Abstract
Background and aims
Water spinach readily uptakes cadmium (Cd), posing a risk to human health. Selecting low-Cd cultivars is a promising mitigation strategy, but its extensive utilization is limited without a clear understanding of the critical plant factors determining Cd accumulation. This study aimed to elucidate the effects and interactions of the radial oxygen loss (ROL) and iron (Fe) plaque on the Cd accumulation in different water spinach cultivars and the underlying mechanisms.
Methods
A pot and four hydroponic experiments using ten water spinach cultivars were conducted with different aeration, Fe supply, and Cd stress treatments.
Results
The Cd accumulation of different cultivars was determined by both root uptake and root-to-shoot transport. There were 4.0-, 3.8- and 6.3-fold differences among different cultivars in ROL, Fe plaque, and shoot Cd accumulation, respectively. Rhizosphere and root Fe plaque reduced root Cd uptake and were regulated by Fe supply and ROL. Increased ROL under stagnant conditions enhanced Fe plaque and induced its redistribution along root axes. Root Cd retention through compartmentalization and chelation inhibited root-to-shoot Cd translocation.
Conclusion
The Fe plaque, ROL, and root Cd retention formed an interactive system to decrease the Cd uptake and accumulation in water spinach. Low-Cd cultivars exhibited higher ROL, greater Fe plaque and more effective root Cd retention, and responded to stagnant conditions with greater ROL increases and more enhanced Fe deposition on root surfaces. This study provides new insights into the Cd accumulation mechanisms in water spinach and a theoretical basis for selecting low-Cd cultivars.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water spinach (Ipomoea aquatica Forsk.) is an important leafy vegetable in East and Southeast Asia (Göthberg et al. 2002). Owing to its geographic origin, water requirement, and specific plant characteristics, water spinach is prone to accumulating deleterious heavy metals, especially cadmium (Cd), posing a severe threat to public health (Kang et al. 2020; Tang et al. 2020). The consumption of contaminated agricultural products, as a major route for chronic Cd exposure, is linked to various human diseases, including bone damage, kidney dysfunction, and cancer (Khan et al. 2017). In recent decades, rapid urbanization and industrial development have exacerbated Cd contamination in farmland soils in many Asian countries, especially in China, where Cd stood as one of the most prevalent soil contaminants (Zhao et al. 2015). With evolving dietary habits and increased vegetable consumption over the past decades, it has become imperative to mitigate the Cd accumulation in water spinach to ensure food safety.
Different water spinach cultivars differed in their Cd accumulation, rendering the selection and cultivation of low-Cd cultivars an effective and eco-friendly strategy to tackle the Cd contamination in this vegetable (Wang et al. 2009; He et al. 2015; Xiao et al. 2015). However, the key processes and plant traits affecting the cultivar-dependent Cd accumulation in water spinach remained poorly understood, which hindered the widespread adoption of this technique. A few studies claimed that the root-to-shoot Cd translocation, rather than root Cd uptake, determined the Cd accumulation in the aerial parts of different water spinach cultivars (Xin et al. 2013). Nevertheless, our prior research observed cultivar-dependent root Cd accumulation in water spinach and hinted at a latent correlation between root and shoot Cd concentrations, implying an overlooked role of root uptake in determining shoot Cd accumulation (Xiao et al. 2015). This highlighted critical root characteristics such as radial oxygen loss (ROL) and iron (Fe) plaque that might affect the Cd accumulation in the edible parts. Systematically evaluating the effects and mechanisms of these characteristics should constitute a fundamental step toward breeding low-Cd water spinach cultivars.
ROL and Fe plaque formation have been linked to root Cd uptake in several wetland plants (Cheng et al. 2014; Huang et al. 2020). ROL signified radial oxygen diffusion from root aerenchyma to the rhizosphere, accounting for approximately 30–40% of the total oxygen supplied to the aerenchyma (Pedersen et al. 2021). Wetland plants adapted to waterlogged conditions by developing greater root aerenchyma and thus typically had higher rates of ROL (Khan et al. 2016; Yamauchi et al. 2018). ROL substantially altered soil chemistry in the rhizosphere and profoundly affected element cycling (Colmer 2003). Specifically, ROL could oxidize the large amounts of Fe2+ in the flooded rhizosphere into Fe(III) (hydr)oxides, which were then deposited on root surfaces and soil particles as root and rhizosphere Fe plaque, respectively (Zandi et al. 2022). Enhanced Fe plaque effectively reduced Cd uptake in several wetland plants because dissolved Cd could be sequestered on amorphous Fe hydroxides via adsorption and co-precipitation (Li et al. 2019; Huang et al. 2022). However, the specific roles of ROL and Fe plaque formation in determining the Cd accumulation of water spinach remain largely unclear.
ROL and Fe plaque formation were adaptive traits regulated by environmental and genetic factors. ROL was determined by lysigenous aerenchyma formation, the development of the barrier to ROL, adventitious root growth, respiratory consumption along roots, and the responses of these characteristics to environmental stresses (Colmer 2003; Yamauchi et al. 2018). The aerenchyma formation could be enhanced by root hypoxia under waterlogged soil conditions, leading to increased root porosity (Pedersen et al. 2021). The ROL barrier on exodermis cell walls facilitated the longitudinal oxygen diffusion towards root tips while impeding oxygen release into the rhizosphere (Jiménez et al. 2021). Wetland plants responded to soil waterlogging by restricting ROL from the basal segments and enhancing ROL from root tips (Abiko et al. 2012; Qi et al. 2020). Fe plaque formation was affected by various plant, soil, and microbial properties, among which ROL and active Fe(II) supply deemed critical (Zandi et al. 2022). Higher rates of ROL and exogenous Fe(II) supplementation could effectively enhance Fe plaque formation (Huang et al. 2020; Yuan et al. 2021). However, these root characteristics varied widely among plant species (Yang et al. 2017), and little is known about their development and impact on Cd uptake in water spinach. On the other hand, it was found in rice that the ROL and Fe plaque formation varied among different cultivars, and the cultivars with higher ROL tended to form stronger Fe plaque and accumulate lower Cd levels (Cheng et al. 2014; Mei et al. 2020). It is thus important to assess the ROL and Fe plaque formation in different water spinach cultivars and their relationship with Cd uptake and accumulation.
