Abstract
This study investigated (a) net Cu uptake kinetics in durum wheat (Triticum turgidum durum L.) exposed to free Cu2+ activities in solution ranging from 0.4 to 2,420 nM and (b) the relative importance of plant uptake and soil’s ability to supply Cu2+ to the roots. Plant Cu flux showed a hyperbolic shape, enabling to estimate the Michaelis–Menten kinetic parameters (F max and K M) for durum wheat. Plant Cu flux was then compared with soil Cu flux as assessed by the Diffusive Gradient in Thin film technique on seven soil samples. This comparison suggested that the rate-limiting process of Cu bioavailability to durum wheat would be plant uptake kinetics in most contaminated soils with the exception of moderately contaminated, calcareous soils. However, theoretical considerations targeted soil’s ability to supply Cu as the rate-limiting process in most soils for Cu (hyper-) accumulator plants with requirement larger than that of common crop species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The determination of metal uptake kinetics is a necessary step toward the modelling of soil–plant transfer in nutrient-poor or contaminated environments (Nowack et al. 2006). For instance, the Barber-Cushman approach was applied to predict plant uptake of a range of metals such as cadmium (Cd), manganese (Mn) and zinc (Zn) (Mullins et al. 1986; Adhikari and Rattan 2000; Sadana and Claassen 2000; Sterckeman et al. 2004).
In the case of copper (Cu), Seuntjens et al. (2004) simulated plant Cu uptake using kinetic parameters F max and K M determined for rice (Lindon and Henriques 1992). However, the choice of kinetic parameters used for this purpose is questionable as there is little available literature on Cu uptake kinetics and these are not environmentally relevant. Most of kinetic parameters were indeed calculated from experiments carried out with free Cu2+ activities, {Cu2+}, varying between 1.6 and 500 µM (Cathala and Salsac 1975; Veltrup 1976; Bowen 1987), which far exceed {Cu2+} usually found in soils even when heavily contaminated (Sauvé et al. 1997; Vulkan et al. 2000). Furthermore, some other studies determined kinetic parameters on the basis of total Cu concentrations in nutrient solution (Nielsen 1976; Lindon and Henriques 1992), while free Cu2+ is usually considered as the major Cu species that plants are able to take up. Only Antunes and Hale (2006) recently carried out a more relevant investigation of Cu uptake in durum wheat (Triticum turgidum durum L.), i.e. accounting for Cu speciation in solution with {Cu2+} from the sub-nanomolar to the micromolar range. However, the experimental procedure used, i.e. only root Cu accumulation in darkness was considered, was rather designed to investigate the effect of diffusive limitations on root Cu uptake than the actual plant uptake kinetics.
In comparison, uptake kinetics of Cd and Zn were recently described for a range of plant species from agricultural crops to metal hyperaccumulator (Hart et al. 1998, 2002; Lombi et al. 2001). In these studies, uptake fluxes were determined on intact plants with metal activities from the nanomolar to micromolar range and by distinguishing the metabolic influx into root symplasm from the passive loading of metal in root apoplasm. Nevertheless, all these experiments were carried out on short-time scales (20 min) and therefore did not account for any longer-term regulation of uptake by internal plant nutrient status (Reid and Liu 2004). In the range of metal toxicity, some modifications of uptake kinetics are expected to occur (Briat and Lebrun 1999). In the particular case of Cu, it was also noted that Cu induced rhizoderm ruptures in cowpea (Vigna unguiculata L.) after 12 h of exposure to 1.1 µM Cu2+ (Kopittke et al. 2008). This result highlights the need to carry out uptake experiments over several days.
Consequently, relevant kinetic parameters for Cu uptake in intact plants are currently lacking and could thus restrict the ability of numerical models to predict soil–plant transfer of Cu. This study therefore aimed at determining the kinetic parameters (F max and K M) of Cu uptake in durum wheat at optimal and toxic ranges of {Cu2+}. The uptake experiment was carried out over 8 d to account for the impact of a potential Cu phytotoxicity. Finally, the relevance of kinetic parameters for soil–plant transfer was assessed by comparing the respective importance of the theoretical plant Cu flux and the soil’s ability to supply Cu2+ at the root surface on a set of seven soil samples showing a wide range of pH and total Cu content.
Material and methods
Plant growth
Durum wheat seeds (cv. Acalou) were germinated for 3 d in the dark on filter paper moistened with CaCl2 600 µM and H3BO3 2 µM. The growth chamber parameters were set at (day/night): 25/20°C, 75/70% of relative humidity and 450 µmol photons m−2 s−1 light intensity. Seedlings were then transferred in 35-dm3 tanks (20 pairs of seedlings per tanks) for four additional days. From the second week, seedlings were exposed for an additional 2-week to a complete nutrient solution (µM): Ca(NO3)2 2000, KNO3 2000, MgSO4 1000, KH2PO4 500, NaFe(III)EDTA 100, H3BO3 10, MnCl2 2, ZnSO4 1, CuCl2 1 and Na2MoO4 0.05. The nutrient solution was renewed every second day.
At the end of this pre-culture stage, 20 homogeneous pairs of plants were selected on the basis of their whole fresh biomass. Five pairs of plants were harvested to determine Cu content and plant root parameters (see below) prior to exposure to a range of Cu concentrations. The remaining 15 pairs of plants were transferred for another 8-d period of exposure in 15 complete nutrient solutions with the same composition as above (with however KH2PO4 reduced to 50 µM) and containing Cu concentrations ranging from 2.6 to 95 µM. The experiment was thus conducted without replication (except for control plants). Solution pH was set at 6.0 (±0.1) and buffered with 1 mM MES (2-morpholinoethanesulphonic acid). Free Cu2+ activities, {Cu2+}, in the nutrient solution were buffered with EDTA (100 µM), supplied as NaFe(III)EDTA (as a source of Fe), to reach 0.4 to 2,420 nM. Free Cu2+ activities were calculated with the aqueous speciation model PHREEQC (version 2.14.3.2411) and the MINTEQ database of thermodynamic constants (Appendix). Nutrient solutions were renewed daily about 6 h after the beginning of the day-period to limit nutrient depletion as well as root-induced alkalisation and exudation of Cu-chelating compounds, especially phytosiderophores which are usually secreted from 3 to 6 h after the onset of light (Reichman and Parker 2007). Solution pH was measured daily just before renewal with a combined glass-electrode (6.0234.110, Metrohm, Switzerland). Total Cu concentration was also measured in each initial solution and after 1, 4 and 8 d of plant growth in the solutions (before renewal) by flame atomic absorption spectrometry (Varian SpectraAA-600, Australia). The quality of speciation modelling was checked by measuring {Cu2+} on five initial nutrient solutions with a Cu ion-selective electrode (Cu-ISE; DX-264, Mettler Toledo, USA) and a double-junction reference electrode (Inlab-302, Mettler Toledo). ISE measurements were calibrated between p{Cu2+} 9.0–5.2 in 10−4 M Cu solutions buffered with iminodiacetic acid and potassium phthalate according to the procedure described by Sauvé et al. (1995). Before measurements, ISE was polished for 30 s with a 3-µm Al2O3 strip then successively soaked for 5 min in H2SO4 0.025 M and Na4EDTA 0.1 M.
