Abstract
Aims
The principal contributor to the cation binding properties of roots is currently considered to be the cell wall or, alternatively, the plasma membrane. The aim of this study was to highlight their respective contributions in the binding properties.
Methods
Cell walls of a dicotyledon (Solanum lycopersicum L.) and monocotyledon (Triticum aestivum L.) were isolated from roots and their binding properties were compared to those of their respective roots. Cell wall and root binding capacities were evaluated by potentiometric titrations and cation exchange capacity measurements, while their biochemical composition was analyzed by 13C-NMR spectroscopy.
Results
The lower binding capacity of isolated cell walls compared to roots revealed that cell plasma membranes had a higher binding site density than cell walls. The significant decrease in some NMR signals, i.e. carbonyl C, N alkyl/methoxyl C and alkyl C regions, suggested that carboxyl, amine and phosphate binding sites, borne by proteins and phospholipid plasma membranes, contribute to the binding capacity.
Conclusions
Cell walls and plasma membranes were found to be jointly involved in root binding properties and their respective contributions seemed vary between plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Trace elements in soil were originally derived from the weathering of parent rock (Dœlsch et al. 2006a), but the development of industrial and agricultural activities and urbanization gave rise to other sources. Mining, agricultural practices such as organic waste spreading, refuse incineration and urban traffic account for substantial anthropogenic contamination (Dœlsch et al. 2006b; Nagajyoti et al. 2010; Legros et al. 2013; Zhou et al. 2013).
Roots are the first plant organs exposed to trace elements in soil. Plants may show various rhizotoxicity symptoms, such as root growth inhibition, root hair damage, local swelling and ruptures in the rhizodermis and outer cortex (Sheldon and Menzies 2005; Kopittke and Menzies 2006; Kopittke et al. 2008). In recent years, root binding properties have often been put forward as a major driver of micronutrient uptake and trace element toxicity in plants (Reid 2001; Thakali et al. 2006; Kopittke et al. 2009a). The root apoplast, i.e. the compartment consisting of water, gas and cell walls, and root cell plasma membranes are, alternatively, considered to be a major contributor to root binding properties (Sattelmacher 2001; Kinraide 2004).
Cell walls exhibit many binding sites associated with carboxylic, phenolic, amine and thiol functional groups borne by a range of chemical polymers tightly woven into the tridimensional meshwork (Sarkar et al. 2009; Krzesłowska 2011). Binding properties closely depend on the cell wall composition, which varies notably with plant age and between species. The root binding capacity is usually higher in dicots (20–50 cmolc.kg−1 or meq.100 g−1) than in monocots (10–20 cmolc.kg−1) (Ram 1980; Sattelmacher 2001; Straczek et al. 2008). This difference in binding capacity is attributed to a higher pectin content in dicot cell walls (20-35 % of the dry mass) than in monocot cell walls (5 % of the dry mass) (Vogel 2008). Free carboxylic groups borne by pectins are usually considered to be responsible for 70-90 % of the root binding properties (Haynes 1980). Experimental studies have shown that these free carboxyl groups play a crucial role in aluminum (Al) accumulation in roots and in the induction of Al rhizotoxicity (Horst et al. 2010). Beyond pH 7, phenolic groups also contribute significantly to root cell wall binding properties (Meychik and Yermakov 2001). Considering that cell wall compounds, especially pectins, seem to control root cation binding properties, model formalisms have been based on descriptions of interactions between trace elements and isolated cell walls, thus overlooking the possible contribution of other root compartments such as cell plasma membranes (Allan and Jarrell 1989; Postma et al. 2005).
However, the plasma membrane bears cation binding sites provided by phospholipid phosphate groups and protein-containing amino acids entrapped in the plasma membrane (Kinraide et al. 1992). The negative charges provided by these sites create a negative plasma membrane electrical potential that controls the activity of ions at the plasma membrane surface (Kinraide 2006). Experimental studies on root protoplasts showed that metals such as copper (Cu), nickel (Ni) or Al have high binding affinity for root cell plasma membranes (Zhang et al. 2001; Vulkan et al. 2004; Kudo et al. 2011). The development of a plant-ion interaction model based on the binding properties of the plasma membranes while ignoring those of cell walls has markedly enhanced prediction of plant uptake and rhizotoxicity of a broad range of ions (Kopittke et al. 2011; Wang et al. 2011). These authors have consistently suggested that plasma membranes substantially contribute to the binding properties of whole roots.
Ultimately, the respective contributions of cell walls and plasma membranes to the binding properties of whole roots have not been clearly established, especially as they are always tested separately. Studies carried out on cation distributions within whole roots using current imaging techniques have not provided any further information on this point. Elements accumulated outside and within the cells can be clearly differentiated (Liu and Kottke 2003; Kopittke et al. 2007; Kopittke et al. 2009b), but it is much harder to distinguish between ions bound in cell walls from those bound on plasma membranes. Nanoscale secondary ion mass spectrometry (NanoSIMS), an analytical tool that provide high spatial resolution combined with high elemental sensitivity (Herrmann et al. 2007), would seem to be able to make such distinctions, but to our knowledge this technique has never been used for this purpose (Smart et al. 2010; Moore et al. 2011).
The present study thus aimed to highlight the respective contributions of cell walls and plasma membranes to the binding properties of whole roots. The properties and chemical nature of cell wall and plasma membrane cation binding sites were assessed by comparing cell walls isolated from roots with cell walls and plasma membranes still in the roots. Multiple procedures using chemical or physical treatments have been previously described for isolating cell walls (Sentenac and Grignon 1981; Masion and Bertsch 1997; Bastías et al. 2010; Yang et al. 2010). All of these procedures are assumed to be capable of removing plasma membranes and the cytoplasm from roots while preserving the cell wall composition and binding properties. The isolation procedure used in this study allowed us to recover cell walls while preserving the shapes and dimensions they originally had in the roots. Experiments were performed on roots of two plant species representative of dicots i.e. Solanum lycopersicum L. and monocots i.e. Triticum aestivum L.. The cell wall and root binding properties were estimated by measuring the cation exchange capacity (CEC) and performing potentiometric titrations. The cell wall and root compositions were characterized by solid-state 13C nuclear magnetic resonance (13C-NMR).