In addition to the root Cd uptake, the upward transport was also considered crucial for the Cd accumulation in the aerial parts of wetland plants and was closely related to the root Cd retention (Uraguchi et al. 2009; Xin et al. 2013). The chelation of Cd by thiols and subsequent sequestration into vacuoles represented a typical detoxification strategy in plants, which eventually inhibited the upward transport of Cd (Nocito et al. 2011; Adrees et al. 2015). Meanwhile, root cell walls rich in negatively charged functional groups had a high affinity for Cd2+ and thus blocked the radial diffusion of Cd into the xylem before being transported upward (Zhang et al. 2020). The deposition of lignin and suberin within the exodermal and endodermal cell walls further enhanced the cell wall sequestration of Cd and inhibited root-to-shoot Cd translocation (Clemens et al. 2013; Huang et al. 2019). Based on these findings, elucidating the root Cd chelation and sequestration as described by the subcellular distribution and chemical forms of Cd is indispensable for comprehending the different Cd accumulation in different water spinach cultivars.
Therefore, in this study, we conducted a series of soil pot and hydroponic experiments (1) to evaluate the cultivar variations in ROL, Fe plaque formation, and Cd accumulation of water spinach and their relationships under diverse growth conditions, (2) to elucidate the mechanisms for the effects of ROL, Fe plaque, and root retention on reducing the Cd accumulation of different cultivars, and (3) to explore the potential influences of critical environmental factors, i.e., Cd levels, aeration conditions, and Fe supply, on these effects of Fe plaque, ROL, and related plant characteristics. This study will provide a theoretical basis and practical methods for understanding the mechanisms of Cd accumulation in water spinach and selecting reliable low-Cd cultivars.
Materials and methods
Experimental materials
Based on our previous research (Xiao et al. 2015), ten water spinach cultivars, including five high-Cd and five low-Cd cultivars, were used in the present study with seeds obtained from local seed companies (Table 1). In each experiment, seeds of the tested water spinach cultivars were surface sterilized with 30% (V/V) H2O2 for 15 min, rinsed with deionized water, and germinated in seedling trays until the three-leaf stage. Soil material (with 0.09 mg kg−1 total Cd concentration) was collected from a paddy field at South China Agricultural University in Guangdong Province, China. The concentrations of total organic matter, nitrogen (N), phosphorus, potassium (K), and Fe of the soil were 8.1, 1.3, 1.1, 0.61, and 26.0 g kg−1, respectively, and soil pH was 6.3. The soil was air-dried, ground, and sieved through an 8-mm mesh before use. All the experiments in this study were carried out in glasshouse conditions (28/18°C day/night temperature, light period 16 h per day with natural sunlight supplemented with sodium vapor lamps).
Experimental design
Pot experiment (PT1)
A pot experiment was carried out to evaluate the variations in ROL, Fe plaque formation, and Cd accumulation among different water spinach cultivars and the relationships between the characteristics above (PT1). The soil was spiked with 30 mg kg−1 Cd (CdCl2) and fertilized with 76.3 mg kg−1 N (urea) and 28.6 mg kg−1 K (KCl). One kilogram of prepared soil was filled in plastic pots (14 cm diameter and 18 cm height) as bulk soil, and a rhizobag (8 cm diameter and 11 cm height) containing 0.5 kg of clean sand and divided into two halves by thin plastic cards was placed in the center of each pot. Uniform seedlings of the ten water spinach cultivars were transplanted into each half of the rhizobags (one plant per half) with four replicates. After transplanting, the plants were grown for 75 d and submergence was initiated at 10 d. One plant in each pot was collected to analyze ROL, and the other was harvested and separated into shoots and roots. The fresh root samples were used for determining the Fe plaque formation. After that, the shoot and root samples were oven-dried for determining the Cd concentrations in the shoot and root tissues and Fe plaque formation. The sand in the rhizobags was also collected and air-dried for the analysis of rhizosphere Fe plaque.
Hydroponic experiments (HP1-4)
Firstly, a hydroponic experiment was conducted to assess the ROL of different water spinach cultivars under contrasting external aeration conditions (HP1). According to previous studies, the aerated treatment was conducted using a 20% Hoagland nutrient solution, and the stagnant treatment was conducted using a 20% Hoagland nutrient solution containing 0.1% agar (W/V), which was deoxygenated with N2 for 12 h before use (Huang et al. 2019). The aerated and stagnant treatments were performed to mimic the drained and flooded soil conditions, respectively, and all the treatments were replicated in triplicate. The nutrient solution was renewed every 3 days, and plants were harvested on 21 d to determine their growth indices, root porosity, and ROL. Afterward, a hydroponic experiment was performed to unveil the response of ROL to Cd stress and its effect on the Cd accumulation of water spinach (HP2). Two high-Cd cultivars (C01 and C05) and two low-Cd cultivars (C09 and C10) were used in this experiment based on the results of PT1 and HP1, and seedlings were exposed to varied Cd concentrations (0, 15, and 50 µM Cd in the form of CdCl2) in stagnant 20% Hoagland nutrient solution (the same as in HP1, renewed every 3 days) with three replicates. Plants were harvested on 21 d to determine the Cd concentrations and ROL. After obtaining two cultivars with contrasting (rates of) ROL and Cd accumulation (C05 and C09), a hydroponic experiment was carried out to elucidate the effects of external aeration and Fe supply on the formation of root Fe plaque and their association with ROL (HP3). In the HP3, seedlings were transplanted into aerated and stagnant 20% Hoagland nutrient solutions and grown for 30 days. Half of the seedlings were collected for ROL analysis, and the other half were exposed to 0 and 30 mg L−1 Fe (FeSO4) in 20% Hoagland nutrient solution for 24 h, which was commonly performed in previous relevant studies (Deng et al. 2010; Lai et al. 2012). Half of the seedlings in each Fe treatment were then harvested to determine the Fe plaque formation in different root segments, while the other half were subjected to 25 µM Cd in 50% Hoagland nutrient solution for 24 h prior to harvest for determining the Cd accumulation in water spinach tissues and Fe plaque. All the treatments were replicated four times. To explore the effects and mechanisms of ROL-related plant traits on the root-to-shoot transport of Cd in water spinach, a hydroponic experiment was conducted using cultivars C05 and C09 (HP4). Seedings were exposed to low-Cd (15 µM) and high-Cd (50 µM) treatment (CdCl2) in a stagnant 25% Hoagland nutrient solution and grown for 28 days. Plants were harvested on 4, 7, 14, 21, and 28 d, respectively, with three replicates. Samples were divided into two halves, and one half was directly frozen in liquid nitrogen and stored at − 70 °C before determining the chemical fractions and subcellular distribution of Cd in water spinach tissues. The other half was oven-dried to measure the root and shoot Cd concentrations.