After 8 d of exposure, the 15 pairs of plants (which were at the tillering stage) were harvested. Plant shoots and roots were separated. Roots were divided in three equivalent sub-samples in order to measure total and symplasmic Cu content as well as root length. Shoots and roots used to measure total Cu content were directly oven-dried at 105°C, while root sub-samples used for symplasmic Cu and length measurements were stored at −20°C before analyses. The five pairs of control plants were handled similarly.
Plant measurements
After thawing, root length was measured on stained roots using a scanner (Epson expression 10000 XL) with a two light sources procedure and the WinRHIZO software (version 2005 b, Regent Instruments, Canada), as previously detailed by Bravin et al. (2009). After root length measurements, root samples were oven-dried at 105°C for dry biomass determination. Relative root length (RRL) calculation was adapted from Kinraide et al. (2004) as followed:
where RL is the root length for a given Cu exposure, RL min and RL max the minimal and maximal root length measured among the range of exposure to Cu.
Copper rhizotoxicity was assessed by fitting the RRL vs. {Cu2+} relationship with the Hill’s equation:
where a is the Hill number and EC 50 the {Cu2+} in solution resulting in a 50% reduction in the maximal RRL. The Excel™ macro REGTOX (version 7.0.5; Vindimian 2001) was used to compute fitting parameters (a and EC 50), EC 25, EC 75, EC 90 and the 99% non-parametric confidence intervals for EC 25, EC 50, EC 75 and EC 90.
Another set of frozen root sub-samples was used to measure symplasmic Cu after the extraction of apoplasmic Cu with HCl on thawn roots. The extraction procedure was previously detailed and tested by Chaignon et al. (2002), based on the earlier work of Iwasaki et al. (1990). Iwasaki et al. (1990) had shown in the first place that HCl did not extract root symplasmic Cu, providing the extraction was not too long and conducted under mildly acidic conditions. Chaignon et al. (2002) later checked that freezing and thawing of roots before the extraction and HCl concentrations (10−3 and 10−2 M for short periods of time) used during the extraction were not affecting the integrity of root plasma membrane, in order to avoid any overestimation of Cu binding in root apoplasm. Although the desorption efficiency of the extraction procedure was not assessed here, a large underestimation of apoplasmic Cu was unlikely to occur as a large part of root Cu was recovered in the apoplasm (see below in the Results section). Roots were finally oven-dried at 105°C for dry biomass determination.
Copper concentration in shoots and roots (for total and symplasmic Cu determination) was determined after grinding and digestion as detailed by Bravin et al. (2009). The accuracy of Cu determination was checked by including blanks and reference materials of maize shoots (Zea mays L., V 463, Bureau InterProfessionnel d’Etudes Analytiques, France) and olive leaves (Olea europaea L., n° 62, Community Bureau of References, Commission of the European Communities) in the procedure.
Plant Cu flux (F plt, ng Cu m−2 root surface area s−1) was calculated for each Cu exposure according to Mullins and Sommers (1986) formula, accounting for an exponential root growth:
where m C and m are the mass of Cu (ng) accumulated in the whole plants after pre-culture (control) and 8-d exposure to Cu, S C and S the root surface area (m2) after pre-culture and 8-day exposure to Cu, k the growth constant (s−1) and t the duration of exposure to Cu (8 d). In a preliminary experiment, we checked that an exponential function can be applied properly to model root growth with or without Cu rhizotoxicity.
Kinetic parameters of Cu uptake by durum wheat were determined from the Michaelis–Menten equation:
where F max is the maximal flux of Cu in plant (ng Cu m−2 s−1) and K M the {Cu2+} at 1/2 F max usually standing for the affinity of Cu for transporters in the plasma membrane of root cells and/or binding sites in root apoplasm. Michaelis–Menten equation was fitted on plant Cu flux accounting for Cu in root apoplasm (uptake flux) or not (absorption flux). Parameters of Michaelis–Menten equation and its statistical significance were calculated with Statistica (version 7, Statsoft) using the Marquardt–Levenberg algorithm. The level of statistical significance was represented by ** and *** for P ≤ 0.01 and P ≤ 0.001, respectively.
Soil measurements
Seven topsoil samples collected in former vineyards of Southern France were selected to cover a large range of pHCaCl2 (4.1–7.8). Soil samples were either strongly acidic (A and B), slightly acidic (C and D) or calcareous (E, F and G) (Table 1). For each pH category, soil samples were either little (B, D and G) or moderately (A, C and F) Cu-contaminated. An additional highly Cu-contaminated, calcareous soils (E) was also included. Additional soil physical and chemical properties can be found in Michaud et al. (2007) and Bravin (2008).
Soil extract with 0.01 M CaCl2 (1:10 soil:solution ratio) is usually suggested as a reasonable surrogate for the determination of total concentration and speciation of Cu in soil solution, while in CaCl2 extracts total Cu concentration tends to be slightly under-estimated and {Cu2+} slightly over-estimated in comparison with measurements made directly in soil solution (Sauvé et al. 1997; Kalis et al. 2007). Consequently, ISE measurements were carried out on 0.01 M CaCl2 extracts (1:10 soil:solution ratio) to estimate {Cu2+} in soil solution. Single measurement of {Cu2+} (no replicate) was performed on each soil as a preliminary experiment on a larger set of soil samples showed that p{Cu2+} deviation was lower than 5% (results not shown). Diffusive gradient in thin film (DGT) technique was also deployed on each soil sample in triplicate to assess Cu flux in soil (Table 1). The procedures followed for ISE and DGT measurements were already detailed by Bravin (2008, p. 84–85). DGT measurements were computed as a flux (F DGT, ng Cu m−2 s−1):
where m is the mass of Cu (ng) accumulated in the resin, A the active surface area of DGT unit (3.14 cm2) and t the deployment time (17 h). The thickness of the diffusive layer, including the diffusive gel and the filter membrane, was equal to 0.093 cm.
