage). At harvest, all rhizobags were transported to a N2-filled box in the laboratory. Soil samples were carefully taken from the rice rhizosphere, then placed and stored in a vacuum tube for further analysis under N2 conditions (Keon et al. 2001). Soil pH and Eh were measured with a pH/Eh meter (TM-39, Germany) and the electrode was calibrated before sample analysis. Readings measured at the same time and under similar conditions allowed better comparisons among different treatments and rice cultivars. After harvest and dithionite-citrate-bicarbonate (DCB) extraction, the samples were washed thoroughly with tap water and deionized water, divided into root and shoot, and oven-dried at 70 °C to a constant weight. A parallel agar-solution trial was designed to observe changes in the rates of ROL from rice roots of two cultivars under different water treatments and at different growth stages. The solution with 0.1% agar was filled with N2 and the solution without agar was filled with O2 to simulate flooded and non-flooded soil conditions, respectively, which allowed the entire rice root system to be removed without damage for measurement of ROL (Wiengweera et al. 1997). The same two cultivar (cv. Suyunuo and Guangluai) seedlings were selected and transplanted into plastic pots (12 cm diameter and 17 cm high, one seedling per pot, four replicates), then grown in the agar solution filled with N2 and non-agar solution filled with O2 and arranged in a randomized complete block design for the growth period. After 30 days, 60 days, and 90 days, the plants were harvested and used for measurement of ROL rates.
Determination of ROL rates
The ROL rate of each seedling was determined according to the titanium (Ti3+)-citrate method described by Kludze et al. (1994) and Mei et al. (2009). Nutrient solution (80 ml) was poured into 100-ml test tubes and then purged with Ar gas for 1200 s to remove dissolved O2. The bases of the plant seedlings, previously washed to remove foreign matter, were coated with paraffin oil to inhibit contamination by atmospheric O2. Control treatments did not contain any plants. All test tubes were kept at 25 °C.
After 6 h, the test tubes were gently shaken, and solution samples were collected with a syringe through a rubber tube introduced into the solution alongside the roots. Absorbance of the partly oxidized Ti3+-citrate solution was measured at 527 nm using a Perkin-Elmer Lambda 3 UV-VIS Spectrophotometer (Perkin-Elmer Corp., USA). The amount of O2 released from the whole-plant root system was determined by extrapolation of the measured absorbance from a standard curve made up of different concentrations of Ti3+ and expressed in the following equation:
where c = initial volume (L) of Ti3+-citrate added to each test tube; y = concentration of Ti3+ (μmol Ti3+ L−1) in control solution (without plants); z = concentration of Ti3+ (μmol Ti3+ plant−1 L−1) in solution after 6 h of treatment with plants; and g = g dry weight of root per plant.
Fe plaque
Here, Fe plaque on the root surface was extracted by DCB-extraction (Taylor and Crowder 1983). Fresh roots were extracted in a solution containing 40 ml of 0.3 M tri-sodium citrate (Na3C6H5O7·2H2O), 5 ml of 1.0 M sodium bicarbonate (NaHCO3), and 3 g of sodium dithionite (Na2S2O4) at room temperature for 3 h. After this, 1 g of dithionite was added each hour to keep the solution anoxic. Roots were then rinsed with 15 ml of deionized water and added to the DCB extract. The resulting extracts were made up to 100 ml with deionized water. The Fe plaque extract was digested in a mixture of sulfur acid and hydrogen peroxide (H2SO4/H2O2) (80/20, v/v) at 360 °C (Ohyama et al. 1991).
Rhizosphere soil solution collection and analysis
Collection of rhizosphere soil solution was performed as per He et al. (2015). Soil adhering to the roots of plants grown in the rhizobags was shaken off, loaded in a syringe, and then centrifuged at 6000 rpm for 20 min at room temperature to collect the rhizosphere soil solution. The collected soil solution was then filtered through 0.45-μm membranes and the concentration of Cd in the filtered solution was measured by atomic absorption spectrometry (AA7000, Shimadzu, Japan).
Chemical analysis of plant and soil samples
Oven-dried grain, shoot, and root samples were separately ground using a Retsch grinder (Type: 2 mm, Germany), and the Cd in plant tissues was extracted by digesting the samples with nitric (HNO3) and perchloric (HClO4) acids (4:1, v/v). Concentrations of Cd in the digests of the plant tissues and Fe plaque extracts for total Cd were determined by atomic absorption spectrometry (AA7000, Shimadzu, Japan). The concentrations of Fe in the DCB extracts were determined by inductively coupled plasma-atomic emission spectrometry (ICP-AES) (Optima 2000DV, Perkin Elmer, USA). For quality assurance, blanks and standard plant materials [GBW-07603 (GSV-2) China Standard Materials Research Center, Beijing, China] were used. Average recovery rates for all metals (Cd, Fe) were within the range of 90% ± 10%.