Material and methods
Plant growth
Bread wheat (T. aestivum L. cv. Premio) and tomato (S. lycopersicum L. cv. Moneymaker) were grown in hydroponic conditions for 21 days with an experimental procedure adapted from Bravin et al. (2010). Briefly, wheat and tomato seeds (5 and 20 seeds per pot, respectively) were germinated in darkness for 7 days in 600 μM CaCl2 and 2 μM H3BO3. Seedlings were then grown for 14 days in a nutrient solution (μM): Ca (NO3) 2 2,000, KNO3 2,000, MgSO4 1,000, KH2PO4 500, NaFe (III) EDTA 100, H3BO3 10, MnCl2 2, CuCl2 1, ZnSO4 1 and Na2MoO4 0.05. The solution was renewed every 2–3 days. The growth chamber parameters were set at (day/night): 25/20 °C, 75/70 % relative humidity and 16/8 h with a photon flux density of 450 μmol photons m−2 s−1 during the day. At harvest, roots were blotted with paper towels before being subdivided into homogenous subsamples and then stored frozen. After thawing, roots were subdivided into two groups: (i) root subsamples were rinsed with 1 mM Ca (NO3) 2 to eliminate cytosolic compounds released during thawing and oven-dried at 50 °C (until a steady mass) and are hereafter referred to as roots; and (ii) remaining root subsamples were maintained moist for subsequent analysis (see below).
Isolation of root cell walls
Cell walls were isolated from wheat and tomato roots using Triton X 100, a non-ionic detergent which enabled us to remove the cellular components by solubilizing plasma membrane phospholipids. The procedure was adapted from Cathala et al. (1978). Thawed wheat and tomato roots were cut into 1–2 cm long pieces, then immersed for 30 days in a solution containing 1 % v/v Triton X 100 and 1 mM Ca (NO3) 2. Triton X 100 was then removed by washing with a 1 mM Ca (NO3) 2 solution that was renewed every day for 10 days. The whole procedure was carried out at 6 (±1)°C under stirring (60 rpm). The isolated cell walls, hereafter referred as to cell walls, were stored at 4 °C in 1 mM Ca (NO3) 2.
To assess the efficiency of the isolation procedure, the loss of calcium (Ca), copper (Cu), iron (Fe), phosphorus (P) and potassium (K) in the cell walls during the 30-day of the procedure was determined. Initial roots and the root material obtained after 30 days of the isolation procedure was oven-dried at 50 °C until a steady mass was achieved, and then ground using a porcelain mortar. A subsample of each root material was digested in a microwave oven (March 5, CEM Corporation) for 15 min (i.e. 10 min at 600 W under 1.2 bar then 5 min at 1,200 W under 10 bar) with 10 ml of aqua regia (i.e. 67 % v/v of 37 % HCl and 33 % of 69 % HNO3). The mineral element concentration in the digest was determined by inductively coupled plasma atomic emission spectroscopy (ICP-AES, Jobin Hyvon Horiba J 38). Measurements were performed in duplicate at 0 and 30 days. Blanks, in-house reference samples and certified reference material were included in the digestions and analyses. The measurement uncertainty ranged from 6 to 15 %.
Determination of the cation exchange capacity of roots and cell walls
The cation exchange capacity (CEC) of wheat and tomato roots and cell walls was determined according to the procedure of Dufey and Braun (1986). A 25 mg mass (dry mass basis) of roots and cell walls was shaken end-over-end in 15 ml of 10 mM CuSO4 for 30 (±1) min. The suspension was filtered (Whatman, grade 4) and roots and cell walls were rinsed three times with 100 ml of 0.1 mM CuSO4 through a Büchner funnel. Roots and cell walls were then shaken end-over-end in 50 ml of 0.1 M HCl for 20 (±1) min. The suspension was finally filtered (Whatman, grade 4) before ICP-AES determination of the Cu concentration in the filtrate. Roots and cell walls were oven-dried at 50 °C and weighed. Measurements were performed in quadruplicate. Blanks were included in the measurements and analyses. Due to its high affinity for root surfaces, Cu is supposed to saturate all cation binding sites onto roots and cell walls. The CEC (cmolc.kg−1) was thus estimated from Cu concentration measured in the acidic filtrate by considering a 1:1 stoichiometric ratio between root binding sites and Cu2+.
Characterization of the acidic properties of roots and cell walls by potentiometric titration
To avoid analytical artefacts, roots and cell walls were first stirred in HNO3 solution -at pH 3 -for 1 h to remove highly bound or precipitated cations (e.g. Fe and Al), then rinsed twice with ultrapure water (18.2 MΩ) for 30 min. Roots and cell walls were then oven-dried at 50 °C until a steady mass was achieved.
The titration procedure was adapted from Garnier et al. (2004a). Briefly, titrations were performed with a Metrohm titration stand with two 719 S Titrino titrators controlled by Tinet 2.4 software. Approximately 0.2 g (dry mass basis) of roots or cell walls was placed in a thermostated cell at 25 (±0.2)°C filled with 100 ml of 10 mM KNO3. The solution was continuously stirred and flushed with an ultrapure nitrogen flow. Roots and cell walls were titrated with 0.1 M KOH (Fischer chemical, titrated three times using potassium hydrogen phthalate to accurately determine the KOH concentration) and 0.2 M HNO3 (from 69 % HNO3, trace analysis grade, Fischer Scientific, titrated three times using the freshly prepared KOH solution). The combined pH-micro-electrode (Ag/AgCl/KCl 3 M, Bioblock Scientific) was calibrated daily with pH-buffer solutions (HANNA 4.01, 7.01 and 10.01 at 25 °C). Titrations were carried out in several steps. The pH was first lowered to 2.5 with HNO3 additions. After 15 min of stirring, the solution was free from carbonates and the pH was then increased step-by-step to 11.5 with the incremental addition of KOH at two different rates: 100 μl from pH 2.5 to 3.5 and from pH 10.5 to 11.5 and 20 μl from pH 3.5 to 10.5.