Results
Chemical stability of nutrient solutions over time
The major parameters driving {Cu2+} in nutrient solutions were total Cu concentration and pH. Total Cu concentration appeared rather stable over time with maximum changes lower than 30% and 20% for exposure treatments 1–5 ([Cu]Tot < 20.3 µM) and 6–15 ([Cu]Tot > 24.7 µM), respectively (results not shown). These variations were attributed to both plant Cu uptake and decrease in solution volume due to water uptake.
Despite the addition of MES, alkalisation occurred and pH increased up to 7.3 in the less Cu-concentrated solutions (results not shown). In the most Cu-concentrated solutions, pH increase was lower likely due to the buffering ability of freshly precipitated Fe oxyhydroxides and a lower root biomass (see below). Such alkalisation induced a large decrease in {Cu2+} (one or two orders of magnitude) and outlined the difficulties to maintain stable chemical conditions in nutrient solutions exposed to plant with large root biomass as stressed by Kopittke and Menzies (2006). As nutrient solutions were renewed daily, results were however discussed on the basis of the initial composition of nutrient solutions at each level of exposure.
Plant Cu content and toxicity
Plant Cu content increased with increasing {Cu2+} in nutrient solutions, showing a hyperbolic pattern with a saturation shape for both shoots and roots (Fig. 1). Copper contents ranged from 9 to 53 mg kg−1 in shoots (Fig. 1a) and from 25 to 1,485 mg kg−1 in roots with 53–81% bound to the apoplasm (Fig. 1b). Copper content in shoots increased almost linearly with the increase in Cu content in the root symplasm (results not shown). However, Cu translocation from root symplasm to shoots, as deduced from shoot to root symplasm Cu content ratio, decreased 20-fold when {Cu2+} increased up to 50 nM, then remained steady for higher {Cu2+} (results not shown).
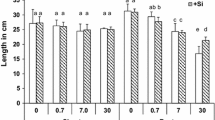
Several symptoms of Cu phytotoxicity were observed. Interveinal chlorosis consistent with Fe deficiency occurred for {Cu2+} in solution equal or larger than 550 nM. Shoot and especially root biomasses were also affected and decreased with increasing {Cu2+} in solution (Fig. 2a). Although no replication was performed at each {Cu2+}, the relative root length (RRL) was clearly affected by Cu in the low range of exposure as RRL decreased by up to 44% for Cu content in the root apoplasm lower than 500 mg kg−1 (Fig. 3a) or {Cu2+} in solution lower than 50 nM (Fig. 3b, see the insert). Above these thresholds, the RRL still decreased with increasing Cu content in the root apoplasm and, more particularly, with increasing {Cu2+} in solution (Fig. 3a and b). The RRL vs. {Cu2+} relationship could be fitted to Hill’s equation (Eq. 2) to determine rhizotoxicity thresholds (Fig. 3b). This revealed that {Cu2+} in solution of 15, 63, 268 and 1,140 nM reduced the RRL by 25, 50, 75 and 90%, respectively, compared to the maximal root length measured (Table 2). Visual symptoms of rhizotoxicity were also observed (Fig. 2b, c and d). The increasing exposure of plants to {Cu2+} in solution induced an increase in lateral root formation. Nevertheless, the growth of these lateral roots appeared strongly restricted and almost completely inhibited at the highest {Cu2+} (Figs. 2c and d).
Relative root length (RRL) as a function of Cu content of root apoplasm (a) and {Cu2+} in solutions (b). Curve and equation in (b) were obtained by fitting the data to Hill’s equation (Eq. 2)
Cu uptake kinetics in plant
Copper fluxes in plant, accounting for the apoplasm pool (uptake flux) or not (absorption flux), could be fitted with the Michaelis–Menten equation (Fig. 4). In both cases, experimental data exhibited a hyperbolic pattern with a sharp and almost linear increase in Cu fluxes for {Cu2+} lower than 100 nM, followed by a saturation shape up to 2,400 nM {Cu2+}. The kinetic parameters (F max and K M) deduced from the Michaelis–Menten equation are given in Table 3. It is noteworthy that accounting for the apoplasm pool involved a 2.3-fold increase in F max and a 1.5-fold decrease in K M, highlighting the affinity of root apoplasm for Cu. As uptake and absorption kinetics were measured over several days of exposure, the measured fluxes obviously corresponded to net Cu fluxes, i.e. integrating influx into the roots and efflux from the roots to the solution.
Activity-dependent kinetics of net Cu fluxes in intact plants. Closed and opened symbols stand for uptake and absorption fluxes, respectively, given that uptake accounts for Cu in root apoplasm while absorption does not. Experimental data were fitted to Michaelis–Menten equation (Eq. 5) for uptake (solid line) and absorption (dashed line) (see Table 3 for corresponding model parameters). The dotted line stands for the diffusion limitation flux that likely drives the plant uptake flux in EDTA-buffered solutions at low {Cu2+} (i.e. {Cu2+} < K M), according to the reaction layer concept (thickness of the reaction layer, µ = 65 µm) (see Discussion section for rationale, Eq. 7)
Comparison between theoretical plant Cu fluxes and DGT Cu flux
The kinetic parameters specifically determined for durum wheat (Table 3) enabled to calculate the theoretical plant Cu flux (F Tplt) in seven soil samples, based on {Cu2+}CaCl2 measured in CaCl2-soil extracts with ISE (Table 1) and Eq. 5. These theoretical plant fluxes can be then compared with corresponding soil fluxes (F DGT), i.e. soil’s ability to supply Cu2+ at the root surface, as assessed by DGT (Table 1). Such a comparison enables to assess whether soil re-supply kinetic or plant uptake kinetic is the rate-limiting process controlling Cu bioavailability to plants. This comparison is made in Fig. 5 where both the DGT Cu flux as a function of {Cu2+}CaCl2 and the Michaelis–Menten model for Cu uptake kinetics in durum wheat (solid line) are plotted. Soil Cu flux was lower than F Tplt only for the slightly acidic, little contaminated soil (D). The largest differences between F Tplt and F DGT was observed for the moderately (F) and highly (E) contaminated, calcareous soils where F Tplt was 10- and 26-fold lower than F DGT, respectively.