Statistical analysis
The arithmetic mean and standard error of four replicates were calculated. Parametric one-way analysis of variance (ANOVA) followed by the post-hoc Tukey-HSD test were used to determine differences among the 20 rice cultivars. Two- and three-way ANOVA were performed to determine interactions among factors (water regime, rice cultivar, and growth stage). The assumptions of the parametric ANOVA were tested, with no data transformation needed. Differences between flooded and non-flooded regimes within the same cultivar or between cultivars within the same treatment were compared by student t-test. All statistical analyses were performed using SPSS v13.0. Relationships among rates of ROL, porosity, Cd tolerance, and concentration of Cd in grain were evaluated by simple correlation coefficients using Origin 9.0 (Origin Lab, USA).
Results
Experiment 1 - soil pot trial with different levels of Cd
Rates of ROL, porosity, Cd tolerance, and Cd accumulation in rice grains of different cultivars
Significant variations in root porosities and ROL rates were found among the 20 rice cultivars (Fig. 1a, b). The biomasses of all rice cultivars grown in Cd-added soil were lower than that of the control. These results indicate that plant growth was significantly reduced in 100 mg kg−1 Cd-added soil. The Cd tolerance index (grain biomass % of control) varied significantly among the cultivars, ranging from 23% to 79% (Fig. 1c). There were also large differences in grain Cd concentrations, which ranged from 0.47 to 2.73 mg kg−1 DW (mean 1.29 mg kg−1 DW) (Fig. 1d). The cultivars (e.g., Suyunuo) with higher rates of ROL usually showed lower grain Cd accumulation and higher Cd tolerance than the cultivars (e.g., Guangluai) with lower rates of ROL grown in the same Cd-added soil.
Rates of radial oxygen loss (ROL) (mmol O2 kg−1 root d.w. h−1) and porosity (%, v/v) of rice cultivars exposed to 0.1% agar with 50% strength Hoagland solution for 30 days; and Cd tolerance (TI) (%, grain biomass) and total Cd concentration (mg kg−1 d.w.) in grains from 20 rice cultivars grown in soil with addition of 100 mg Cd kg−1 (as CdCl2.2.5 H2O) (mean ± S.E., n = 4). Only first eight letters are used to name cultivars
Correlations among Cd tolerance, Cd accumulation, root porosities, and ROL of rice
The rates of ROL were negatively correlated to grain Cd, but significantly positively correlated with root porosities and Cd tolerance indices of plants (Fig. 2). These results demonstrated that cultivars with higher root porosities usually exhibited higher rates of ROL, and the rates of ROL had significant impacts on Cd tolerance and accumulation in rice.
Correlations between Cd tolerance (TI) (%, grain biomass) and total Cd concentration (mg kg−1 d.w.) in grains of rice grown in soils with 100 mg Cd kg−1; and porosity (%, v/v) and rates of radial oxygen loss (ROL) (mmol O2 kg−1 root d.w. h−1) of rice grown in 0.1% agar with 50% strength Hoagland solution for 30 days (r = 20, p < 0.05)
Experiment 2 - Rhizobag trial with Cd-contaminated soil
Biomass and rates of ROL in rice plants grown under different water regimes and at different growth stages
Higher dry shoot and root weights were recorded in the low Cd-accumulating cultivar (Suyunuo) than in the high Cd-accumulating cultivar (Guangluai), irrespective of water regimes and growth stages (Table 2). Compared to the non-flooded regime, the flooded regime increased the biomass of both cultivars, with an increasing trend of seedling < tillering < booting stage. The low Cd-accumulating cultivar showed a higher rate of ROL compared with the high Cd-accumulating cultivar at all growth stages, irrespective of water regime. The rates of ROL were higher under the flooded regime (7.41 to 16.3 mmol O2 kg−1 root d.w. h−1) than under the non-flooded regime (3.22 to 10.1 mmol O2 kg−1 root d.w. h−1) and increased with growth stage for all tested cultivars (Table 2). Three-way ANOVA also showed significant differences in shoot biomass (F = 19.3, p < 0.05) and rates of ROL (F = 36.1, p < 0.05) among the three treatments (p < 0.05) but not in root biomass (F = 0.19, p > 0.05) (Table 1S).
Concentration of Cd in shoot and root tissues of rice under different water regimes and growth stages
Concentrations of Cd in shoot and root tissues were significantly different between the high and low Cd-accumulating cultivars grown in the rhizobags filled with Cd-contaminated soil (2.18 mg kg−1 Cd in soil) under both flooded and non-flooded regimes at different growth stages. Concentrations of Cd in shoot tissues under the flooded regime (0.13 to 1.01 mg Cd kg−1) for both cultivars at all growth stages were significantly lower than those under the non-flooded regime (0.68 to 1.39 mg Cd kg−1) (p < 0.05), but with an increasing trend of seedling < tillering < booting stage. Furthermore, compared to the cultivar with higher Cd accumulation, the cultivar with lower grain Cd accumulation in the soil pot trial also accumulated lower Cd in shoot tissues in the rhizobag trial with Cd-contaminated soil at all growth stages, irrespective of water regime (Table 3). Based on the three-way ANOVA results, significant differences were found in the concentrations of Cd in the shoot and root tissues (p < 0.05) among the three factors (i.e., Cultivar × Water regime × Growth stage) (Table 2S).