Measurements and chemical analyses
The whole root system was used for measuring the root porosity and ROL. The root porosity of water spinach seedlings was measured using the pycnometer method described by Mei et al. (2009). The ROL was measured colorimetrically by the titanium(III) (Ti(III) citrate method (Kludze et al. 1994). In brief, water spinach seedlings were immersed in a tube with 25% deoxygenated Hoagland nutrient solution, and the nutrient solution was coated with paraffin oil to hinder atmospheric O2 contamination after Ti(III) citrate stock buffer was injected. The plants were then transported to a climate chamber and incubated for 6 h (Cheng et al. 2012). The absorbance of the partly oxidized Ti(III) citrate solution was measured using a spectrophotometer at 527 nm (Cheng et al. 2014). The ROL was calculated using the following equations:
where ROL is the total ROL from entire roots (µM O2 plant−1 h−1) and the rate of ROL is the ROL per unit root mass (µM O2 g−1 root DW h−1), v is the initial volume of Ti(III) added to each tube (L), y is the Ti concentration in the control tube without plants (µM Ti L−1), z is the Ti concentration in the test tube after 6 h incubation (µM Ti L−1), and m is the root dry weight (Wang et al. 2014).
The Fe plaque on fresh root surfaces and in the rhizosphere was extracted using the dithionite-citrate-bicarbonate (DCB) method, and the Cd and Fe concentrations in Fe plaque were expressed as their contents per kilogram of root/soil dry weight (Otte et al. 1989; Wang et al. 2014). A sequential extraction procedure was performed using frozen tissues to extract 6 Cd fractions, including (1) inorganic Cd (F1, extracted with 80% ethanol), (2) water-soluble Cd (F2, extracted with deionized water), (3) Cd integrated with pectates and protein (F3, extracted with 1 M NaCl solution), (4) undissolved Cd-phosphate complexes (F4, extracted with 2% acetic acid), (5) Cd oxalate (F5, extracted with 0.6 M HCl solution) and (6) residual Cd (F6) (Xin et al. 2017). The subcellular distribution of Cd in the root was quantified following the method of Huang et al. (2019). In brief, the apoplastic fluid fraction was extracted by shaking the frozen root segments with deionized water and was separated by centrifugation. Afterward, the root segments were ground in liquid nitrogen and extracted with a cold buffer solution that contained 50 mM HEPES (C8H18N2O4S), 1 mM DTT (C4H10O2S2), 0.5 M sucrose, and 5 mM ascorbic acid (adjusted to pH 7.5 with NaOH). The homogenate was centrifuged, the supernatant was designated as the symplastic fraction, and the pellet was the cell wall fraction. All the extracts and residues were digested with concentrated HNO3 and HClO4 (5:1, V/V) prior to the determination of Cd concentration.
Shoot and root samples were digested with concentrated HNO3 and HClO4 (5:1, V/V) before determining the Cd concentrations. Standard reference material GBW-07604 (China Standard Materials Research Center, Beijing, China) and reagent blanks were included in each batch, and the recoveries of Cd all ranged between 90 and 110%. The Cd concentration in the extracts and digests was determined by atomic absorption spectrometry (AAS; AA240FS, Varian, CA, USA), and the Fe concentrations were determined using inductively coupled plasma-optical emission spectrometry (ICP-OES; Optima 2000 DV, Perkin Elmer, MA, USA).
Statistical analysis
Statistical differences among different water spinach cultivars or treatments were analyzed by analysis of variance (ANOVA), followed by post-hoc analysis based on the least significant difference (LSD) test at p < 0.05 level. A paired t-test was employed to compare the differences in plant characteristics between aerated and stagnant conditions in the HP1. Pearson correlation analysis was performed, and the correlation coefficient was calculated to determine the relationships between different plant characteristics and element concentrations. All these statistical analyses were performed using R software (Version 4.2.2), and artwork was created using the ‘ggplot2’ package.
Results
Variations in ROL, Fe plaque, and Cd accumulation among cultivars
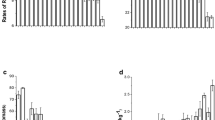
In the hydroponic experiment HP1, root porosity, ROL, and the rate of ROL varied significantly among the ten tested water spinach cultivars in both aerated and stagnant treatments (p < 0.05), except for the rate of ROL in the aerated treatment (Fig. 1a). Cultivar C09 exhibited the highest levels in these characteristics, whereas C05 showed the lowest levels. The root porosity, ROL, and rates of ROL of the low-Cd cultivar group were averagely 48.8%, 36.9%, and 32.9% higher, respectively, than those of the high-Cd cultivars in different treatments. All these characteristics were increased by the stagnant treatment (p < 0.01; Fig. 1a). Compared with the aerated treatment, the stagnant treatment increased root porosity, ROL, and the rate of ROL by 49.5%, 97.8%, and 80.7%, respectively. Besides, root porosity was positively correlated with ROL and the rate of ROL, especially under stagnant conditions (p < 0.01; Fig. 1b).
Root porosity, ROL and the rate of ROL of the tested water spinach cultivars in the aerated and stagnant treatments (mean ± SE, n = 3) (a) and Pearson correlations of root porosity with ROL and the rate of ROL (b) in the hydroponic experiment HP1. The overall correlation coefficient (R2) was calculated based on the complete dataset. ** indicates the correlation reaches significance at p < 0.01 level. The scales of y-axes are different among different indices. DW, dry weight
In the pot experiment PT1, the concentrations of Fe and Cd in the Fe plaque on root surfaces differed significantly among the ten water spinach cultivars (p < 0.01; Fig. 2a), indicating cultivar-dependent formation and Cd immobilization of root Fe plaque. The low-Cd cultivars established greater root Fe plaque, showing 80.1% and 62.4% higher root-plaque Fe and Cd than the high-Cd cultivars, respectively. The root-plaque Fe and Cd concentrations of C09, as the highest among the tested cultivars, were 2.7- and 1.3-fold higher, respectively, than those of the lowest cultivar (C05). Similar variations among cultivars and between the low-Cd and high-Cd groups were also observed in rhizosphere Fe plaque (Fig. S1). Results of the PT1 also presented significant differences in root and shoot Cd concentrations, as well as the root-to-shoot Cd translocation rate among the tested cultivars (p < 0.05; Fig. 2a). Shoot and root Cd concentrations ranged from 2.2 to 13.5 mg kg−1 and from 16.6 to 38.2 mg kg−1, respectively, and Cd translocation rates were 0.13–0.39. The root and shoot Cd concentrations and Cd translocation rate of the high-Cd group were 158.8%, 91.5%, and 35.3% higher, respectively, than those of the low-Cd group.