Comparison between the theoretical uptake fluxes of Cu in plants (lines) and the ability of seven soil samples to supply Cu2+ at the root surface as measured by DGT (data points). Solid line stands for the uptake flux of Cu in durum wheat determined in the present study (F max = 43 ng Cu m−2 s−1; K M = 86 nM). Dotted line stands for the unbiased uptake flux of Cu in durum wheat, taking into account a plausible “unbiased” K M (F max = 43 ng Cu m−2 s−1; K M* = 1 nM). Dashed line stands for the expected uptake flux of Cu in plants showing large Cu requirements, i.e. with a large F max (F max* = 170 ng Cu m−2 s−1; K M* = 1 nM). See Discussion section for the rationale about F max* and K M* values. Closed circles stand for non calcareous soils with pH < 5 and crosses stand for non calcareous soils with pH > 5. Opened triangles stand for calcareous soils
Due to uncertainty on the K M value (see Discussion section for rationale), a similar comparison between F Tplt and F DGT was also performed by recalculating the plant uptake kinetics with a plausible “unbiased” K M* value (K M* = 1 nM) while keeping F max unchanged (Fig. 5, dotted line). In this case, F DGT was lower than F Tplt not only for the slightly acidic, little contaminated soil (D), but also for the little and moderately contaminated, calcareous soils (F and G). Plant Cu flux was lower than F DGT only for the strongly acidic soils (A and B) and the highly contaminated calcareous soil (E), while F Tplt and F DGT were comparable for the slightly acidic, moderately contaminated soil (C).
This kind of theoretical comparison also enables to investigate plant Cu requirements that differ from that of durum wheat. A last calculation of the plant uptake kinetics was therefore performed by considering a new F max value (F max* = 170 ng Cu m−2 s−1), about 4-fold larger than F max value determined for durum wheat (see Discussion section for rationale), and K M* (Fig. 5, dashed line). In this last case, F DGT was lower than F Tplt for all soils except for the highly contaminated, calcareous soil (E) where F Tplt and F DGT were comparable.
Discussion
Activity-dependent kinetics and the associated parameters, F max and K M, are needed for the modelling of metal uptake by plants. However, in the case of Cu, the reliability and the environmental relevance of kinetic parameters reported in the literature are questionable. In the present study, such parameters were determined for durum wheat in a more relevant range of {Cu2+}, 0.4–2,420 nM. The interest of these kinetic parameters for plant uptake modelling was also further addressed by comparing the theoretical plant uptake flux to the ability of soil to supply Cu2+ at the root surface.
Copper phytotoxicity for environmentally relevant {Cu2+}
Compared with previous studies about uptake kinetics of Cu by plants (Cathala and Salsac 1975; Veltrup 1976; Bowen 1987), the range of {Cu2+} investigated in the present work was several orders of magnitude lower and thus more environmentally relevant with respect to typical {Cu2+} found in soils, including contaminated soils, usually ranging from 10−12 to 10−5 M (Sauvé et al. 1997; Vulkan et al. 2000). Even though relatively low Cu concentrations were used in the present study, strong Cu phytotoxicity symptoms were observed, altering the growth of the whole plant.
Copper contents of 9 to 20 mg kg−1 in shoots and 25 to 90 mg kg−1 in the whole roots when plants were exposed to {Cu2+} lower than 10 nM suggest that {Cu2+} in the nanomolar or sub-nanomolar range is non-toxic to durum wheat. This is support by Michaud et al. (2008) who suggested that Cu content of about 100–150 mg kg−1 is the critical level in the whole roots for the occurrence of Cu rhizotoxicity to durum wheat. Nevertheless, root length was altered by {Cu2+} as low as 15 nM which induced a 25% reduction in root elongation, while {Cu2+} of about 60 nM reduced root elongation by 50% (Table 2). These values appeared lower than phytotoxic thresholds previously reported in the literature. Kopittke et al. (2007) mentioned a 10% reduction in root or shoot biomasses of cowpea for 1,000 nM {Cu2+}, while Michaud et al. (2008) reported a 50% reduction in root elongation of durum wheat for 600 nM {Cu2+}. However, Cu2+ phytotoxicity for a given {Cu2+} is known to increase with increasing pH (Zhao et al. 2006). Kopittke et al. (2007) and Michaud et al. (2008) experiments were carried out at pH 4.5 and 5.5 respectively, which may explain the lower phytotoxicity measured in comparison with our experiment at pH 6. The visual symptoms of rhizotoxicity, i.e. an increased number of poorly developed lateral roots with increasing {Cu2+} (Fig. 2c and d), were previously mentioned by Kopittke and Menzies (2006) and Kopittke et al. (2007) for cowpea. These authors further interpreted the reduction of root elongation of cowpea by an increased rigidity of root cell wall in which Cu was accumulated. Such accumulation led to cell rupturing in the elongation zone for {Cu2+} around 1 µM, in a similar way as for the more documented case of Al rhizotoxicity (Kopittke et al. 2008). These alterations of root structure could be responsible for nutrient deficiencies such as iron deficiency, consistent with the interveinal chlorosis observed in our study and elsewhere (Kopittke and Menzies 2006; Michaud et al. 2008). They may also dramatically impact uptake kinetics. This conclusion therefore justifies carrying out kinetic experiments over longer durations than couples of minutes or hours in order to account for plant adaptative response to growth conditions.
Copper uptake kinetics in durum wheat
The activity-dependent kinetics of Cu absorption in durum wheat, which strictly speaking excludes the portion of root Cu adsorbed onto the cell walls (apoplasm), showed a hyperbolic shape with a saturable component for {Cu2+} above 500 nM (Fig. 4). Hart et al. (1998, 2002) and Lombi et al. (2001) also described a saturable component for the absorption of Cd and Zn by several plant species. This result suggests that Cu absorption in durum wheat is metabolically driven by transport protein(s) in the plasma membrane of root cells. Compared with other metals such as Zn and Fe, transporter(s) involved in Cu absorption into roots of graminaceous species is (are) poorly described. Although Cu can efficiently compete with Fe to form metal-phytosiderophore complex (Reichman and Parker 2005), Cu-phytosiderophore absorption mediated by yellow stripe1 transporter may be of limited significance in the primary absorption of Cu by graminaceous species (Roberts et al. 2004; Yruela 2009). Alternatively, Cu absorption in durum wheat could be mediated by the high-affinity Cu+ transporter COPT1 and the Cu2+ transporter ZIP2 as recently described for the non graminaceous species Arabidopsis thaliana (Puig et al. 2007; Burkhead et al. 2009; Yruela 2009). When considering the Cu uptake flux in durum wheat, i.e. including the apoplasmic Cu in roots, the shape of the kinetics also exhibited a saturable component with a higher maximal flux (F max). The difference between the uptake and the absorption kinetics was steady for {Cu2+} above 500 nM. This suggests the saturation of the Cu binding capacity of root apoplasm, reaching about 1 g Cu kg−1 dry root biomass (results not shown). This is in line with former results of Antunes and Hale (2006) who observed a saturation trend of Cu accumulation in the roots of 11 day-old durum wheat exposed to {Cu2+} in the micromolar range.