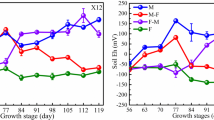
Eh and pH in rhizosphere soil solution
The Eh values in the soil solution collected from the rhizosphere of both cultivars under the flooded regime were significantly lower than those under the non-flooded regime at all growth stages. The Eh values in the rhizosphere soil solution of the high Cd-accumulating cultivar were often lower than those of the low Cd-accumulating cultivar, irrespective of water regime, with values ranging from 17.8 to 49.4 mv, 29.7 to 66.6 mv, and 61.7 to 101 mv at the seedling, tillering, and booting stages, respectively. In contrast, the pH in the rhizosphere soil solution under the flooded regime was significantly higher than that under the non-flooded regime, with a difference of 0.48–0.90 pH units. The low Cd-accumulating cultivar tended to have lower pH values in the rhizosphere soil solution than the high Cd-accumulating cultivar, irrespective of water regimes, which decreased with growth stage. Three-way ANOVA showed significant differences in pH (F = 16.1, p < 0.05) or Eh (F = 4.88, p < 0.05) values among the three different factors (i.e., Cultivar × Water regime × Growth stage) (Table 2S).
Fe plaque formation and concentrations of Cd on root surfaces and in rhizosphere soil solution
Compared with the non-flooded regime, the flooded regime significantly induced more Fe plaque formation on the root surfaces for both cultivars, which increased with growth stage. Significantly higher formation of Fe plaque was also found on the root surface of the low Cd-accumulating cultivar compared with the high Cd-accumulating cultivar for the same water regime and growth stage. The concentration of Cd on the root surfaces under the flooded regime was often much higher than that under the non-flooded regime, irrespective of growth stage, and more Cd was absorbed/solidified on the root surface of the low Cd-accumulating cultivar than the high Cd-accumulating cultivar. However, in the rhizosphere soil solution, significantly lower concentrations of Cd were found in both cultivars under the flooded regime than under the non-flooded regime at the same growth stages. The low Cd-accumulating cultivar tended to show lower Cd in the rhizosphere soil solution than the high Cd-accumulating cultivar, irrespective of water regime, with the order of seedling > tillering > booting stage (Table 3). In addition, based on three-way ANOVA, we found significant differences in Fe plaque formation (F = 8.96, p < 0.05) and in Cd concentration on the root surface and rhizosphere soil solution among the three different factors (i.e., Cultivar × Water regime × Growth stage) (Table 2S).
Discussion
Relationships among ROL, Cd tolerance, and Cd accumulation
Our results showed that Cd tolerance and Cd concentration in the rice grains varied considerably among cultivars (Fig. 1). Tolerance to Cd varied by about 3-fold in grain biomass % and concentrations of Cd in grain varied by about 6-fold under soil with the addition of 100 mg Cd kg−1. Similar results have been reported in previous studies (Liu et al. 2003, 2006). Variation in Cd tolerance and accumulation among different cultivars may be related to intrinsic internal factors, such as root porosity and ROL (Armstrong 1971; Mei et al. 2012), as well as environmental factors, especially rhizosphere properties (Arao et al. 2009; Chen et al. 2018c; Zhao and Wang 2019; Zheng and Zhang 2011).
Wetland plants, such as rice grown in waterlogged conditions, have evolved special and species-specific properties, such as root porosity and ROL, to facilitate survival in anoxic environments (Armstrong 1979; Mei et al. 2012). In the current study, the different cultivars demonstrated different root porosities and rates of ROL, ranging from 21.4%–33.1% and 6.52–13.2 mmol O2 kg−1 root d.w. h−1, respectively (Fig. 1), similar to that reported in previous research (Mei et al. 2009, 2012). The present results also showed that the rates of ROL were positively correlated with Cd tolerance and negatively correlated with grain Cd concentrations (Fig. 1). These results suggest that rice cultivars with higher porosity/ROL rate tended to have a greater ability to limit Cd transport to above-ground parts, and thus enhanced Cd tolerance. Our results also suggest that cultivars possessing higher porosity can transport more O2 from the shoot to root, leading to the release of more O2 from roots to the rhizosphere, and thus stronger effects on their rhizospheres.