Concentrations of Fe and Cd in root Fe plaque, shoot and root Cd concentrations and root-to-shoot Cd translocation rate of the tested water spinach cultivars (mean ± SE, n = 4) (a); Pearson correlations of the Fe concentration in root Fe plaque with the rate of ROL, Cd concentration in root Fe plaque and shoots (b) in the pot experiment (PT1). The overall correlation coefficient (R2) was calculated based on the complete dataset. ** and * indicate the correlations reach significance at p < 0.01 and p < 0.05 level, respectively. The scales of y-axes are different among different elements. DW, dry weight
Fe plaque and its effect on Cd accumulation
In the experiment PT1, shoot Cd concentration was negatively correlated with the Fe concentration in root Fe plaque, particularly in the low-Cd cultivars (p < 0.01; Fig. 2b), suggesting a mitigation effect of Fe plaque formation on the shoot Cd accumulation of water spinach. The root-plaque Fe was strongly positively correlated with the Cd concentration in root Fe plaque and was negatively correlated with root Cd concentration (p < 0.01; Fig. 2b and S2d). These implied enhanced Cd immobilization and reduced root Cd uptake by root Fe plaque. The rhizosphere Fe plaque exerted similar effects on Cd immobilization and plant Cd uptake because the Fe concentration in rhizosphere Fe plaque also showed an inverse relation with shoot Cd concentration (p < 0.01; Fig. S2e).
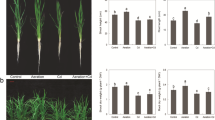
Results of the hydroponics HP3 presented varied spatial patterns of Fe plaque along root axes across different aeration treatments, with the enrichment of Fe deposition on root basal segment in the aerated treatment and on the apical segment in the stagnant treatment (Fig. 3a). Specifically, the stagnant treatment increased the root-plaque Fe concentrations on the middle and apical segments by 1.4- and 5.0-fold, respectively, when compared with the aerated treatment, but reduced the value on the basal segment by 31.1%. Compared with the high-Cd cultivar C05, the root Fe plaque of the low-Cd cultivar C09 exhibited a lesser reduction on the basal segment but more pronounced enhancements on the middle and apical segments (Fig. 3a). With respect to the Cd accumulation, the stagnant treatment reduced shoot Cd concentration by 21.0% and this effect in C09 reached significance with Fe supplementation (p < 0.05; Fig. 3b). The Fe addition treatment (30 mg L−1) also effectively mitigated shoot Cd accumulation, which reduced the shoot Cd of C05 and C09 by 22.7% and 39.0%, respectively, when compared with the control.
The Fe concentration in Fe plaque on the surfaces of different root segments with Fe addition (a) and effects of Fe addition (30 mg L−1) on shoot Cd concentration (b) in the two water spinach cultivars in the hydroponic experiment (HP3) (mean ± SE, n = 4). Different uppercase letters indicate significant difference between the aerated and stagnant treatments and different lowercase letters indicate significant difference between Fe addition treatments or among root segments, respectively, at p < 0.05 level. The scales of y-axes are different between the two cultivars
ROL and its relationship with Cd accumulation
Based on the hydroponics HP2, the ROL of the four water spinach cultivars was diminished by increasing Cd exposure (Fig. 4a). Compared with the control, 15 and 50 µM Cd treatments decreased the rate of ROL by 23.2% and 38.2%, respectively. Among the four cultivars, C05 exhibited minimum decreases of 7.8% and 14.5% in the rate of ROL in the 15 and 50 µM Cd treatments, respectively. Meanwhile, the 50 µM Cd treatment increased shoot Cd concentration by an average of 1.9-fold compared to the 15 µM Cd treatment. However, the root-to-shoot Cd translocation rates in the two treatments were comparable.
Results of the PT1 presented a positive correlation between the rate of ROL and the Fe concentration in root Fe plaque (p < 0.01; Fig. 2b), suggesting a close connection between ROL and Fe plaque formation. More importantly, the rate of ROL exhibited negative correlations with shoot Cd concentration in both the PT1 and HP2 experiments (p < 0.01; Figs. S2f and 4b). Furthermore, the rate of ROL was negatively correlated with root Cd in the PT1 and was negatively correlated with the root-to-shoot Cd translocation rate in the HP2 (p < 0.01; Fig. 4b). These results indicated significant effects of ROL on the Cd uptake and accumulation in water spinach.
Pearson correlations of the rate of ROL with shoot Cd concentration and the root-to-shoot Cd translocation rate (a) and values of these indices (mean ± SE, n = 3) of the four water spinach cultivars (b) in the hydroponic experiment HP2. Different uppercase letters indicate significant difference among treatments and different lowercase letters indicate significant difference among cultivars, respectively, at p < 0.05 level. The overall correlation coefficient (R2) was calculated based on the complete dataset. ** and * indicate the correlations reach significance at p < 0.01 and p < 0.05 level, respectively. The scales of y-axes are different among different indices. DW, dry weight
Root Cd chelation and compartmentalization
In the hydroponic experiment HP4, both C05 and C09 exhibited temporal increases in shoot Cd concentration and root-to-shoot Cd translocation rate over the experimental period of 28 days (Fig. 5a and b). The low-Cd cultivar C09 generally showed a steadily lower shoot Cd and root-to-shoot Cd translocation rate than the high-Cd cultivar C05, except in the initial stage. Interestingly, within the first week, the root Cd of C09 was consistently higher than that of C05 in both the 15 µM and 50 µM Cd treatments (Fig. S3).