Compared with the literature on Cu uptake kinetics in plants (accounting for apoplasmic pool or not), the maximal fluxes (F max) determined on short-term experiments (from 20 min to 4 h) were 7- to 1,400-fold larger than the F max value determined for durum wheat in the present experiment (Cathala and Salsac 1975; Veltrup 1976; Bowen 1987). Veltrup (1976) reported for Hordeum distichum L. a 20-fold decrease in F max when measured over 2 h instead of 20 min and an additional 2-fold decrease in F max when measured over 24 h. This suggests that F max parameter was highly sensitive to the duration of uptake experiments, which could be related to the time required for physiological adaptation of plants to environmental constraints such as metal toxicity. Conversely, K M values found in the literature did not seem to be related to the duration of the experiments. In contrast, it varied considerably with the range of tested Cu2+ concentrations, ranging from 1.6 up to 500 µM (Cathala and Salsac 1975; Veltrup 1976; Bowen 1987). In these former studies, tested Cu2+ concentrations were all extremely high compared with concentrations reported to occur in soils, even in highly contaminated soils, and their environmental relevance is thus questionable. We decided to investigate a narrower range of concentrations, more likely to be experienced by soil-grown plants, which may explain why we found smaller K M values than those reported earlier. Our kinetic parameters could be compared more reliably to those achieved by Antunes and Hale (2006) for durum wheat exposed to {Cu2+} ranging from 0.03 to 830 nM. Although this study mainly addressed the Cu binding properties of root apoplasm rather than the determination of true uptake kinetic parameters, we can estimate F max and K M values equal to about 30–100 ng Cu m−2 s−1 and 0.2 nM, respectively. This F max value is therefore comparable to ours while K M is 430-fold lower. The large discrepancy in the K M value could be partly explained by differences in the experimental design. Antunes and Hale (2006) used younger durum wheat seedlings of only 11-day-old and exposed the plants to Cu2+ in the darkness.
The Cu kinetic parameters determined for durum wheat can also be compared with those reported for Cd uptake as plants usually take up very small amounts of Cd as for Cu. Investigating Cd uptake in durum wheat for environmentally relevant exposure, Hart et al. (1998, 2002) obtained F max values which were 6- to 8-fold larger than those found for Cu in our experiment. This discrepancy could be explained by either the occurrence of different uptake pathways (transporters) for Cd and Cu or by the shorter duration (20 min) of their experiments as already discussed above. Conversely, K M values of 40–70 nM obtained by these authors for Cd were in close agreement with our results for Cu. The new set of Cu uptake kinetic parameters that we determined in durum wheat therefore appeared more environmentally relevant than those available in the literature. Nevertheless, it should be kept in mind that the determination of such kinetic parameters is partly dependent on plant species/genotypes (Lombi et al. 2001), plant age (Chen et al. 2008) and experimental conditions such as the composition of the nutrient solution.
For the latter, Degryse et al. (2006) recently showed that plant uptake kinetics of a range of metals including Cu was driven in the low concentration range by diffusion limitations that occurred during the supply of free metals to roots, even when free metal activities are buffered with chelators such as EDTA. Although the determination of F max value remains driven by physiological processes, K M value is therefore likely overestimated by several orders of magnitude, i.e. the affinity of metals to roots is substantially underestimated, in most studies due to diffusion limitations (Degryse et al. 2009b). In chelator-buffered solutions, the diffusion limitation flux, F diff (ng Cu m−2 s−1), can be estimated using the reaction layer concept as follows:
where D is the diffusion coefficient of free Cu2+ in solution (5 10−10 m2 s−1), µ the thickness (m) of the reaction layer and M Cu the molar mass (63.5 g mol−1) of Cu. The reaction layer is the zone close to the root surface where the dissociation rate of the metal-chelator complex is not fast enough to re-supply the pool of free metal readily taken up by the root, thereby leading to the depletion of free metal activity towards the root surface. For a more detailed discussion about this concept, readers should refer to Degryse et al. (2006). Within the low {Cu2+} range, i.e. when {Cu2+} < K M, the Michaelis–Menten model (Eq. 5) can be reduced to a linear model:
Assuming that diffusion limitations occurred in this low {Cu2+} range, F plt becomes equal to F diff and, consequently, µ can be calculated by combining Eqs. 7 and 8:
The corresponding model for F diff is depicted in the Fig. 4. The order of magnitude thus calculated for µ supports the occurrence of diffusion limitations in our EDTA-buffered solutions (Degryse et al. 2009a). Recently, Degryse et al. (2009a, b) suggested “unbiased” K M value equal to about 1 nM for Zn and Cd uptake by Spinacia oleracea L.. As the range of the rate constants for water loss by metal aqua-complexes (M(H2O) 2+6 ) are fairly similar for Cd, Cu and Zn (Morel and Hering 1993), this suggests that the “unbiased” K M value characterising Cu uptake kinetics in durum wheat is also likely close to 1 nM, i.e. about 80-fold lower than the value determined in the present work (Table 3). Although the study of Antunes and Hale (2006) was not properly designed to determine uptake kinetic parameters of Cu in durum wheat, their findings even suggested a K M value in the sub-nanomolar range. In this study, the authors used NTA to buffer {Cu2+}. The lability of Cu-NTA complex is more than 50-fold larger than that of Cu-EDTA complex (Degryse et al. 2006), which could partly explain the 430-fold lower K M value than that we found in the present study.