Effects of ROL on pH, Eh, Fe plaque formation, and Cd in rice rhizosphere soil solution
In the rhizosphere of wetland plants, oxygen concentration [O2], pH, and Eh are important physicochemical parameters of biogeochemical processes (Begg et al. 1994; Bravin et al. 2008; Hinsinger et al. 2009). In addition, ROL is important for wetland plants to adapt to waterlogged and stressful environments (Colmer 2003a, b; Pezeshki 2001; Visser et al. 2003). Consequently, ROL can change the rhizosphere O2, pH, Eh, and Fe plaque formation of wetland plants (Blossfeld et al. 2011; Mei et al. 2014) and O. sativa L. (Mei et al. 2012). In the present study, the low Cd-accumulating cultivar, with higher rates of ROL, exhibited higher Eh, but lower pH in its rhizosphere than the high Cd-accumulating cultivar, irrespective of water regime or growth stage (Tables 2 and 3). Similar results have been reported in the rhizosphere of rice plants grown in arsenic (As)-polluted soil (Mei et al. 2012). Although simultaneous readings rather than successive readings of Eh were measured in the present study, our preliminary work showed that Eh values under the same treatment were relatively stable during the study period. On the other hand, based on a 27-month study, Dušek et al. (2008) found that Eh values in a subsurface-flow wetland planted with Phragmites australis change periodically, ranging from −400 to +800 mV over a few hours in flooded soil when light starts inducing photosynthesis. The hourly based Eh data obtained from Bustamante et al. (2011) also showed that redox potential varies with light and temperature, with significantly higher Eh (around +80 mV) during the day when photosynthesis is active than under dark conditions, although Eh remains relatively stable during the illumination period. Mei et al. (2014) also found that rhizosphere environments in wetland plants with higher ROL and Fe plaque are more acidic (lower pH) and less reduced (lower negative Eh). María-Cervantes et al. (2010) further reported that the reed P. australis, with a highly developed aerenchyma and intense ROL, exhibits higher Eh in rhizosphere soil than the saltmarsh shrub Sarcocornia fruticosa, which does not develop aerenchyma. Thus, results from both present and previous studies suggest that ROL from root systems plays an important role in changing [O2], pH, and Eh in rice rhizosphere.
In the present study, the low Cd-accumulating rice cultivar with higher rates of ROL tended to show increased Fe plaque formation and higher Cd accumulation on the root surface than that of the high Cd-accumulating cultivar with lower rates of ROL under both water regimes and at all growth stages (Tables 2 and 3). The current study also showed that the low Cd-accumulating cultivar had lower Cd levels in the rhizosphere solution and lower Cd transportation to the shoot tissues than the high Cd-accumulating cultivar (Tables 2 and 3). Similar results have also been reported for different wetland plants, such as water spinach (Xiao et al. 2015). Rice cultivars with higher rates of ROL release more O2, resulting in a larger influence on the rhizosphere zone and higher degree of oxidation in the rhizosphere soil (Mei et al. 2012). Water flow, ion transport by convection and diffusion processes, plant uptake, changes in pH and Eh, and root exudation all alter the chemical composition at the soil/root interface and may result in precipitation phenomena, favoring pollutant immobilization in the rhizosphere (Fitz and Wenzel 2002). Thus, increased [O2] in the rhizosphere likely induced additional Fe2+ oxidation, greater Fe plaque formation on the root surfaces and in the rhizosphere, greater Cd fixation by co-precipitation on the root surfaces, less Cd in the rhizosphere soil solution (Table 3), and thus less Cd transportation to aboveground parts of rice.
Effects of water regime and growth stage on biomass, ROL, and Cd in rice tissues
In the present study, the flooded regime induced higher rates of ROL compared with the non-flooded regime, suggesting that other environmental factors may influence the rates of ROL. Similarly, previous studies have indicated that high strength wastewater significantly decreases ROL but induces greater Fe plaque formation in other wetland plants (Mei et al. 2014; Pi et al. 2010). This may be related to the toxicity of flooding on microbial activity, leading to greater demand for O2 in rhizosphere soil. These results also indicate that although ROL is mainly controlled genetically, as suggested by previous researchers (Jackson et al. 1985), environmental stressors such as wastewater and flooding regimes can change this property.
Additionally, compared with the non-flooded regime, the flooded regime not only exhibited higher rates of ROL, but also exhibited higher biomass, Eh, and Fe plaque formation, and lower Cd concentration in the rhizosphere soil solution and Cd accumulation in shoot tissues of both tested cultivars, irrespective of growth stage (Tables 2 and 3). Liu et al. (2009) also found the biomass of wetland plants to be positively correlated with ROL. Tian et al. (2019) reported that as soil Eh increases from 0 to 150 mv (which is within the range found in the present study), exchangeable Cd content in soil decreases. When soil is kept moist (non-flooded), total Cd is more likely to transform into water-soluble and exchangeable Cd content in the soil (Khaokaew et al. 2011). The mean Cd concentration in the shoots of the two cultivars in the flooded treatment was 0.56 mg Cd kg−1, compared to 1.00 mg Cd kg−1 in the non-flooded treatment (Table 3). Previous research has also reported that flooding decreases Cd concentration in rice grains (Arao et al. 2009; Chen et al. 2018c; Rizwan et al. 2018; Tian et al. 2019; Zhao and Wang 2019). A decrease in Cd in shoots of plants under flooded conditions may be related to several factors, such as enhanced rates of ROL from rice roots (Mei et al. 2012), dilution effect of increased biomass (Wang et al. 2014), and Cd bioavailability in the rhizosphere (Liu et al. 2008). Additionally, water regimes can change both Eh and pH in soil, and subsequently affect Cd solubility and availability and thus manipulate its accumulation in plant tissues (Honma et al. 2016; Xiao et al. 2015).