Shoot Cd concentration (n = 3) (a) and root-to-shoot Cd translocation rate (n = 4) (b) in different Cd stress treatments over the experimental period of 28 days (mean ± SE); chemical fractions of Cd in shoots and roots (c) and subcellular distribution of Cd in roots (d) at 21 d (mean, n = 4) of the two water spinach cultivars in the hydroponic experiment (HP4). F1, inorganic Cd; F2, water soluble Cd; F3, Cd integrated with pectates and protein; F4, undissolved Cd-phosphate complexes; F5, Cd oxalate; F6, residual Cd; Cd15, 15 µM Cd treatment, Cd50, 50 µM Cd treatment
The Cd accumulation in the shoots exhibited a pronounced difference between C09 and C05 at 21 d. Meanwhile, the dramatic increase from 21 d to 28 d, especially in the 50 µM Cd treatment, probably implied an acute toxic response. Therefore, the samples collected at 21 d were used in the sequential extraction procedure. Results indicated that the chemical forms of Cd in roots and shoots differed between C05 and C09. In the root, C09 had lower proportions of the inorganic (F1-Cd) and water-soluble Cd (F2-Cd) but a higher proportion of the Cd integrated with pectates and protein (F3-Cd) than C05. In the shoot where the F1-Cd and F2-Cd were minimal, C09 had a lower proportion of the F3-Cd and higher proportions of the undissolved Cd-phosphate complexes (F4-Cd) and Cd oxalate (F5-Cd) than C05. The F3-Cd dominated the Cd accumulated in both cultivars, accounting for 51.0-69.8% in the root and 53.1-77.5% in the shoot (Fig. 5c). Compared with the 15 µM Cd treatment, 50 µM Cd raised the proportion of the F3-Cd in both cultivars and diminished those of the F1-Cd and F2-Cd in the root (Fig. 5c). By extracting the Cd in subcellular components of the roots, it was observed that the cell wall fraction (FCW-Cd) constituted the major proportion of root Cd, which averaged 52.1% and 75.7% in C05 and C09, respectively. The two tested cultivars showed different subcellular distribution patterns of Cd in the root (Fig. 5d). C09 contained more FCW-Cd than C05, especially under the 50 µM Cd treatment.
Discussion
Cultivar variations in ROL, Fe plaque and Cd accumulation
This study revealed a strong dependence of root porosity and (rate of) ROL on the cultivar in water spinach (Fig. 1a). This aligns with the observations in other wetland plants (Colmer 2003; Tong et al. 2023). Root porosity was governed by the aerenchyma tissue, and enhanced aerenchyma formation increased ROL by facilitating oxygen diffusion to root tips (Colmer 2003; Mei et al. 2009). In our study, the positive relations of root porosity with (rate of) ROL (Fig. 1b) demonstrated a substantial effect of root aerenchyma formation on the cultivar-dependent ROL of water spinach, particularly under anoxic conditions. The relative root porosity and ROL of water spinach cultivars could remain stable despite changes in aeration conditions, as shown by the significant relation in each characteristic between different experimental treatments (Fig. S4). For brevity, subsequent analyses mainly adopted the rate of ROL to represent the ROL of water spinach cultivars based on the robust relationship between the two traits.
The Fe plaque formation of water spinach also varied among the tested cultivars (Fig. 2a). The cultivar variations in root and rhizosphere Fe plaque could be connected to ROL, as indicated by the positive correlations between ROL and the Fe concentrations in both root and rhizosphere Fe plaque (Figs. 2b and S2a). The Fe plaque of wetland plants typically formed through the oxidation of rhizosphere Fe(II) by O2 from ROL, especially in submerged environments (Xu et al. 2018; Zhong et al. 2021). Factors that promoted ROL, e.g., plant growth and selenium addition, also enhanced Fe plaque and its capability to sequester and immobilize heavy metal(loid)s in the rhizosphere (Wang et al. 2013; Huang et al. 2020). Moreover, our study highlights pronounced cultivar effects on Cd uptake, transport and accumulation in water spinach (Fig. 2a). Notably, shoot Cd concentration positively correlated with both root Cd and root-to-shoot Cd translocation rate (Figs. S2b and S2c), emphasizing the combined effects of root uptake and upward transport on the Cd accumulation in the shoot. While previous studies claimed an exclusive role of root-to-shoot Cd transport (Xin et al. 2013), the present results provide essential updates to understanding the mechanisms of Cd accumulation in water spinach, featuring key root characteristics (e.g., ROL and Fe plaque) in determining the Cd levels in the edible parts.
Effects of Fe plaque on Cd accumulation
The distinct Fe plaque formation between the low-Cd and high-Cd cultivars suggested a substantial mitigation effect of Fe plaque on shoot Cd accumulation, which was further evidenced by the negative correlations of shoot Cd concentration with the Fe concentrations in root and rhizosphere Fe plaque (Figs. 2, S1 and S2e). As these relationships were notably more pronounced in the low-Cd group, Fe plaque seemed to particularly contribute to reducing the Cd accumulation in these cultivars. This effect could be attributed to the adsorption and co-precipitation of Cd by Fe (hydr)oxides in the Fe plaque due to the high affinity of Fe hydroxides for Cd2+, which subsequently inhibited root Cd uptake (Huang et al. 2022; Zandi et al. 2022). In the PT1, root-plaque Fe showed a positive correlation with root-plaque Cd and an inverse relationship with root Cd, further exemplifying this mechanism (Figs. 2b and S2d). Depending on soil and plant factors, e.g., cultivar and root physiological activities, the role of the Fe plaque in Cd uptake might vary from a barrier to a facilitator according to previous reports (Lai et al. 2012; Huang et al. 2017; Gao et al. 2023). The present study presents compelling evidence for the mitigation effects of Fe plaque on the Cd uptake and accumulation in water spinach.
Based on the HP3, the Fe plaque formed heterogeneous patterns along root axes, and the Fe deposition on different root segments was differently affected by the external aeration conditions (Fig. 3a). The analogous patterns of rice Fe plaque were primarily ascribed to the longitudinal variation of ROL (Limmer et al. 2021; Zandi et al. 2023). Changes in the spatial distribution of Fe plaque were also associated with variations in the spatial pattern of ROL under distinct aeration conditions (Wu et al. 2012). The ROL patterns were determined by several root traits, among which the barrier to ROL formed in the root basal segment and stimulated by root hypoxia might play a key role (Yamauchi et al. 2018; Pedersen et al. 2021). These findings highlight the need for more comprehensive investigation into root anatomical characteristics to elucidate the regulation of Fe plaque formation in water spinach. More importantly, compared with the high-Cd cultivar C05, the low-Cd cultivar C09 exhibited a lesser reduction in the Fe plaque formation on the basal segment and a greater enhancement on the tips under stagnant conditions (Fig. 3a). This suggested that the low-Cd water spinach cultivar not only established stronger overall root Fe plaque but also developed more favorable patterns of Fe plaque under varied external aerations.
The HP3 illustrated an effective reduction in the Cd accumulation of water spinach by Fe supplementation and stagnant treatment (Fig. 3b). The availability of active Fe(II) was proved vital for both the formation and Cd-immobilizing capacity of Fe plaque (Li et al. 2019; Huang et al. 2022). Stagnant conditions were found to enhance Fe plaque formation and subsequent Cd sequestration by increasing ROL (Zhong et al. 2021). The difference in shoot Cd accumulation between C05 and C09 increased markedly from 32.2% in the aerated treatment without Fe supply to 101.0% in the stagnant treatment with 30 mg L−1 Fe (Fig. 3b). This indicated the pivotal role of Fe plaque in shaping the different Cd accumulation among water spinach cultivars. Therefore, the Fe plaque formation could be considered as a critical criterion for selecting low-Cd water spinach cultivars, which should exhibit greater root Fe plaque with more favorable spatial patterns under varying aeration conditions.