The acidic properties of roots and cell walls were determined by fitting the experimental data with PROSECE software (Garnier et al. 2004b). Briefly, PROSECE is based on a discrete site distribution model where each site is defined by a site density (L Hi , cmolc.kg−1) and a stability constant (pKai). The fitting procedure highlights the number of sites for optimal fitting as well as their optimal density and pKa.
Identification of the chemical structure of roots and cell walls by solid-state 13C-NMR spectroscopy
Solid-state 13C-1H cross-polarization magic angle spinning nuclear magnetic resonance (13C CP-MAS NMR) analyses were carried out at 101.6 MHz on a Bruker Avance WB 400-MHz Spectrometer. Roots and cell walls (150–200 mg, dry mass basis) were packed into a 4 mm Zirconia rotor and spun at 10 kHz in a MAS probe. Cross-polarization was performed with ramped 1H pulse to circumvent Hartmann-Hahn mismatches. All spectra were obtained with a 2 ms contact time and 2 s recycling time. To improve the resolution, dipolar decoupling was applied on protons during acquisition. Chemical shifts were referenced to tetramethylsilane. 12–18 k scans were conducting depending on the amount of sample.
Spectra were divided in chemical shift regions in which the chemistry of the C atoms were similar: alkyl C (0–45 ppm), N-alkyl and methoxyl C (45–60 ppm), carbohydrate C (60–90 ppm), anomeric C (90–110 ppm), Aryl C (110–145 ppm), O-aryl C (145–165 ppm) and carbonyl C (165–190 ppm) (Wershaw and Mikita 1987). For a semi-quantitative comparison between the root and cell wall spectra for each species, the anomeric region (90–110 ppm) was defined as the internal reference. The anomeric bond is a marker of polysaccharides which mostly occur in the cell walls (Vogel 2008). We therefore considered that the anomeric region was quantitatively preserved in the cell walls in comparison with the corresponding roots of each species. Spectra were thus normalized to the maximal intensity of the anomeric region and numerical ratios between the area of each region and the area of the reference were calculated.
Spectra deconvolution was performed with IGOR PRO 5.0 software. It uses Gaussian type functions to perform the fitting. The baseline was corrected by the software. For each generated Gaussian type function, the consistency of the position, amplitude and standard deviation was thoroughly investigated.
Results
Loss of mineral elements during the isolation procedure
The mineral element loss patterns during wheat and tomato cell wall isolation were very similar (Fig. 1). Potassium was no longer detected after the 30-day isolation procedure. P, Ca and Fe losses were 90–95, 86–80 and 77-80 % for tomato and wheat, respectively. The weakest loss was noted for Cu, with 52 and 62 % for tomato and wheat, respectively.
Cation exchange capacity of roots and cell walls
For wheat and tomato, the root CEC was threefold higher than that of the respective cell walls when expressed on an initial root mass basis (Fig. 2). The CEC of tomato roots (73 ± 2 cmolc.kg−1) and cell walls (23 ± 3 cmolc.kg−1) was ca. 2.5-fold higher than that of wheat roots (29 ± 4 cmolc.kg−1) and cell walls (10 ± 1 cmolc.kg−1), respectively.
Cation exchange capacity (CEC) of roots (filled bars) and cell walls (empty bars) of wheat and tomato. Error bars stand for the standard deviation (n = 4). Different lower case letters stand for a significant difference between roots and cell walls of a given species, while different upper case letters stand for a significant difference between roots and cell walls of wheat and tomato (P ≤ 0.05, Mann–Whitney U test)
Acidic properties of roots and cell walls
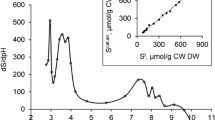
The experimental potentiometric titration data for wheat and tomato roots and cell walls were accurately fitted with PROSECE by parameterising four proton binding functional groups for wheat and tomato roots and cell walls (Fig. 3; Table 1). These sites were characterised by distinct pKa. The pKa of site 1 (L1) were fitted at 3.4 and 3.6 for wheat and tomato cell walls and 4.1 and 4.4 for roots. The pKa of site 2 (L2) ranged from 5.0 to 5.3 for wheat and tomato cell walls and tomato roots, while it was fitted at 7.2 for wheat roots. The pKa of site 3 (L3) were fitted at 7.4 and 8.1 for tomato cell walls and roots and at 8.7 and 9.1 for wheat cell walls and roots. The pKa of site 4 (L4) consistently ranged from 9.8 to 10.1 for all samples. The estimated site density markedly differed depending on the root material (Table 1). For tomato, the site density was consistently distributed for cell walls and roots, with LH4T > LH1T > LH2T > LH3T. For wheat, no consistent pattern was observed in the site density distribution for cell walls and roots. The total site density estimated for wheat and tomato roots was respectively 2.4- and 3.4-times higher than those estimated for cell walls (Table 1).
These results are consistent with the total site density estimated on the basis of CEC, as the CEC measured in wheat and tomato cell walls and roots was linearly correlated with the total site density estimated on the basis of potentiometric titration data (R2 = 0.98, data not shown). The total site density estimated by potentiometric titration was slightly higher than that estimated on the basis of the CEC (regression slope: 1.25).