The present results also showed that as the concentration of Cd in the rhizosphere soil solution decreased, the rates of ROL and Fe plaque formation increased from the seedling to booting stage, with the lowest levels found in the rhizosphere soil solution and rice shoots during the booting stage, irrespective of rice cultivar or water regime (Table 3). The increasing trend of ROL from seedling to booting stage may be partly due to increasing biomass, as the shoot and root biomasses of most cultivars increased with plant growth and were higher under flooded conditions than under non-flooded conditions. Liu et al. (2009) reported that wetland plant biomass is positively correlated with ROL. Moreover, MacFarlane and Burchett (2002) also indicated that the concentration of photosynthetic pigments and efficiency of photosynthetic processes are important sources of O2 for ROL. These results suggest that an increase in biomass, particularly that of leaves, will induce photosynthesis and consequently increase ROL from the roots of all wetland plants. The low levels of dissolved Cd observed during the post-heading three-week period were likely because the rice grain concentrations were most sensitive to dissolved Cd (Li et al. 2017). Previous studies have shown that solution Cd content in soil increases with higher Eh values under oxidation conditions (Frohne et al. 2011; Honma et al. 2016), which could be attributed to a decrease in ROL induced by oxidation conditions (non-flooded) and lower Fe plaque formation generated on rice roots. The ROL of rice decreased under non-flooding conditions (oxidation), which may be due to the decrease in O2 requirements in the rice rhizosphere (Mei et al. 2014; Pi et al. 2010). Conversely, an increase in O2 released from roots may stimulate the formation of Fe plaque and change the rhizosphere O2, metal, pH, and Eh levels due to the oxidation of Fe2+ to Fe3+ on the root surfaces (Mei et al. 2012). Consequently, here, Cd2+ was fixed by co-precipitation and the concentration of Cd in the rhizosphere soil solution decreased and the low level of Cd in the rhizosphere soil solution led to lower Cd transportation to the above-ground parts of the rice plants (Table 3). It has been reported that ROL-induced Fe plaque promotes Cd deposition on the root surface, leading to limited Cd transfer and distribution in rice (Cheng et al. 2014). These results suggest that the booting stage is crucial for reducing Cd uptake and accumulation and rhizosphere soil solution plays an important role in Cd accumulation.
Based on three-way ANOVA, we found significant differences in the rates of ROL, Fe plaque formation, and Cd concentration in the rhizosphere soil solution and rice tissues among the three factors (i.e., Cultivar × Water regime × Growth stage) (p < 0.05) (Table 1S and Table 2S). These results indicate that the three factors had significant effects on the rates of ROL, Fe plaque formation, and Cd concentration in the rhizosphere soil solution and rice tissues. The common mechanism of cultivars, water regimes, and rice growth stages in reducing Cd accumulation is that ROL induced greater Fe plaque formation, which, in turn, bound more Cd in Fe plaque, limited soil mobility of Cd in the rhizosphere, and minimized Cd uptake by roots and accumulation in shoots.
Conclusions
This study investigated the effects of cultivars, water regimes, and growth stages on Cd accumulation in rice with different ROL. Our results demonstrated large variations in porosity, Cd tolerance, and Cd accumulation in grains, which were significantly correlated with ROL. The flooded regime markedly increased shoot biomass and rates of ROL but decreased shoot Cd accumulation. The rhizosphere characteristics of the cultivars had important roles in Cd accumulation. The low Cd-accumulating cultivar showed low Cd accumulation in edible parts, higher rates of ROL, higher Cd combined with Fe plaque formation, and lower Cd bioavailability in the rhizosphere soil. The later stage was also shown to be crucial for reducing Cd uptake and accumulation, with an important influence on the modification of rhizospheres via the release of more O2. The fact that the flooded regime and later growth stages markedly reduced Cd accumulation may be due to a reduction in Cd mobility and bioavailability in the rhizosphere. Our study suggests that Cd accumulation can be significantly reduced in rice grains by selecting appropriate cultivars and adopting a suitable water regime at appropriate growth stages when rice is grown in Cd-contaminated soils.