Effects of ROL on Fe plaque and Cd accumulation
The present study demonstrates the mitigating effect of ROL on the Cd accumulation in water spinach. This effect was evidenced by the negative correlations between shoot Cd concentration and ROL (Figs. S2f and 4b), along with the different ROL between the low-Cd and high-Cd cultivars (Fig. 1a). Enhanced Fe plaque formation likely accounted for this effect, supported by the positive correlations of the Fe concentrations in root and rhizosphere Fe plaque with ROL (Fig. 2b and S2a). Although the combined roles of ROL and Fe plaque in reducing plant Cd accumulation were reported previously (Wang et al. 2013; Zhang et al. 2022), their contribution to the varied Cd accumulation among crop cultivars, especially in water spinach, remained uncertain. Our findings provide the first evidence that water spinach cultivars with higher ROL (e.g., C09) formed stronger Fe plaque, resulting in lower Cd accumulation in the edible parts. Increased ROL under stagnant conditions further stimulated Fe plaque formation and alleviated shoot Cd accumulation in water spinach (Figs. 1a and 3a and Table S1). Notably, the low-Cd cultivar C09 exhibited a greater increase in ROL than the high-Cd cultivar C05 in response to stagnant conditions, which likely contributed to its more favorable pattern of Fe plaque (Fig. 3a and Table S1). This suggested that low-Cd cultivars tended to respond more actively to varied aeration conditions.
The strong negative correlation between ROL and the root-to-shoot translocation rate in the HP2 suggested potential involvement of ROL in the Cd upward transport (Fig. 4a). This association might stem from root anatomical characteristics that hindered the radial transport of Cd into the root stele, subsequently restraining root-to-shoot Cd translocation (Qi et al. 2020; Xiao et al. 2021). To date, the regulation mechanism of ROL in water spinach has been rarely reported, warranting further exploration of relevant anatomical and physiological traits.
Cd chelation and compartmentalization
Due to its higher ROL, greater Fe plaque, and a more favorable Fe-plaque pattern, C09 exhibited consistently lower shoot Cd and root-to-shoot Cd translocation rate compared to C05 in the HP4 (Fig. 5a and b). Nonetheless, the discrepancy in root Cd uptake between the two cultivars appeared to diminish in the HP4 (Fig. S3), possibly due to inhibited Fe plaque formation resulting from Fe deficiency in the nutrient solution. Collectively, these findings underscore the combined roles of ROL and Fe plaque as an integrated system for determining the Cd accumulation in different water spinach cultivars.
Compared with C05, the low-Cd cultivar C09 exhibited a higher proportion of F3-Cd and lower proportions of F1-Cd and F2-Cd (Fig. 5c). The transformation of F1-Cd and F2-Cd into F3-Cd represented a detoxification mechanism in plants, concurrently restricting Cd upward translocation (Xin et al. 2017; Zhang et al. 2020; Lu et al. 2020). In our study, elevated Cd exposure reduced the proportions of the labile F1-Cd and F2-Cd while increased that of F3-Cd, resulting in lower Cd translocation rates (Fig. 5b and c). Moreover, the subcellular distribution pattern of Cd in the roots revealed that C09 had a larger proportion of FCW-Cd than C05 (Fig. 5d). Plant cell walls typically contained rich negatively charged functional groups capable of binding Cd2+, and enhanced cell walls facilitated root Cd retention to alleviate the phytotoxicity (Cui et al. 2017; Xin et al. 2017; Zhang et al. 2020). Consequently, the increased proportions of FCW-Cd under higher Cd stress also contributed to decreased Cd translocation efficiency (Fig. 5b and d). These results suggest that the low-Cd water spinach cultivar might be more effective in retaining Cd in the roots, thereby reducing Cd transport into the edible parts.
Conclusion
This study presented apparent variations in ROL and Fe plaque formation among different cultivars and provided conclusive evidence for the critical effects of these root characteristics on the Cd accumulation of water spinach. The root and rhizosphere Fe plaque effectively sequestered Cd and reduced root Cd uptake, while the formation of Fe plaque strongly depended on exogenous Fe availability and ROL. Cultivars with higher ROL generally had greater Fe plaque formation, and ROL increases under stagnant conditions enhanced the Fe plaque and prompted its redistribution along root axes. Root retention of Cd via chelation and compartmentalization significantly reduced root-to-shoot Cd translocation, particularly under elevated Cd exposure. Therefore, ROL, Fe plaque, and root Cd retention formed an integrated system that mitigated the Cd uptake and accumulation in different water spinach cultivars (Fig. 6). Low-Cd water spinach cultivars exhibited higher ROL, greater Fe plaque formation, and more effective root Cd retention. These cultivars also responded to stagnant conditions with greater increases in ROL and more favorable spatial patterns of the Fe plaque. These features can serve as useful criteria for cultivar selection. Furthermore, planting water spinach under prolonged flooding with adequate Fe supply could effectively mitigate the Cd accumulation in the edible parts. These findings provide a theoretical basis for the controlling mechanism of Cd accumulation in water spinach and offer practical methods for selecting and breeding low-Cd cultivars to improve food quality and safeguard public health.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Abiko T, Kotula L, Shiono K et al (2012) Enhanced formation of aerenchyma and induction of a barrier to radial oxygen loss in adventitious roots of Zea nicaraguensis contribute to its waterlogging tolerance as compared with maize (Zea mays ssp. mays). Plant Cell Environ 35:1618–1630. https://doi.org/10.1111/j.1365-3040.2012.02513.x
Adrees M, Ali S, Rizwan M et al (2015) Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: a review. Ecotoxicol Environ Saf 119:186–197. https://doi.org/10.1016/j.ecoenv.2015.05.011