Comparison of Cu uptake kinetics in plants and soil’s ability to supply Cu
Most of the few studies dealing with the modelling of soil–plant metal transfer concluded that soil’s ability to supply metals at the root surface was usually lower than plant requirement and consequently that soil supply was the rate-limiting process of metal phytoavailability (Mullins et al. 1986; Adhikari and Rattan 2000; Sterckeman et al. 2004). As a result, former model predictions of metal uptake in plants were poorly sensitive to uptake kinetic parameters in comparison with soil parameters. In the case of Cu, the uptake kinetic parameters determined for durum wheat suggest that plant requirement are very low, which is consistent with the low concentrations of Cu usually found in plants (compared with other micronutrients). Thus, it appeared relevant to compare the theoretical plant uptake flux of Cu (F Tplt) to soil Cu flux (F DGT), i.e. soil’s ability to supply Cu2+ at the root surface as assessed with the DGT technique. This comparison was made in Fig. 5 on a set of seven soil samples exhibiting a wide range of pH and total Cu content, by plotting both the DGT Cu flux as a function of {Cu2+}CaCl2 and different Michaelis–Menten models for Cu uptake kinetics in plants. Three sets of Cu uptake kinetics were considered in Fig. 5 with either (a) the uptake kinetic parameters determined for durum wheat (F max and K M; solid line), (b) uptake kinetic parameters of durum wheat modified to account for an “unbiased” K M (F max and K M*; dotted line) or (c) expected uptake kinetic parameters for a plant with a large Cu requirement, i.e. with a large F max (F max* and K M*; dashed line). Consequently, if F DGT is lower than F Tplt, i.e. if data points (F DGT vs. {Cu2+}CaCl2) are below the Michaelis–Menten curve (Fig. 5), soil metal supply is expected to be the rate-limiting process. Conversely, if F Tplt is equal or lower than F DGT, plant uptake flux is expected to be the rate-limiting process.
When using the uptake kinetic parameters of durum wheat determined in the present study, it is noteworthy that F DGT was lower than F Tplt only for the little Cu-contaminated, slightly acidic soil (D, Table 1). This is consistent with the large increase in F DGT noted either in strongly acidic soils (pH < 5) at a given level of soil contamination or with increasing total soil Cu (Table 1; Bravin 2008). It is also striking that the ability of soil to supply Cu2+ was 10- and 26-fold larger than F Tplt for the two Cu-contaminated, calcareous soils (E and F, Table 1), in spite of the very low {Cu2+} measured in such calcareous soils. The importance of F DGT in contaminated calcareous soils in comparison with plant uptake could be attributed to both the ability of dissolved organic matter (DOM) to mobilise Cu from the soil solid-phase to the solution at high pH and the lability of organically-bound Cu in solution. Consequently, this comparison suggests that the rate-limiting process for durum wheat would be plant uptake kinetics rather than soil Cu supply flux in most soils. Exceptions could occur for little contaminated, slightly acidic soils that exhibit a poor ability to supply Cu2+ in solution compared with plant requirement.
However, as discussed above, the K M value determined herein for Cu uptake kinetics in durum wheat is likely overestimated and thus deserves further discussion. A similar comparison between F Tplt and F DGT was performed by recalculating the Michaelis–Menten curve with a plausible “unbiased” K M value, K M*, set at 1 nM while keeping F max unchanged (Fig. 5, dotted line). It was not surprising to observe that F DGT remained larger than F Tplt for the strongly acidic soils as plant uptake kinetics is drastically limited by internal plant regulations at such high {Cu2+} in solution, corresponding to the saturation component of the Michaelis–Menten model. Conversely, K M* had a huge impact for calcareous soils as F DGT became lower than F Tplt both in weakly and moderately contaminated, calcareous soils. When considering a more suitable K M value, these results lead to conclude that for plant species with Cu requirement similar to that of durum wheat, i.e. non (hyper-) accumulator plants including the most common crop species (Chaignon et al. 2002, 2003, 2009; Faucon et al. 2007; Bravin et al. 2009), plant uptake kinetics would be the rate-limiting process in most of contaminated soils. Exceptions could occur for moderately contaminated, calcareous soils. These conclusions support those of Degryse et al. (2009a) who recently investigated the relation between soil DGT fluxes and plant uptake for metals. These authors suggested that plant uptake of Zn and Cu would be limited by soil re-supply mostly in moderately or uncontaminated soils at high pH. On the other hand, as regards to the impact of K M value on the nature of the rate-limiting process, this also justifies strengthening our efforts to determine more accurately and broadly metal uptake kinetics in plants.
To check the impact of larger Cu requirements by plants, as would occur in e.g. (hyper-) accumulator species, on the nature of the rate-limiting processes, Michaelis–Menten was recalculating using K M* and a larger F max, F max*, set at 170 ng Cu m−2 s−1 (Fig. 5, dashed line). The F max* value was chosen on the basis of the 4-fold increase in F max observed for Cd uptake when comparing durum wheat (Hart et al. 1998, 2002) with the hyperaccumulator species Thlaspi caerulescens ecotype Ganges (Lombi et al. 2001). Except for the highly contaminated, calcareous soils, F DGT was lower than F Tplt for all soils including the two strongly acidic soils. Plant species with a high Cu requirement, such as Cu (hyper-) accumulator, would therefore be expected to deplete Cu2+ in the rhizosphere, i.e. Cu phytoavailability would mainly depend on soil’s ability to supply Cu2+, except when grown in highly contaminated soils. Such calculations may explain why DGT measurements were the best predicator of shoot Cu content in the indicator, accumulator species Lepidium heterophyllum Benth which was shown to be able to accumulate very large amount of Cu in its shoots (up to about 800 to 900 mg kg−1; Zhang et al. 2001), while DGT measurement was a lesser predicator than total soil Cu of shoot Cu content in bread wheat, which is presumably a plant species with a low Cu requirement (Nolan et al. 2005) in as much as durum wheat and the most common crop species.