References
Alloway BJ (2013) Sources of heavy metals and metalloids in soils. Heavy metals in soils: Trace Metals and Metalloids in Soils and their Bioavailability, pp 11–50
Arao T, Kawasaki A, Baba K, Mori S, Matsumoto S (2009) Effects of water management on cadmium and arsenic accumulation and dimethylarsinic acid concentrations in Japanese rice. Environ Sci Technol 43:9361–9367
Armstrong W (1971) Radial oxygen losses from intact rice roots as affected by distance from the apex, respiration and waterlogging. Physiol Plant 25:192–197
Armstrong W (1979) Aeration in higher plants. In: Woolhouse HW (ed) Advances in botanical research, vol 7. Academic Press, London, pp 225–332
Begg CB, Kirk GJD, Mackenzie AF, Neue HU (1994) Root-induced iron oxidation and pH changes in the lowland rice rhizosphere. New Phytol 128:469–477
Blossfeld S, Gansert D, Thiele B, Kuhn AJ, Lösch R (2011) The dynamics of oxygen concentration, pH value, and organic acids in the rhizosphere of Juncus spp. Soil Biol Biochem 43:1186–1197
Bravin MN, Travassac F, Le FM, Hinsinger P, Garnie JM (2008) Oxygen input controls the spatial and temporal dynamics of arsenic at the surface of a flooded paddy soil and in the rhizosphere of lowland rice (Oryza sativa L.): a microcosm study. Plant Soil 312:207–218
Bustamante MAO, Mier MV, Estrada JAE, Domíguez CD (2011) Nitrogen and potassium variation on contaminant removal for a vertical subsurface flow lab scale constructed wetland. Bioresour Technol 102:7745–7754
Chen HP, Tang Z, Wang P, Zhao F-J (2018a) Geographical variations of cadmium and arsenic concentrations and arsenic speciation in Chinese rice. Environ Pollut 238:482–490
Chen HP, Yang X, Wang P, Wang Z, Li M, Zhao F-J (2018b) Dietary cadmium intake from rice and vegetables and potential health risk: a case study in Xiangtan, southern China. Sci Total Environ 639:271–277
Chen HP, Zhang W, Yang X, Wang P, McGrath SP, Zhao F-J (2018c) Effective methods to reduce cadmium accumulation in rice grain. Chemosphere 207:699–707
Cheng H, Wang MY, Wong MH, Ye ZH (2013) Does radial oxygen loss and iron plaque formation on roots alter Cd and Pb uptake and distribution in rice plant tissues? Plant Soil 375:137–148
Colmer TD (2003a) Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ 26:17–36
Colmer TD (2003b) Aerenchyma and an inducible barrier to radial oxygen loss facilitate root aeration in upland, paddy and Deepwater rice (Oryza sativa L.). Ann Bot 91:301–309
Dušek J, Picek T, Čížková H (2008) Redox potential dynamics in a horizontal subsurface flow constructed wetland for wastewater treatment: Diel, seasonal and spatial fluctuations. Ecol Eng 34:223–232
Fitz WJ, Wenzel WW (2002) Arsenic transformation in the soil-rhizosphere-plant system: fundamentals and potential application to phytoremediation. J Biol 99:259–278
Frohne T, Rinklebe J, Diaz-Bone RA, Laing GD (2011) Controlled variation of redox conditions in a floodplain soil: impact on metal mobilization and biomethylation of arsenic and antimony. Geoderma 160:414–424
He BY, Ling L, Zhang LY, Li MR, Li QS, Mei XQ, Li H, Tan L (2015) Cultivar-specific differences in heavy metal (Cd, Cr, Cu, Pb, and Zn) concentrations in water spinach (Ipomoea aquatic ‘Forsk’) grown on metal-contaminated soil. Plant Soil 386:251–262
Hinsinger P, Bengough AG, Vetterlein D, Young IM (2009) Rhizosphere: biophysics, biogeochemistry and ecological relevance. Plant Soil 321:117–152
Hoagland DR, Arnon DI (1950) The water culture method for growing plants without soil. Calif Agric Exp Sta Circ 347, Riverside, CA
Honma T, Ohba H, Kaneko-kadokura A, Makino T, Nakamura K, Katou H (2016) Optimal soil Eh, pH, and water management for simultaneously minimizing arsenic and cadmium contaminations in rice grains. Environ Sci Technol 50:4178–4185
Houben D, Evrard L, Sonnet P (2013) Mobility, bioavailability and pH-dependent leaching of cadmium, zinc and lead in a contaminated soil amended with biochar. Chemosphere 92:1450–1457
Hu YA, Cheng HF, Tao S (2016) The challenges and solutions for cadmium-contaminated rice in China: a critical review. Environ Int 92-93:515–532
Jackson MB, Armstrong W (1999) Formation of aerenchyma and the processes of plant ventilation in relation to soil flooding and submergence. Plant Biol 1:274–287
Jackson MB, Fenning TM, Jenkins W (1985) Aerenchyma (gas space) formation in adventitious roots of rice is not controlled by ethylene of small partial pressures of oxygen. J Exp Bot 36(10):1566–1572
Jensen CR, Luxmoore RJ, Van Gundy SD, Stolzy LH (1969) Root air space measurements by a pycnometer method. Agron J 61:474–475
Jiang YX, Chao SH, Liu JW, Yang Y, Chen YJ, Zhang AC, Cao HB (2018) Source apportionment and health risk assessment of heavy metals in soil for a township in Jiangsu Province, China. Chemosphere 168:1658–1668