Cheng H, Chen D-T, Tam NF-Y et al (2012) Interactions among Fe2+, S2–, and Zn2+ tolerance, root anatomy, and radial oxygen loss in mangrove plants. J Exp Bot 63:2619–2630. https://doi.org/10.1093/jxb/err440
Cheng H, Wang M, Wong MH, Ye Z (2014) Does radial oxygen loss and iron plaque formation on roots alter cd and pb uptake and distribution in rice plant tissues? Plant Soil 375:137–148. https://doi.org/10.1007/s11104-013-1945-0
Clemens S, Aarts MGM, Thomine S, Verbruggen N (2013) Plant science: the key to preventing slow cadmium Poisoning. Trends Plant Sci 18:92–99. https://doi.org/10.1016/j.tplants.2012.08.003
Colmer TD (2003) Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ 26:17–36. https://doi.org/10.1046/j.1365-3040.2003.00846.x
Cui J, Liu T, Li F et al (2017) Silica nanoparticles alleviate cadmium toxicity in rice cells: mechanisms and size effects. Environ Pollut 228:363–369. https://doi.org/10.1016/j.envpol.2017.05.014
Deng D, Wu S-C, Wu F-Y et al (2010) Effects of root anatomy and Fe plaque on arsenic uptake by rice seedlings grown in solution culture. Environ Pollut 158:2589–2595. https://doi.org/10.1016/j.envpol.2010.05.015
Gao Y, Tong H, Zhao Z et al (2023) Effects of Fe oxides and their redox cycling on cd activity in paddy soils: a review. J Hazard Mater 456:131665. https://doi.org/10.1016/j.jhazmat.2023.131665
Göthberg A, Greger M, Bengtsson B-E (2002) Accumulation of heavy metals in water spinach (Ipomoea aquatica) cultivated in the Bangkok region, Thailand. Environ Toxicol Chem 21:1934–1939. https://doi.org/10.1002/etc.5620210922
He B, Ling L, Zhang L et al (2015) Cultivar-specific differences in heavy metal (cd, cr, Cu, Pb, and zn) concentrations in water spinach (Ipomoea aquatic ‘Forsk’) grown on metal-contaminated soil. Plant Soil 386:251–262. https://doi.org/10.1007/s11104-014-2257-8
Huang X, Tang K, Xu X, Cai C (2017) Interaction of Fe-Mn plaque and Arthrobacter echigonensis MN1405 and uptake and translocation of cd by Phytolacca acinosa Roxb. Chemosphere 174:585–592. https://doi.org/10.1016/j.chemosphere.2017.02.012
Huang L, Li WC, Tam NFY, Ye Z (2019) Effects of root morphology and anatomy on cadmium uptake and translocation in rice (Oryza sativa L). J Environ Sci 75:296–306. https://doi.org/10.1016/j.jes.2018.04.005
Huang G, Ding C, Li Y et al (2020) Selenium enhances iron plaque formation by elevating the radial oxygen loss of roots to reduce cadmium accumulation in rice (Oryza sativa L). J Hazard Mater 398:122860. https://doi.org/10.1016/j.jhazmat.2020.122860
Huang G, Pan D, Wang M et al (2022) Regulation of iron and cadmium uptake in rice roots by iron(III) oxide nanoparticles: insights from iron plaque formation, gene expression, and nanoparticle accumulation. Environ Sci Nano 9:4093–4103. https://doi.org/10.1039/D2EN00487A
Jiménez JDLC, Clode PL, Signorelli S et al (2021) The barrier to radial oxygen loss impedes the apoplastic entry of iron into the roots of Urochloa humidicola. J Exp Bot 72:3279–3293. https://doi.org/10.1093/jxb/erab043
Kang Z, Zhang W, Qin J et al (2020) Yield advantage and cadmium decreasing of rice in intercropping with water spinach under moisture management. Ecotoxicol Environ Saf 190:110102. https://doi.org/10.1016/j.ecoenv.2019.110102
Khan N, Seshadri B, Bolan N et al (2016) Root iron plaque on wetland plants as a dynamic pool of nutrients and contaminants. Adv Agron 138:1–96. https://doi.org/10.1016/bs.agron.2016.04.002
Khan MA, Khan S, Khan A, Alam M (2017) Soil contamination with cadmium, consequences and remediation using organic amendments. Sci Total Environ 601–602:1591–1605. https://doi.org/10.1016/j.scitotenv.2017.06.030
Kludze HK, DeLaune RD, Patrick WH (1994) A colorimetric method for assaying dissolved oxygen loss from container-grown rice roots. Agron J 86:483–487. https://doi.org/10.2134/agronj1994.00021962008600030005x
Lai Y, Xu B, He L et al (2012) Cadmium uptake by and translocation within rice (Oryza sativa L.) seedlings as affected by iron plaque and Fe2O3. Pak J Bot 44:1557–1561
Li J, Liu J, Yan C et al (2019) The alleviation effect of iron on cadmium phytotoxicity in mangrove A. marina. Alleviation effect of iron on cadmium phytotoxicity in mangrove Avicennia marina (Forsk.) Vierh. Chemosphere 226:413–420. https://doi.org/10.1016/j.chemosphere.2019.03.172
Limmer MA, Evans AE, Seyfferth AL (2021) A new method to capture the spatial and temporal heterogeneity of aquatic plant iron root plaque in situ. Environ Sci Technol 55:912–918. https://doi.org/10.1021/acs.est.0c02949
Lu R-R, Hu Z-H, Zhang Q-L et al (2020) The effect of Funneliformis mosseae on the plant growth, cd translocation and accumulation in the new Cd-hyperaccumulator Sphagneticola Calendulacea. Ecotoxicol Environ Saf 203:110988. https://doi.org/10.1016/j.ecoenv.2020.110988
Mei XQ, Ye ZH, Wong MH (2009) The relationship of root porosity and radial oxygen loss on arsenic tolerance and uptake in rice grains and straw. Environ Pollut 157:2550–2557. https://doi.org/10.1016/j.envpol.2009.02.037
Mei X, Li Q, Wang H et al (2020) Effects of cultivars, water regimes, and growth stages on cadmium accumulation in rice with different radial oxygen loss. Plant Soil 453:529–543. https://doi.org/10.1007/s11104-020-04634-w
Nocito FF, Lancilli C, Dendena B et al (2011) Cadmium retention in rice roots is influenced by cadmium availability, chelation and translocation: cadmium retention in rice roots. Plant Cell Environ 34:994–1008. https://doi.org/10.1111/j.1365-3040.2011.02299.x
Otte ML, Rozema J, Koster L et al (1989) Iron plaque on roots of Aster tripolium L.: Interaction with zinc uptake. New Phytol 111:309–317. https://doi.org/10.1111/j.1469-8137.1989.tb00694.x
Pedersen O, Sauter M, Colmer TD, Nakazono M (2021) Regulation of root adaptive anatomical and morphological traits during low soil oxygen. New Phytol 229:42–49. https://doi.org/10.1111/nph.16375