However, these comparisons between F Tplt and F DGT should be interpreted with caution. Firstly, although F Tplt can be larger than F DGT at the scale of the whole root system, the conclusion could be more balanced at the scale of root fragment as the physiological activity of root fragments greatly varied along the root axis (Puig et al. 2007). In comparison, F DGT is much more homogeneous over the whole DGT surface area. As for plant uptake kinetics that can greatly differ for different plant species and/or experimental conditions (see the above discussion), soil metal flux as measured by DGT can be also altered by a range of abiotic and biotic factors as already discussed by Degryse et al. (2009a). Among these, DGT measurement largely depends on the thickness of the diffusive layer as F DGT increases with decreasing thickness of the diffusive layer (Lehto et al. 2006). In our experiment, the thickness of the diffusive layer was arbitrarily set at 0.9 mm according to Zhang et al. (2001). The deployment time of DGT can also affect measurements as F DGT was found to decrease as much as 5-fold after several days (Nowack et al. 2004). The advection flux of metal due to plant water uptake (i.e. mass flow) was also neglected in the present calculations. However, Oliver and Barber (1966) and Lehto et al. (2006) estimated that mass flow would significantly contribute to Cu and Zn uptake in plants, especially when grown in soils with a poor ability to re-supply free metal ions to soil solution, i.e. with a low buffering capacity and/or a slow metal desorption kinetics. Our calculations also do not account for root distribution in soil and the consequent competition between roots for Cu uptake (Barber 1995), which may result in altered {Cu2+} in the vicinity of neighbouring roots. Finally, other rhizosphere processes could greatly alter metal chemistry at the soil–root interface, such as pH changes and exudation of metal-complexing compounds (Hinsinger and Courchesne 2008; Bravin et al. 2009). The present study therefore provides new but still incomplete knowledge of the potential rate-limiting processes driving Cu phytoavailability. Additional processes listed above should be accounted for in a more integrative way in future studies.
References
Adhikari T, Rattan RK (2000) Modelling zinc uptake by rice crop using a Barber-Cushman approach. Plant Soil 227:235–242
Antunes PMC, Hale BA (2006) The effect of metal diffusion and supply limitations on conditional stability constants determined for durum wheat roots. Plant Soil 284:229–241
Barber SA (1995) Soil nutrient bioavailability: a mechanistic approach (2nd edn). Wiley-Interscience, New York
Bowen JE (1987) Physiology of genotypic differences in zinc and copper uptake in rice and tomato. Plant Soil 99:115–125
Bravin MN (2008) Rhizosphere processes controlling the bioavailability of copper to durum wheat cultivated in former vineyard soils. PhD Thesis, Montpellier SupAgro Available via the corresponding author
Bravin MN, Marti AL, Clairotte M, Hinsinger P (2009) Rhizosphere alkalisation—a major driver of copper bioavailability over a wide range of pH in an acidic, copper-contaminated soil. Plant Soil 318:257–268
Briat JF, Lebrun M (1999) Plant responses to metal toxicity. Compte-Rendu de l’Académie des Sciences 322:43–54
Burkhead JL, Reynolds KAG, Abdel-Ghany SE, Cohu CM, Pilon M (2009) Copper homeostasis. New Phytol 182:799–816
Cathala N, Salsac L (1975) Absorption du cuivre par les racines de maïs (Zea mays L.) et le tournesol (Helianthus annuus L.). Plant Soil 42:65–83
Chaignon V, Di Malta D, Hinsinger P (2002) Fe-deficiency increases Cu acquisition by wheat cropped in a Cu-contaminated former vineyard soil. New Phytol 154:121–130
Chaignon V, Sanchez-Neira I, Herrmann P, Jaillard B, Hinsinger P (2003) Copper bioavailability and extractability as related to chemical properties of contaminated soils from a vine-growing area. Environ Pollut 123:229–238
Chaignon V, Quesnoit M, Hinsinger P (2009) Copper availability and bioavailability are controlled by rhizosphere pH in rape grown in an acidic Cu-contaminated soil. Environ Pollut 157:3363–3369
Chen W, Li L, Chang AC, Wu L, Kwon SI, Bottoms R (2008) Modeling uptake kinetics of cadmium by field-grown lettuce. Environ Pollut 152:147–152
Degryse F, Smolders E, Parker DR (2006) Metal complexes increase uptake of Zn and Cu by plants: implications for uptake and deficiency studies in chelator-buffered solutions. Plant Soil 289:171–185
Degryse F, Smolders E, Zhang H, Davison W (2009a) Predicting availability of mineral elements to plants with the DGT technique: a review of experimental data and interpretation by modelling. Environ Chem 6:198–218
Degryse F, Verheyen L, Smolders E (2009b) Reported Michaelis constants (KM) for Cd and Zn uptake by plants reflect diffusion limitations around roots, not the affinity of metal transporters. 10th ICOBTE conference, 13–18 July 2009, Chihuahua, Mexico
Faucon MP, Ngoy Shutcha M, Meerts P (2007) Revisiting copper and cobalt concentrations in supposed hyperaccumulators from SC Africa: influence of washing and metal concentrations in soil. Plant Soil 301:29–36
Hart JJ, Welch RM, Norwell WA, Sullivan LA, Kochian LV (1998) Characterization of cadmium binding, uptake, and translocation in intact seedlings of bread and durum wheat cultivars. Plant Physiol 116:1413–1420
Hart JJ, Welch RM, Norwell WA, Kochian LV (2002) Transport interactions between cadmium and zinc in roots of bread and durum wheat seedlings. Physiol Plantarum 116:73–78
Hinsinger P, Courchesne F (2008) Biogeochemistry of metals and metalloids at the soil–root interface. In: Violante A, Huang PM, Gadd GM (eds) Biophysic–chemical processes of heavy metals and metalloids in soil environments. Wiley, Hoboken, pp 267–311
Iwasaki K, Sakurai K, Takahashi E (1990) Copper binding by the root cell walls of Italian ryegrass and red clover. Soil Sci Plant Nutr 36:431–440
Kalis EJJ, Temminghoff EJM, Visser A, van Riemsdijk WH (2007) Metal uptake by Lolium perenne in contaminated soils using a four-step approach. Environ Toxicol Chem 26:335–345
Kinraide TB, Pedler JF, Parker DR (2004) Relative effectiveness of calcium and magnesium in the alleviation of rhizotoxicity in wheat induced by copper, zinc, aluminium, sodium and low pH. Plant Soil 259:201–208
Kopittke PM, Menzies NW (2006) Effect of Cu toxicity on growth of cowpea (Vigna unguiculata). Plant Soil 279:287–296
Kopittke PM, Dart PJ, Menzies NW (2007) Toxic effects of low concentrations of Cu on nodulation of cowpea (Vigna unguiculata). Environ Pollut 145:309–315