Kahr G, Madsen FT (1995) Determination of the cation exchange capacity and the surface area of bentonite, illite and kaolinite by methylene blue adsorption. Appl Clay Sci 9:327–336
Keon NE, Swartz CH, Brabander DJ, Harvey C, Hemond HF (2001) Validation of an arsenic sequential extraction method for evaluating mobility in sediments. Environ Sci Technol 35:2778–2784
Khaokaew S, Chaney RL, Landrot G, Ginder-Vogel M, Sparks DL (2011) Speciation and release kinetics of cadmium in an alkaline paddy soil under various flooding periods and draining conditions. Environ Sci Technol 45:4249–4255
Kludze HK, DeLaune RD, Patrick WH (1993) Aerenchyma formation and methane and oxygen exchange in rice. Soil Sci Soc Am J 51:386–391
Kludze HK, Delaune RD, Patrick J (1994) A colorimetric method for assaying dissolved oxygen loss from container-grown rice roots. Agron J 86:483–487
Li B, Wang X, Qi XL, Huang L, Ye ZH (2012) Identification of rice cultivars with low brown rice mixed cadmium and lead contents and their interactions with the micronutrients iron, zinc, nickel and manganese. J Environ Sci 24:1790–1798
Li H, Luo N, Li YW, Cai QY, Li HY, Mo CH, Wong MH (2017) Cadmium in rice: transport mechanisms, influencing factors, and minimizing measures. Environ Pollut 224:622–630
Liu JG, Liang JS, Li KQ, Zhang ZJ, Yu BY, Lu XL, Yang JC, Zhu QS (2003) Correlation between cadmium and mineral nutrients in absorption and accumulation in various genotypes of rice under cadmium stress. Chemosphere 52:1467–1473
Liu WJ, Zhu YG, Hu Y, Williams PN, Gault AG, Meharg AA, Charnock JM, Smith FA (2006) Arsenic sequestration in iron plaque, its accumulation and speciation in mature rice plants (Oryza sativa L.). Environ Sci Technol 40:5730–5736
Liu HJ, Zhang JL, Christie P, Zhang FS (2008) Influence of iron plaque on uptake and accumulation of Cd by rice (Oryza sativa L.) seedlings grown in soil. Sci Total Environ 394(2–3):361–368
Liu Y, Tam NFY, Yang JX, Pi N, Wong MH, Ye ZH (2009) Mixed heavy metals tolerance and radial oxygen loss in mangrove seedlings. Mar Pollut Bull 58:1843–1849
MacFarlane GR, Burchett MD (2002) Toxicity, growth and accumulation relationships of copper, lead and zinc in the grey mangrove Avicennia marina (Forsk.) Vierh. Mar Environ Res 54:65–84
Malik AI, English JP, Colmer TD (2009) Tolerance of Hordeum marinum accessions to O2 deficiency, salinity and these stresses combined. Ann Bot 103(2):237–248
María-Cervantes A, Conesa HM, Gonzálea-Alcaraz MN, Alvarez-Rogel J (2010) Rhizosphere and flooding regime as key factors for the mobilization of arsenic and potentially harmful metals in basic, mining-polluted salt marsh soils. Appl Geochem 25:1722–1733
McDonald MP, Galwey NW, Colmer TD (2001) Waterlogging tolerance in the tribe Triticeae: the adventitious roots of Critesion marinum have a relatively high porosity and a barrier to radial oxygen loss. Plant Cell Environ 24(6):585–596
Mehmood K, Ahmad HR, Abbas R, Murtaza S & G (2019) Heavy metals in urban and peri-urban soils of a heavily-populated and industrialized city: assessment of ecological risks and human health repercussions. Hum Ecol Risk Assess https://doi.org/10.1080/10807039.2019.1601004, 1, 18
Mei XQ, Ye ZH, Wong MH (2009) The relationship of root porosity and radial oxygen loss on arsenic tolerance and uptake in rice grains and straw. Environ Pollut 157:2550–2557
Mei XQ, Wong MH, Yang Y, Dong HY, Qiu RL, Ye ZH (2012) The effects of radial oxygen loss on arsenic tolerance and uptake in rice and on its rhizosphere. Environ Pollut 165:109–117
Mei XQ, Yang Y, Tam NFY, Wang YW, Li L (2014) Roles of root porosity, radial oxygen loss, Fe plaque formation on nutrient removal and tolerance of wetland plants to domestic wastewater. Water Res 50:147–159
Ohyama T, Ito M, Kobayashi K, Araki S, Yasuyoshi S, Sasaki O, Yamazaki T, Soyama K, Tanemura R, Mizuno Y, Ikarashi T (1991) Analytical procedures of N, P, K concentrations in plant and manure materials using H2SO4-H2O2 Kjeldahl digestion method. Jpn Bull Faculty Agric Niigata Univ 43:111–120
Pan YY, Bonten LTC, Koopmans GF, Song J, Luo YM, Temminghoff EJM, Comans RNJ (2016) Solubility of trace metals in two contaminated paddy soils exposed to alternating flooding and drainage. Geoderma 261:59–69
Pezeshki SR (2001) Wetland plant responses to soil flooding. Environ Exp Bot 46:299–312
Pi N, Tam NFY, Wong MH (2010) Effects of wastewater discharge on formation of Fe plaque on root surface and radial oxygen loss of mangrove roots. Environ Pollut 158:381–387
Qi YB, Huang B, Darilek JL (2014) Effect of drying on heavy metal fraction distribution in rice paddy soil. PLoS One 9:1–8