13C-NMR spectra of roots and cell walls
Overall, the spectra shape was very similar for wheat and tomato cell walls and roots (Fig. 4a and b). All spectra exhibited a quantitatively substantial carbohydrate region (between ca. 60 and 110 ppm). The NMR spectra intra-species comparison revealed that the total area of the cell wall spectra accounted for 76 and 85 % of the total area of the root spectra for tomato and wheat, respectively (Fig. 4). For both species, the area of the cell wall carbohydrate region was very similar to that of the roots, i.e. the cell wall to root signal ratio was over 90 % (Fig. 5). The carbonyl C and the N alkyl/-methoxyl C regions exhibited a cell wall to root signal ratio ranging from 39 to 44 % in wheat and from 55 to 65 % in tomato. The lowest cell wall to root signal ratio was obtained for the alkyl C region, with a cell wall to root signal of only 35 %. The aryl C and O-aryl C regions were not studied because the signal intensity was too weak to be confidently distinguished from the baseline.
Discussion
Root cation binding properties are alternatively attributed to the properties of cell walls, particularly pectins, or of root cell plasma membranes. To assess the respective contributions of these two root materials, we investigated the properties and chemical nature of cation binding sites of isolated cell walls compared to those of whole roots still containing cell walls and plasma membranes.
Efficiency of the cell wall isolation procedure
Theoretically, an efficient root cell wall isolation procedure should: (i) entirely remove the root symplasm by solubilization of cell plasma membranes, and (ii) quantitatively and qualitatively preserve the cell wall compounds. Although the quality of the isolation procedure is seldom checked, this aspect can be assessed in various ways, e.g. electronic microscopy observation (Sentenac and Grignon 1981) or measurement of ATPase and Cyt C activities (Masion and Bertsch 1997). We assessed the efficiency of the isolation procedure by probing the loss of mineral elements in isolated cell walls compared to the roots (Fig. 1).
The removal of 90-95 % of P, which is a representative membrane phospholipid constituent, in tomato and wheat cell walls compared to their respective roots suggested that the plasma membranes were efficiently solubilized by our isolation procedure using Triton X 100 (Fig. 1). The low percentage of P remaining in cell walls could be attributed to the P-containing proteins embedded in the cell walls (Kaida et al. 2010). The loss of almost 70 % of the alkyl-C NMR signal, presumably largely corresponding to the long aliphatic chains of membrane phospholipids, in tomato and wheat cell walls compared to their respective roots further suggests that plasma membranes were efficiently solubilized and removed from isolated cell walls (Figs. 4 and 5). Cathala et al. (1978) also reported that optical and microscopic observations of root cell walls (isolated with a Triton X 100 procedure very similar to the one we used) did not show any remaining plasma membrane fragments.
Following the removal of plasma membranes, the cell wall isolation procedure should allow removal of the cytoplasmic content. The complete loss of K, a soluble cation almost entirely contained in the cytoplasm (Marschner 1995), during the cell wall isolation procedure illustrated efficient removal of the cytoplasmic content of root cells (Fig. 1). Cathala et al. (1978) similarly reported that K recovered in cell walls isolated using Triton X 100 represented less than 3 % of the K initially measured in the roots of several dicots and monocots.
The partial recovery of Ca, Fe and Cu contrasted with the loss of P and K from the isolated cell walls (Fig. 1). Calcium, Fe and even more so Cu are well-known to highly interact and accumulate in root cell walls. Bravin et al. (2010) reported a percentage of apoplastic Cu ranging between 50 to 80 % for durum wheat grown under hydroponic conditions very similar to those we used to grow tomato and wheat. Strasser et al. (1999) reported a percentage of apoplastic Fe ranging from 10 to 50 % for both dicots and monocots. The recovery of Ca in isolated cell walls was harder to accurately interpret as it depends concomitantly on the initial distribution of Ca between the root symplast and apoplast and the desorption of Ca from cell walls during the isolation procedure following the decrease in Ca concentration from the hydroponic solution (i.e. 2 mM) to the isolation solution (i.e. 1 mM).
Finally, the 13C-NMR determination allows us to assess the qualitative preservation of cell wall compounds (Figs. 4 and 5). We noted that the peak observed in the anomeric C region conserved a maximum intensity of close to 105 ppm, which corresponds to inter-chain glycosidic bonds (Fig. 5). This highlighted the absence of any pecto-cellulosic chain degradation.
The above-cited results concomitantly suggested that the major components of the material isolated from roots were preserved, even though the absence of any loss of quantitatively minor cell wall components could not be definitely ascertained. In comparison with isolated cell walls, the roots did contain both cell walls and plasma membranes. Isolated cell walls and roots were consequently adequate for evaluating the respective contributions of cell walls and plasma membranes to the root cation binding properties.
Limited contribution of cell walls to the total binding capacity of roots
The binding capacity of wheat and tomato cell walls, when expressed as a function of the initial root mass, was 2.4- and 3.4-fold lower than the binding capacity of wheat and tomato roots, respectively (Figs. 2 and 3; Table 1). These results mean that root material removed during cell wall isolation, i.e. cell plasma membranes and cytosolic compounds, accounted for 60 and 70 % of the total binding capacity of wheat and tomato roots, respectively. Determination of the concentration of major organic acids extracted from frozen-thawed roots showed that their global contributions to the CEC of tomato and wheat roots were lower than 5 % (results not shown). As these organic acids were mainly found within the cytosol, this suggests that the difference in the binding capacity of roots and cell walls could be mainly attributed to plasma membranes. These results contradict those generally reported in the literature, i.e. indicating that 70-90 % of the cation binding capacity of roots is mainly provided by carboxylic groups borne by cell wall pectins (Haynes 1980; Sattelmacher 2001; Krzesłowska 2011). In contrast, our results suggest that root cell plasma membranes are the main contributors to the total binding capacity of roots. This hypothesis is supported by the reported measurements of significant surface charge densities and ion binding affinities of plasma membranes as reviewed by Kinraide (2001).