Kopittke PM, Blamey FPC, Menzies NW (2008) Toxicities of soluble Al, Cu, and La include ruptures to rhizodermal and root cortical cells of cowpea. Plant Soil 303:217–227
Lehto NJ, Davison W, Zhang H, Tych W (2006) Theoretical comparison of how soil processes affect uptake of metals by diffusive gradients in thinfilms and plants. J Environ Qual 35:1903–1913
Lindon FC, Henriques FS (1992) Effects of increasing concentrations of Cu on metal uptake kinetics and biomass yields. Soil Sci 154:44–49
Lombi E, Zhao FJ, McGrath SP, Young SD, Sacchi GA (2001) Physiological evidence for high-affinity cadmium transporter highly expressed in thlaspi caerulescens ecotype. New Phytol 149:53–60
Martell AE, Smith RM (2003) NIST Standard Reference Database 46. NIST Standard Reference Data, Gaithersburg
Michaud AM, Bravin MN, Galleguillos M, Hinsinger P (2007) Copper uptake and phytotoxicity as assessed in situ for durum wheat (Triticum turgidum durum L.) cultivated in Cu-contaminated, former vineyard soils. Plant Soil 298:99–111
Michaud AM, Chappellaz C, Hinsinger P (2008) Copper phytotoxicity affects root elongation and iron nutrition in durum wheat (Triticum turgidum durum L.). Plant Soil 310:151–165
Morel FMM, Hering JG (1993) Principles and applications of aquatic chemistry (2nd edn). Wiley, New York
Mullins GL, Sommers LE (1986) Cadmium and zinc influx characteristics by intact corn (Zea mays L.) seedlings. Plant Soil 96:153–164
Mullins GL, Sommers LE, Barber SA (1986) Modeling the plant uptake of cadmium and zinc from soils treated with sewage sludge. Soil Sci Soc Am J 50:1245–1250
Nielsen NE (1976) A transport kinetic concept for ion uptake by plants: III. Test of the concept by results from water culture and pot experiments. Plant Soil 45:659–677
Nolan AL, Zhang H, McLaughlin MJ (2005) Prediction of zinc, cadmium, lead, and copper availability to wheat in contaminated soils using chemical speciation, diffusive gradients in thin films, extraction, and isotopic dilution techniques. J Environ Qual 34:496–507
Nowack B, Koehler S, Schulin R (2004) Use of diffusive gradients in thin films (DGT) in undisturbed field soils. Environ Sci Technol 38:1133–1138
Nowack B, Mayer KU, Oswald SE, van Beinum W, Appelo CAJ, Jacques D, Seuntjens P, Gerard F, Jaillard B, Schnepf A, Roose T (2006) Verification and intercomparison of reactive transport codes to describe root-uptake. Plant Soil 285:305–321
Oliver S, Barber SA (1966) Mechanisms for the movement of Mn, Fe, B, Cu, Zn, Al and Sr from one soil to the surface of soybean roots (Glycine max L.). Soil Sci Soc Am J 30:468–470
Puig S, Andres-Colas N, Garcia-Molina A, Penarrubia L (2007) Copper and iron homeostasis in Arabidopsis: responses to metal deficiencies, interactions and biotechnological applications. Plant Cell Environ 30:271–290
Reichman SM, Parker DR (2005) Metal complexation by phytosiderophores in the rhizosphere. In: Huang PM, Gobran GR (eds) Biogeochemisty of trace elements in the rhizosphere. Elsevier, Amsterdam, pp 129–156
Reichman SM, Parker DR (2007) Probing the effects of light and temperature on diurnal rhythms of phytosiderophore release in wheat. New Phytol 174:101–108
Reid R, Liu J (2004) Measurement of trace metal influx in plants: a case study with Co. Funct Plant Biol 31:941–947
Roberts LA, Pierson AJ, Panaviene Z, Walker AL (2004) Yellow stripe1. Expanded roles for the maize iron-phytosiderophore transporter. Plant Physiol 135:112–120
Sadana US, Claassen N (2000) Manganese dynamics in the rhizosphere and Mn uptake by different crops evaluated by a mechanistic model. Plant Soil 218:233–238
Sauvé S, McBride MB, Hendershot WH (1995) Ion-selective electrode measurements of copper(II) activity in contaminated soils. Arch Environ Contam Toxicol 29:373–379
Sauvé S, McBride MB, Norvell WA, Hendershot WH (1997) Copper solubility and speciation of in situ contaminated soils: Effects of copper level, pH and organic matter. Water Air Soil Pollut 100:133–149
Seuntjens P, Nowack B, Schulin R (2004) Root-zone modeling of heavy metal uptake and leaching in the presence of organic ligands. Plant Soil 265:61–73
Sterckeman T, Perriguey J, Cael M, Schwartz C, Morel JL (2004) Applying a mechanistic model to cadmium uptake by Zea mays and Thlaspi caerulescens: Consequences for the assessment of the soil quantity and capacity factors. Plant Soil 262:289–302
Veltrup W (1976) Concentration dependent uptake of copper by barley roots. Plant Physiol 36:217–220
Vindimian E (2001) REGTOX: macro Excel™ pour dose-réponse. Available via. http://web.cena.usp.br/apostilas/Regina/PG/QuimicaAmbiental/REGTOX_EV7.0.4.xls Accessed 13 Aug 2009
Vulkan R, Zhao FJ, Barbosa-Jefferson V, Preston S, Paton GI, Tipping E, McGrath S (2000) Copper speciation and impacts on bacterial biosensors in the pore water of copper-contaminated soils. Environ Sci Technol 34:5115–5121
Yruela I (2009) Copper in plants: acquisition, transport and interactions. Funct Plant Biol 36:409–430
Zhang H, Zhao FJ, Sun B, Davison W, McGrath SP (2001) A new method to measure effective soil solution concentration predicts copper availability to plants. Environ Sci Technol 35:2602–2607
Zhao FJ, Rooney CP, Zhang H, McGrath SP (2006) Comparison of soil solution speciation and diffusive gradients in thin-films measurement as an indicator of copper bioavailability to plants. Environ Toxicol Chem 25:733–742
Acknowledgements
We thank Yves Dudal for introduction to PHREEQC, Jean-Claude Davidian, Catherine Curie and Alain Mollier for their advices about the design of the uptake kinetic experiment and Pierre Berthomieu to provide free access to F-AAS. Didier Arnal, Michael Clairotte and Lucien Roger are also acknowledged for technical assistance. We thanked the two anonymous reviewers for their very constructive comments that substantially increased the quality of the manuscript. Financial support was provided by the PNETOX programme of the French Ministry of Ecology and Sustainable Development.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Robert Reid.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 88 kb)
Rights and permissions
About this article
Cite this article
Bravin, M.N., Le Merrer, B., Denaix, L. et al. Copper uptake kinetics in hydroponically-grown durum wheat (Triticum turgidum durum L.) as compared with soil’s ability to supply copper. Plant Soil 331, 91–104 (2010). https://doi.org/10.1007/s11104-009-0235-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-009-0235-3