Rehman MZ, Rizwan M, Ghafoor A, Naeem A, Ali S, Sabir M, Qayyum MF (2015) Effect of inorganic amendments for in situ stabilization of cadmium in contaminated soils and its phyto-availability to wheat and rice under rotation. Environ Sci Pollut Res 22:16897–16906
Rizwan M, Ali S, Abbas T, Adrees M, Zia-ur-Rehman M, Ibrahim M, Abbas F, Qayyum MF, Nawaz R (2018) Residual effects of biochar on growth, photosynthesis and cadmium uptake in rice (Oryza sativa L.) under Cd stress with different water conditions. J Environ Manag 206:676–683
Sun L, Chen S, Chao L, Sun TH (2007) Effects of flooding on changes in Eh, pH and speciation of cadmium and lead in contaminated soil. Bull Environ Contam Toxicol 79:514–518
Taylor GJ, Crowder AA (1983) Use of the DCB technique for extraction of hydrous iron oxides from roots of wetland plants. Am J Bot 70(8):1254–1257
Tian T, Zhou H, Gu JF, Jia RY, Li HC, Wang QQ, Zeng M, Liao BH (2019) Cadmium accumulation and bioavailability in paddy soil under different water regimes for different growth stages of rice (Oryza sativa L.). Plant Soil. https://doi.org/10.1007/s11104-019-04094-x
Ueno D, Yamaji N, Kono I, Huang CF, Ando T, Yano M, Ma JF (2010) Gene limiting cadmium accumulation in rice. Proc Natl Acad Sci 107(38):16500–16505
Visser EJW, Voesenek L, Vartapetian BB, Jackson MB (2003) Flooding and plant growth. Ann Bot 91(2):107–109
Wang MY, Chen AK, Wong MH, Qiu RL, Cheng H, Ye ZH (2011) Cadmium accumulation in and tolerance of rice (Oryza sativa L.) varieties with different rates of radial oxygen loss. Environ Pollut 159:1730–1736
Wang X, Ye ZH, Li B, Huang LN, Meng M, Shi JB, Jiang GB (2014) Growing rice aerobically markedly decreases mercury accumulation by reducing both hg bioavailability and the production of MeHg. Environ Sci Technol 48:1878–1885
Wang P, Chen HP, Kopittke PM, Zhao F-J (2019) Cadmium contamination in agricultural soils of China and the impact on food safety. Environ Pollut 249:1038–1048
Wiengweera A, Greenway H, Thomson CJ (1997) The use of agar nutrient solution to simulate lack of convection in waterlogged soils. Ann Bot 80:115–123
Wuana RA, Okieimen FE (2011) Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecology. https://doi.org/10.5402/2011/402647
Xiao QQ, Wong MH, Huang L, Ye ZH (2015) Effects of cultivars and water management on cadmium accumulation in water spinach (Ipomoea aquatica Forsk.). Plant Soil 391:33–49
Xue SG, Shi LZ, Wu C, Wu H, Qin YY, Pan WS, Willian H, Cui MQ (2017) Cadmium, lead, and arsenic contamination in paddy soils of a mining area and their exposure effects on human HEPG2 and keratinocyte cell-lines. Environ Res 156:23–30
Yang WT, Gu JF, Zou JL, Zhou H, Zeng QR, Liao BH (2016) Impacts of rapeseed dregs on Cd availability in contaminated acid soil and cd translocation and accumulation in rice plants. Environ Sci Pollut Res 23:20853–20861
Yang QQ, Li ZY, Lu XN, Duan QN, Huang L, Bi J (2018) A review of soil heavy metal pollution from industrial and agricultural regions in China: pollution and risk assessment. Sci Total Environ 642(15):690–700
Yu LL, Zhu JY, Huang QQ, Su DC, Jiang RF, Li HF (2014) Application of a rotation system to oilseed rape and rice fields in Cd-contaminated agricultural land to ensure food safety. Ecotoxicol Environ Saf 108:287–293
Zhao F-J, Wang P (2019) Arsenic and cadmium accumulation in rice and mitigation strategies. Plant Soil 446:1–21. https://doi.org/10.1007/s11104-019-04374-6
Zhao F-J, Ma YB, Zhu YG, Tang Z, McGrath SP (2015) Soil contamination in China: current status and mitigation strategies. Environ Sci Technol 49:750–759
Zheng SN, Zhang MK (2011) Effect of moisture regime on the redistribution of heavy metals in paddy soil. J Environ Sci 23:434–443
Zhou H, Zeng M, Zhou X, Liao BH, Peng PQ, Hu M, Zhu W, Wu YJ, Zou ZJ (2015) Heavy metal translocation and accumulation in iron plaques and plant tissues for 32 hybrid rice (Oryza sativa L.) cultivars. Plant Soil 386:317–329
Acknowledgments
This work was funded by the National Natural Science Foundation of China (41401365, 31670409), National Key Research and Development Program of China (2016YFD0800300, 2018YFD0800700), Postdoctoral Science Special Foundation of China (2014 M552282, 2015 T80939) and the Natural Science Foundation of Guangdong, China (2016A030313273).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Antony Van der Ent.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 28 kb)
Rights and permissions
About this article
Cite this article
Mei, X., Li, Q., Wang, H. et al. Effects of cultivars, water regimes, and growth stages on cadmium accumulation in rice with different radial oxygen loss. Plant Soil 453, 529–543 (2020). https://doi.org/10.1007/s11104-020-04634-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-020-04634-w