The limited contribution of the cell walls to the root binding capacity could thus be partly explained by the loss of root material bearing binding sites during the cell wall isolation procedure, as 48-50 % of the initial root mass was lost (results not shown). However, it is noteworthy that this loss of root material was still lower than the 60 and 70 % decrease in the binding capacity of cell walls compared to that of wheat and tomato roots, respectively (Table 1). This means that the binding site density in cell walls, i.e. 29.2 and 57.7 cmolc.kg−1 of dry cell walls for wheat and tomato, was lower than that of the root material lost during this isolation procedure, i.e. presumably plasma membranes, i.e. 44.5 and 136.2 cmolc.kg−1 of dry mass for wheat and tomato. The result was particularly striking for tomato roots as it suggested that the cell wall binding site density was 2-fold lower than that of plasma membranes.
Cathala et al. (1978) reported very similar CEC in roots and the corresponding cell walls of maize and sunflower. Although the percentage of root material lost during the cell wall isolation procedure was not specified, the findings of that study suggested, like our results, that cell walls do not contribute to more than 50 % of the total binding capacity of roots with a substantial concomitant contribution of plasma membranes. Based on a comparison of the electrical potentials of root cell walls and plasma membranes, Shomer et al. (2003) also suggested that the ion-binding strength of plasma membranes is higher than that of cell walls.
Distinct acidic properties of roots and cell walls related to the chemical nature of binding sites
While the total binding capacity of cell walls was shown to be substantially lower than the total binding capacity of roots, it remains unclear whether the acidic properties of cell walls and roots differed and whether these acidic properties could be related to the chemical nature of the binding sites in cell walls and roots.
As already mentioned, the 13C-NMR spectra of roots and cell walls were qualitatively identical, i.e. the same chemical C-bonds were detected (Fig. 4). Nevertheless, the quantity of chemical C-bonds differed significantly between cell walls and roots. The substantial (i.e. 35-55 %) decrease in the carbonyl C signal, visible ca. 173 ppm (Figs. 4 and 5), is particularly noteworthy. This spectral region consists of contributions from uronic acids, proteins and fatty acids of phospholipids. Uronic acid, which is a component of cell wall pectins that bears cation binding sites, is considered to be a major source of carboxyl groups in plant roots (Grignon and Sentenac 1991). As the spectral contributions of pectins were also noted in the carbohydrate C region (60–110 ppm, Fig. 4), which presented an NMR signal close to that of the roots, it was unlikely that there was any loss of carboxyl groups from uronic acid during the isolation procedure. Alternatively, the disappearance of carbonyl groups from isolated cell walls could be attributed to the removal of proteins embedded in plasma membranes. Indeed, proteins are composed of one or more chains of amino acids linked by peptide bonds and ending by both carboxyl and amine groups. These proteins can represent as much as half of the plasma membrane mass (Gupta 2004; Taiz and Zeiger 2006). Lamport and Várnai (2013) recently reported on the binding capacity towards Ca of carboxyl groups borne by periplasmic arabinogalactan glycoproteins (AGPs). These AGPs may contribute significantly to the binding capacity of plasma membranes. The spectral contribution of proteins was also noted at 56 ppm (N alkyl/methoxyl C) and between 0 and 40 ppm (alkyl C), i.e. two other NMR regions also presenting a signal loss (Fig. 4). Consequently, the lower binding capacity of cell walls was likely partly associated to the substantial loss of carboxyl and amine binding sites borne by proteins embedded in plasma membranes.
The disappearance of 65 % of the alkyl-C signal in cell walls compared to that in roots could also have been partly associated with the removal of phospholipids, i.e. the primary constituent of plasma membranes, during the cell wall isolation procedure. The chemical structure of phospholipids consists of a phosphate group covalently linked to a glycerol molecule which in turn is covalently linked to two fatty acids. A variable head group is further attached to the phosphate group. Phospholipids of the outer part of the plasma membrane can therefore exhibit two to three cation binding sites borne by the head group and phosphate group. For instance, phosphatidylserine contains a serine as head group, a molecule having one carboxyl group and one amine group (Taiz and Zeiger 2006). Cohen and Cohen (1981) and McLaughlin et al. (1981) showed that these functional groups were responsible for the adsorption of monovalent and divalent cations by membrane phospholipids. Consequently, the lower binding capacity of cell walls is likely partly associated with the substantial loss of phosphate, carboxyl and amine binding sites borne by plasma membrane phospholipids.
The potentiometric titrations coupled with PROSECE modeling generated further insight into the identification of functional groups responsible for the binding properties of roots and cell walls. Four types of binding sites were identified for each root material with specific acidic properties, i.e. a stability constant (pKa) and a site density (Table 1). The pKa ranges of carboxylic, phosphate, amine and phenolic groups are respectively 3.4 to 7.5, 5.7 to 7.2 and 8 to 11 for the two latter groups (Meychik and Yermakov 1999; Guiné et al. 2006; Ahmady-Asbchin et al. 2008). Binding sites were consequently grouped into two distinct families, i.e. the carboxyl/-phosphate-like (C-P) sites and the phenolic/-amine-like (Φ-A) sites, with pKa respectively lower and higher than ca. 7.5. The C-P/Φ-A ratio was respectively 0.5 and 1.2 in wheat roots and cell walls, indicating that Φ-A sites were the most abundant in roots and that C-P sites were the most abundant in cell walls. The C-P/Φ-A ratio was respectively 0.9 and 1.2 in tomato roots and cell walls, revealing a similar although less marked site dominance pattern than noted in wheat. The switch in the dominance between Φ-A and C-P sites from roots to cell walls could be explained by a higher loss of Φ-A sites as compared to the loss of C-P sites during the cell wall isolation procedure.
The assignment of a chemical nature to each type of binding site identified by potentiometric titration is still, however, complicated. C-P and Φ-A site denominations are only indicative of the actual reactivity of roots and cell walls as the chemical environment of a functional group may substantially alter its pKa. For example, the pKa of a low molecular acid such as acrylic acid is 4.3 but the pKa of ionogenic groups of acrylic acid in the cross-linked polymer structure may range from 5 to 7.5 (Meychik and Yermakov 1999). Likewise, polygalacturonic acid carboxylic groups may have a pKa in the range of that of Φ-A sites (Lenoble et al. 2008). Accurate knowledge on the chemical structure is thus necessary. The NMR signal loss effectively reflected the decrease in the total site density observed by both potentiometric titration and CEC measurement. However, due to the substantial chemical heterogeneity in the roots and cell walls, the NMR spectra did not generate more accurate results or highlight a straightforward link between the identified functional groups and the pKa.