Qi X, Tam NF, Li WC, Ye Z (2020) The role of root apoplastic barriers in cadmium translocation and accumulation in cultivars of rice (Oryza sativa L.) with different Cd-accumulating characteristics. Environ Pollut 264:114736. https://doi.org/10.1016/j.envpol.2020.114736
Tang L, Hamid Y, Liu D et al (2020) Foliar application of zinc and selenium alleviates cadmium and lead toxicity of water spinach – Bioavailability/cytotoxicity study with human cell lines. Environ Int 145:106122. https://doi.org/10.1016/j.envint.2020.106122
Tong S, Kjær JE, Peralta Ogorek LL et al (2023) Responses of key root traits in the genus Oryza to soil flooding mimicked by stagnant, deoxygenated nutrient solution. J Exp Bot 74:2112–2126. https://doi.org/10.1093/jxb/erad014
Uraguchi S, Mori S, Kuramata M et al (2009) Root-to-shoot cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. J Exp Bot 60:2677–2688. https://doi.org/10.1093/jxb/erp119
Wang J, Yuan J, Yang Z et al (2009) Variation in cadmium accumulation among 30 cultivars and cadmium subcellular distribution in 2 selected cultivars of water spinach (Ipomoea aquatica Forsk). J Agric Food Chem 57:8942–8949. https://doi.org/10.1021/jf900812s
Wang X, Yao H, Wong MH, Ye Z (2013) Dynamic changes in radial oxygen loss and iron plaque formation and their effects on cd and as accumulation in rice (Oryza sativa L). Environ Geochem Health 35:779–788. https://doi.org/10.1007/s10653-013-9534-y
Wang X, Li B, Tam NF-Y et al (2014) Radial oxygen loss has different effects on the accumulation of total mercury and methylmercury in rice. Plant Soil 385:343–355. https://doi.org/10.1007/s11104-014-2239-x
Wu C, Ye Z, Li H et al (2012) Do radial oxygen loss and external aeration affect iron plaque formation and arsenic accumulation and speciation in rice? J Exp Bot 63:2961–2970. https://doi.org/10.1093/jxb/ers017
Xiao Q, Wong MH, Huang L, Ye Z (2015) Effects of cultivars and water management on cadmium accumulation in water spinach (Ipomoea aquatica Forsk). Plant Soil 391:33–49. https://doi.org/10.1007/s11104-015-2409-5
Xiao A, Chen D, Li WC, Ye Z (2021) Root morphology and anatomy affect cadmium translocation and accumulation in rice. Rice Sci 28:594–604. https://doi.org/10.1016/j.rsci.2021.03.003
Xin J, Zhao X, Tan Q et al (2017) Comparison of cadmium absorption, translocation, subcellular distribution and chemical forms between two radish cultivars (Raphanus sativus L). Ecotoxicol Environ Saf 145:258–265. https://doi.org/10.1016/j.ecoenv.2017.07.042
Xin J, Huang B, Yang Z et al (2013) Comparison of cadmium subcellular distribution in different organs of two water spinach (Ipomoea aquatica Forsk.) Cultivars. Plant Soil 372:431–444. https://doi.org/10.1007/s11104-013-1729-6
Xu Y, Sun X, Zhang Q et al (2018) Iron plaque formation and heavy metal uptake in Spartina alterniflora at different tidal levels and waterlogging conditions. Ecotoxicol Environ Saf 153:91–100. https://doi.org/10.1016/j.ecoenv.2018.02.008
Yamauchi T, Colmer TD, Pedersen O, Nakazono M (2018) Regulation of root traits for internal aeration and tolerance to soil waterlogging-flooding stress. Plant Physiol 176:1118–1130. https://doi.org/10.1104/pp.17.01157
Yang J, Zheng G, Yang J et al (2017) Phytoaccumulation of heavy metals (pb, zn, and cd) by 10 wetland plant species under different hydrological regimes. Ecol Eng 107:56–64. https://doi.org/10.1016/j.ecoleng.2017.06.052
Yuan P, Peng C, Shi J et al (2021) Ferrous ions inhibit Cu uptake and accumulation via inducing iron plaque and regulating the metabolism of rice plants exposed to CuO nanoparticles. Environ Sci Nano 8:1456–1468. https://doi.org/10.1039/D0EN01241F
Zandi P, Yang J, Darma A et al (2022) Iron plaque formation, characteristics, and its role as a barrier and/or facilitator to heavy metal uptake in hydrophyte rice (Oryza sativa L). Environ Geochem Health. https://doi.org/10.1007/s10653-022-01246-4
Zandi P, Xia X, Yang J et al (2023) Speciation and distribution of chromium (III) in rice root tip and mature zone: the significant impact of root exudation and iron plaque on chromium bioavailability. J Hazard Mater 448:130992. https://doi.org/10.1016/j.jhazmat.2023.130992
Zhang S, Ni X, Arif M et al (2020) Salinity influences cd accumulation and distribution characteristics in two contrasting halophytes, Suaeda Glauca and Limonium Aureum. Ecotoxicol Environ Saf 191:110230. https://doi.org/10.1016/j.ecoenv.2020.110230
Zhang F, Peng D, Liu L et al (2022) Cultivar-dependent rhizobacteria community and cadmium accumulation in rice: effects on cadmium availability in soils and iron-plaque formation. J Environ Sci 116:90–102. https://doi.org/10.1016/j.jes.2021.08.021
Zhao F-J, Ma Y, Zhu Y-G et al (2015) Soil contamination in China: current status and mitigation strategies. Environ Sci Technol 49:750–759. https://doi.org/10.1021/es5047099
Zhong S, Li X, Li F et al (2021) Water management alters cadmium isotope fractionation between shoots and nodes/leaves in a soil-rice system. Environ Sci Technol 55:12902–12913. https://doi.org/10.1021/acs.est.0c04713
Funding
This work was supported by the National Natural Science Foundation of China (41977329 and 41501355), the Anhui Quality Engineering Project Practice Education Base (2021xqhzsjjd069), and Hefei University Quality Engineering Project Course Ideological and Political Demonstration (2021hfukcsz09).
Author information
Authors and Affiliations
Contributions
Qingqing Xiao: conceptualization, material preparation, data collection, and experiments. Yuanyuan Tang: review, editing, and funding acquisition. Lu Huang: review and editing. Yihan Chi: data curation, formal analysis and visualization, and writing. Zhihong Ye: conceptualization, review, and funding acquisition. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Al Imran Malik.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiao, Q., Tang, Y., Huang, L. et al. Radial oxygen loss and iron plaque function as an integrated system to mitigate the cadmium accumulation in water spinach. Plant Soil 498, 243–258 (2024). https://doi.org/10.1007/s11104-023-06432-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-06432-6