Conclusion
While, based on previous reports, it is often commonly considered that root binding properties are mainly dependent on the cell wall composition, our results conversely suggest that cell walls does not contribute to more than 50 % of the total binding capacity of roots. Because of the apparent efficiency of the cell wall isolation procedure in removing plasma membranes, we noted that binding sites borne by plasma membranes contributed markedly to the total binding capacity of roots.
The distinct binding properties of isolated cell walls and roots should no longer be neglected and should be considered carefully in studies focused on root-ion interactions. Cell walls and plasma membranes should be separated especially to gain insight into processes taking place within cell walls, such as root elongation mechanisms. Conversely, cell walls and plasma membranes have to be seen as a continuum in the overall issue of rhizotoxicity because of their respective contributions in the binding properties of roots. From a more operational standpoint, the biotic ligand model will be more effective if cell walls and plasma membranes are considered as separate cation binding sites. Further in-depth studies are nevertheless required to investigate the binding of trace elements by each type of root material in order to identify the extents of participation of their respective binding sites.
References
Ahmady-Asbchin S, Andrès Y, Gérente C, Cloirec PL (2008) Biosorption of Cu (II) from aqueous solution by Fucus serratus: Surface characterization and sorption mechanisms. Bioresource Technology 99(14):6150–6155. doi:10.1016/j.biortech.2007.12.040
Allan DL, Jarrell WM (1989) Proton and Copper Adsorption to Maize and Soybean Root Cell Walls. Plant Physiol 89(3):823–832. doi:10.1104/pp. 89.3.823
Bastías E, Alcaraz-López C, Bonilla I, Martínez-Ballesta MC, Bolaños L, Carvajal M (2010) Interactions between salinity and boron toxicity in tomato plants involve apoplastic calcium. Journal of Plant Physiology 167(1):54–60. doi:10.1016/j.jplph.2009.07.014
Bravin M, Merrer B, Denaix L, Schneider A, Hinsinger P (2010) Copper uptake kinetics in hydroponically-grown durum wheat (Triticum turgidum durum L.) as compared with soil’s ability to supply copper. Plant and Soil 331(1–2):91–104. doi:10.1007/s11104-009-0235-3
Cathala N, Ghorbal MH, Lamant A, Salsac L (1978) Obtention de parois cellulosiques à l’aide d’un détergent : étude préliminaire de leur composition minérale. C R Acad Sc Paris t 286:1025–1027
Cohen JA, Cohen M (1981) Adsorption of monovalent and divalent cations by phospholipid membranes. The monomer-dimer problem Biophysical journal 36(3):623–651
Dœlsch E, Deroche B, Van de Kerchove V (2006b) Impact of sewage sludge spreading on heavy metal speciation in tropical soils (Réunion, Indian Ocean). Chemosphere 65 (2):286–293. doi:10.1016/j.chemosphere.2006.02.046
Doelsch E, Van de Kerchove V, Saint Macary H (2006a) Heavy metal content in soils of Réunion (Indian Ocean). Geoderma 134 (1–2):119–134. doi:10.1016/j.geoderma.2005.09.003
Dufey JE, Braun R (1986) Cation Exchange Capacity of Roots : Tirtation Sum of Exchangeable Cations, Copper Adsorption. Journal of Plant Nutrition 9(8):1147–1155
Garnier C, Mounier S, Benaïm JY (2004a) Influence of dissolved organic carbon content on modelling natural organic matter acid–base properties. Water Research 38 (17):3685–3692. doi:10.1016/j.watres.2004.05.019
Garnier C, Pižeta I, Mounier S, Benaı̈m JY, Branica M (2004b) Influence of the type of titration and of data treatment methods on metal complexing parameters determination of single and multi-ligand systems measured by stripping voltammetry. Analytica Chimica Acta 505 (2):263–275. doi:10.1016/j.aca.2003.10.066
Grignon C, Sentenac H (1991) pH and Ionic Conditions in the Apoplast. Annual Review of Plant Physiology and Plant Molecular Biology 42 (1):103–128. doi:10.1146/annurev.pp.42.060191.000535
Guiné V, Spadini L, Sarret G, Muris M, Delolme C, Gaudet JP, Martins JMF (2006) Zinc Sorption to Three Gram-Negative Bacteria: Combined Titration, Modeling, and EXAFS Study. Environmental Science & Technology 40(6):1806–1813. doi:10.1021/es050981l
Gupta GP (2004) Plant Cell Biology. Discovery Publishing House, New Delhi
Haynes RJ (1980) Ion exchange properties of roots and ionic interactions within the root apoplasm: Their role in ion accumulation by plants. Bot Rev 46(1):75–99. doi:10.1007/bf02860867
Herrmann AM, Ritz K, Nunan N, Clode PL, Pett-Ridge J, Kilburn MR, Murphy DV, O’Donnell AG, Stockdale EA (2007) Nano-scale secondary ion mass spectrometry — A new analytical tool in biogeochemistry and soil ecology: A review article. Soil Biology and Biochemistry 39 (8):1835–1850. doi:10.1016/j.soilbio.2007.03.011
Horst WJ, Wang Y, Eticha D (2010) The role of the root apoplast in aluminium-induced inhibition of root elongation and in aluminium resistance of plants: a review. Annals of Botany 106(1):185–197. doi:10.1093/aob/mcq053
Kaida R, Serada S, Norioka N, Norioka S, Neumetzler L, Pauly M, Sampedro J, Zarra I, Hayashi T, Kaneko TS (2010) Potential Role for Purple Acid Phosphatase in the Dephosphorylation of Wall Proteins in Tobacco Cells. Plant Physiology 153(2):603–610. doi:10.1104/pp. 110.154138
Kinraide TB (2001) Ion fluxes considered in terms of membrane-surface electrical potentials. Australian Journal of Plant Physiology 28(7):605–616. doi:10.1071/pp 01019
Kinraide TB (2004) Possible Influence of Cell Walls upon Ion Concentrations at Plasma Membrane Surfaces. Toward a Comprehensive View of Cell-Surface Electrical Effects upon Ion Uptake, Intoxication, and Amelioration. Plant Physiol 136(3):3804–3813. doi:10.1104/pp. 104.043174
Kinraide TB (2006) Plasma membrane surface potential (ψpm) as a determinant of ion bioavailability: A critical analysis of new and published toxicological studies and a simplified method for the computation of plant ψpm. Environmental Toxicology and Chemistry 25(12):3188–3198. doi:10.1897/06-103r.1
Kinraide TB, Ryan PR, Kochian LV (1992) Interactive Effects of Al3+, H+, and Other Cations on Root Elongation Considered in Terms of Cell-Surface Electrical Potential. Plant Physiol 99(4):1461–1468. doi:10.1104/pp. 99.4.1461
Kopittke P, Blamey F, Menzies N (2008) Toxicities of soluble Al, Cu, and La include ruptures to rhizodermal and root cortical cells of cowpea. Plant and Soil 303(1):217–227. doi:10.1007/s11104-007-9500-5
Kopittke P, McKenna B, Blamey F, Wehr J, Menzies N (2009a) Metal-induced cell rupture in elongating roots is associated with metal ion binding strengths. Plant and Soil 322(1):303–315. doi:10.1007/s11104-009-9917-0
Kopittke P, Menzies N (2006) Effect of Cu Toxicity on Growth of Cowpea (<i > Vigna unguiculata</i>). Plant and Soil 279(1):287–296. doi:10.1007/s11104-005-1578-z
Kopittke PM, Asher CJ, Blamey FPC, Menzies NW (2009b) Toxic effects of Cu2+ on growth, nutrition, root morphology, and distribution of Cu in roots of Sabi grass. Science of The Total Environment 407(16):4616–4621. doi:10.1016/j.scitotenv.2009.04.041
Kopittke PM, Asher CJ, Kopittke RA, Menzies NW (2007) Toxic effects of Pb2+ on growth of cowpea (Vigna unguiculata). Environmental Pollution 150(2):280–287. doi:10.1016/j.envpol.2007.01.011
Kopittke PM, Kinraide TB, Wang P, Blamey FPC, Reichman SM, Menzies NW (2011) Alleviation of Cu and Pb Rhizotoxicities in Cowpea (Vigna unguiculata) as Related to Ion Activities at Root-Cell Plasma Membrane Surface. Environmental Science & Technology 45(11):4966–4973. doi:10.1021/es1041404
Krzesłowska M (2011) The cell wall in plant cell response to trace metals: polysaccharide remodeling and its role in defense strategy. Acta Physiologiae Plantarum 33(1):35–51. doi:10.1007/s11738-010-0581-z
Kudo H, Kudo K, Ambo H, Uemura M, Kawai S (2011) Cadmium sorption to plasma membrane isolated from barley roots is impeded by copper association onto membranes. Plant Science 180(2):300–305. doi:10.1016/j.plantsci.2010.09.008
Lamport DTA, Várnai P (2013) Periplasmic arabinogalactan glycoproteins act as a calcium capacitor that regulates plant growth and development. New Phytologist 197(1):58–64. doi:10.1111/nph.12005
Legros S, Doelsch E, Feder F, Moussard G, Sansoulet J, Gaudet JP, Rigaud S, Doelsch IB, Macary HS, Bottero JY (2013) Fate and behaviour of Cu and Zn from pig slurry spreading in a tropical water–soil–plant system. Agriculture, Ecosystems & Environment 164 (0):70–79. doi:10.1016/j.agee.2012.09.008
Lenoble V, Garnier C, Masion A, Ziarelli F, Garnier JM (2008) Combination of 13C/113Cd NMR, potentiometry, and voltammetry in characterizing the interactions between Cd and two models of the main components of soil organic matter. Analytical and Bioanalytical Chemistry 390(2):749–757. doi:10.1007/s00216-007-1678-0
Liu D, Kottke I (2003) Subcellular localization of Cd in the root cells ofAllium sativum by electron energy loss spectroscopy. J Biosci 28(4):471–478. doi:10.1007/bf02705121
Marschner H (1995) Mineral Nutrition of higher plants. Academic Press, second edition
Masion A, Bertsch PM (1997) Aluminium speciation in the presence of wheat root cell walls: a wet chemical study. Plant, Cell & Environment 20(4):504–512. doi:10.1046/j.1365-3040.1997.d01-86.x
McLaughlin S, Mulrine N, Gresalfi T, Vaio G, McLaughlin A (1981) Adsorption of divalent cations to bilayer membranes containing phosphatidylserine. The Journal of General Physiology 77(4):445–473. doi:10.1085/jgp.77.4.445
Meychik NR, Yermakov IP (1999) A new approach to the investigation on the tonogenic groups of root cell walls. Plant and Soil 217(1):257–264. doi:10.1023/a:1004675309128
Meychik NR, Yermakov IP (2001) Ion exchange properties of plant root cell walls. Plant and Soil 234(2):181–193. doi:10.1023/a:1017936318435
Moore KL, Schröder M, Wu Z, Martin BGH, Hawes CR, McGrath SP, Hawkesford MJ, Feng Ma J, Zhao F-J, Grovenor CRM (2011) High-Resolution Secondary Ion Mass Spectrometry Reveals the Contrasting Subcellular Distribution of Arsenic and Silicon in Rice Roots. Plant Physiology 156(2):913–924. doi:10.1104/pp. 111.173088
Nagajyoti P, Lee K, Sreekanth T (2010) Heavy metals, occurrence and toxicity for plants: a review. Environmental Chemistry Letters 8(3):199–216. doi:10.1007/s10311-010-0297-8
Postma JWM, Keltjens WG, van Riemsdijk WH (2005) Calcium-(Organo) aluminum-proton Competition for Adsorption to Tomato Root Cell Walls: Experimental Data and Exchange Model Calculations. Environmental Science & Technology 39(14):5247–5254. doi:10.1021/es048138v
Ram LC (1980) Cation exchange capacity of plant roots in relation to nutrients uptake by shoot and grain as influenced by age. Plant and Soil 55(2):215–224. doi:10.1007/bf02181801
Reid RJ (2001) Mechanisms of micronutrient uptake in plants. Functional Plant Biology 28(7):661–668. doi:10.1071/PP01037
Sarkar P, Bosneaga E, Auer M (2009) Plant cell walls throughout evolution: towards a molecular understanding of their design principles. Journal of Experimental Botany 60(13):3615–3635. doi:10.1093/jxb/erp245
Sattelmacher B (2001) The apoplast and its significance for plant mineral nutrition. New Phytologist 149(2):167–192. doi:10.1046/j.1469-8137.2001.00034.x
Sentenac H, Grignon C (1981) A Model for Predicting Ionic Equilibrium Concentrations in Cell Walls. Plant Physiol 68:415–419
Sheldon A, Menzies N (2005) The Effect of Copper Toxicity on the Growth and Root Morphology of Rhodes Grass (<i > Chloris gayana</i > Knuth.) in Resin Buffered Solution Culture. Plant and Soil 278(1):341–349. doi:10.1007/s11104-005-8815-3
Shomer I, Novacky AJ, Pike SM, Yermiyahu U, Kinraide TB (2003) Electrical Potentials of Plant Cell Walls in Response to the Ionic Environment. Plant Physiology 133(1):411–422. doi:10.1104/pp. 103.024539
Smart KE, Smith JAC, Kilburn MR, Martin BGH, Hawes C, Grovenor CRM (2010) High-resolution elemental localization in vacuolate plant cells by nanoscale secondary ion mass spectrometry. The Plant Journal 63(5):870–879. doi:10.1111/j.1365-313X.2010.04279.x
Straczek A, Sarret G, Manceau A, Hinsinger P, Geoffroy N, Jaillard B (2008) Zinc distribution and speciation in roots of various genotypes of tobacco exposed to Zn. Environmental and Experimental Botany 63(1–3):80–90. doi:10.1016/j.envexpbot.2007.10.034
Strasser O, Köhl K, Römheld V (1999) Overestimation of apoplastic Fe in roots of soil grown plants. Plant and Soil 210(2):179–189. doi:10.1023/a:1004650506592
Taiz L, Zeiger E (2006) Plant Physiology, 4th edn. Sinauer Associates Sunderland, MA
Thakali S, Allen HE, Di Toro DM, Ponizovsky AA, Rooney CP, Zhao F-J, McGrath SP (2006) A Terrestrial Biotic Ligand Model. 1. Development and Application to Cu and Ni Toxicities to Barley Root Elongation in Soils. Environmental Science & Technology 40(22):7085–7093. doi:10.1021/es061171s
Vogel J (2008) Unique aspects of the grass cell wall. Current Opinion in Plant Biology 11(3):301–307. doi:10.1016/j.pbi.2008.03.002
Vulkan R, Yermiyahu U, Mingelgrin U, Rytwo G, Kinraide TB (2004) Sorption of Copper and Zinc to the Plasma Membrane of Wheat Root. Journal of Membrane Biology 202(2):97–104. doi:10.1007/s00232-004-0722-7
Wang P, Kinraide TB, Zhou D, Kopittke PM, Peijnenburg WJGM (2011) Plasma Membrane Surface Potential: Dual Effects upon Ion Uptake and Toxicity. Plant Physiol 155(2):808–820. doi:10.1104/pp. 110.165985
Wershaw R, Mikita M (1987) NMR of humic substances and coal. Lewis Publishers, Chelsea
Yang Z-B, Eticha D, Rao IM, Horst WJ (2010) Alteration of cell-wall porosity is involved in osmotic stress-induced enhancement of aluminium resistance in common bean (Phaseolus vulgaris L.). Journal of Experimental Botany 61(12):3245–3258. doi:10.1093/jxb/erq146
Zhang Q, Smith A, Sekimoto H, Reid R (2001) Effect of membrane surface charge on nickel uptake by purified mung bean root protoplasts. Planta 213(5):788–793. doi:10.1007/s004250100555
Zhou H, Zeng M, Zhou X, Liao B-H, Liu J, Lei M, Zhong Q-Y, Zeng H (2013) Assessment of heavy metal contamination and bioaccumulation in soybean plants from mining and smelting areas of southern Hunan Province, China. Environmental Toxicology and Chemistry 32(12):2719–2727. doi:10.1002/etc.2389
Acknowledgments
The authors are grateful to French Environment and Energy Management Agency (ADEME) and the French Centre of Agricultural Research for Development (CIRAD) for funding the PhD scholarship of Stéphanie Guigues and INSU (CNRS) for funding the study via the EC2CO-CYTRIX call. The authors thank Patrick Cazevieille and Claire Chevassus-Rosset (CIRAD) for their technical support during the plant growth phase, Hélène Miche (CEREGE) for providing access to ICP-AES and Jean-Claude Davidian (Montpellier SupAgro) for his advice on root cell wall isolation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Robert Reid.
Rights and permissions
About this article
Cite this article
Guigues, S., Bravin, M.N., Garnier, C. et al. Isolated cell walls exhibit cation binding properties distinct from those of plant roots. Plant Soil 381, 367–379 (2014). https://doi.org/10.1007/s11104-014-2138-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-014-2138-1