Abstract
Low concentrations of Al, Cu and La rapidly decrease root elongation and cause transverse ruptures to the rhizodermis and outer cortex, but it is not known if other trace metals have similar effects. Six trace metals, Ga, Gd, Hg, In, Ru, and Sc, decreased cowpea root growth and caused ruptures similar to those caused by Al, Cu and La. Calculated speciation of the metals showed that only Gd was almost exclusively present as the trivalent ion (Gd3+), but the other test solutions were dominated by Ga(OH)2 +, HgCl2 0, either In3+, In(OH)2+, In(OH)2 +, In(OH)3 0, or InCl2+, and Sc3+ or ScOH2+ (no thermodynamic constants were available for Ru). The results from this and other studies suggest that the ability of these trace metals (plus Al, Cu, and La) to cause ruptures is related to the strength to which the trace metals bind to the cell wall. Therefore, it is proposed that the toxic effects of trace metals results from (1) the strength of binding (either ionically or covalently), and (2) other toxic effects of the metals not dependent on cell wall interactions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Trace metals are natural components of the geosphere, hydrosphere, biosphere and atmosphere, and are present in varying amounts in the environmental media surrounding plants and animals including humans (Thornton 1995). However, the presence of trace metals at elevated concentrations is often problematic (Babula et al. 2008). In some cases, such as aluminium (Al), the impact is primarily on plants. Elevated concentrations of soluble Al in acid soils cause phytotoxicity, reducing crop yields (Kinraide 1991), but there is little transfer of toxic Al into the food chain. However, for other trace metals such as mercury (Hg), elevated concentrations are of concern not only due to their phytotoxicity, but also due to their transfer from the soil reservoir into the food chain via plant uptake; Hg is highly toxic to animals.
Trace metals often have similar phytotoxic effects, including oxidative stress and “bonding… with the structures of proteins and other bioactive compounds” (Babula et al. 2008). Interestingly, a number of metals decrease plant root growth in a manner similar to Al. Clarkson (1965), for example, found that effects of gallium (Ga), indium (In), and lanthanum (La) on onion (Allium cepa L.) “were similar in every respect” to those of Al. In addition, La, praseodymium (Pr), europium (Eu), gadolinium, (Gd), terbium (Tb), erbium (Er) and ytterbium (Yb) stimulate malate efflux from root apices of Al-tolerant wheat (Triticum aestivum L.) in a manner similar to that activated by Al (Kataoka et al. 2002). Research has shown that soluble Al, Cu, and La all result in the formation of markedly similar ruptures caused by the breaking and tearing of cells in the rhizodermis and outer cortex in cowpea (Vigna unguiculata (L.) Walp.) (Kopittke et al. 2008). It was proposed that the binding of Al, Cu, and La to the cell wall (and the resulting increase in cell wall rigidity and eventual cell rupturing) is an important toxic effect of these three metals. It is unknown if other trace metals cause ruptures similar to those observed for Al, Cu, and La (Kopittke et al. 2008).
Therefore, the aims of this study were to determine which other trace metals decrease growth and cause ruptures in cowpea roots, and to determine the range of concentrations and the length of exposure times required to cause rupturing. Light microscopy and scanning electron microscopy (SEM) were used to examine the morphology and timing of the ruptures. Computer modeling was used to estimate the speciation of the metals in the test solutions. These approaches were used to determine the similarities and differences among the trace metals to improve understanding of the underlying mechanisms of toxicity.
Materials and methods
Experimental procedures
Seedlings were grown as described by Kopittke et al. (2008). Cowpea (cv. Caloona) seeds were germinated in rolled paper towels suspended vertically with the base in tap water. After 3 days, seedlings were placed in 10 mm wide Perspex strips (seven seedlings per strip placed across the top of a 600 mL glass beaker containing 650 mL of 1,000 μM CaCl2 and 5 μM H3BO3 (pH 5.4). After ca. 24 h acclimatisation in these solutions, the seedlings were transferred to solutions of Ca and B (as above) plus one trace metal. Root length was calculated from digital images (Kopittke et al. 2008) which were captured at predetermined times. Samples of trace metal solutions were taken from the beakers at the beginning (i.e. immediately after mixing) and at the end of the growth period, filtered to 0.22 μm (Millipore, Millex-GS, SLGS033SS) (analyses had revealed that filtration to 0.025 μm did not capture additional particulate material), acidified to pH < 2.0 using 20 μL of concentrated HCl (98%), and refrigerated (3.5°C) before analysis. Analysis was by inductively coupled plasma-optical emission spectrometry (ICP-OES) for Ca, Ru, and Sc, inductively coupled plasma-mass spectrometry (ICP-MS) for Ga, Gd, and In, and flow injection mercury system-atomic absorption spectroscopy (FIMS-AAS) for Hg. Unless otherwise stated, all metal concentrations listed are the averages of the values measured at the beginning and end of the growth period. Selected concentrations are presented in Table 1.
Preliminary experiments
Preliminary experiments examined if a range in concentrations of Cd (II), Co (II), Ga (III), Gd (III), Hg (II), In (III), Ni (II), Pb (II), Ru (III), Sc (III), Tl (I), or Zn (II) reduced root growth and caused ruptures as had been observed with Al, Cu and La (Kopittke et al. 2008). Cowpea seedlings were grown for 48 h in solutions (without pH adjustment) with metals added at 0, 0.1, 0.5, 1, 5, 10, 25, 50, 100, and 200 μM (metal concentrations not measured). A second preliminary experiment was conducted based upon these results with 10 treatments designed to cover the concentration range of each metal which decreased root growth. After 48 h growth, roots were examined using a dissecting light microscope (see below). Ruptures were evident in roots exposed to selected concentrations of Ga, Gd, Hg, In, Ru, and Sc, with no ruptures observed at any concentration of the other metals tested.
Experiment 1
Based upon these preliminary observations, Experiment 1 investigated the concentrations of Ga, Gd, Hg, In, Ru, and Sc which reduced root elongation and ruptured roots of cowpea. A total of 37 treatments were established, consisting of a control and six concentrations of each of the six metals. Aliquots of stock solution (6.5 mM Ga(NO3)3, 6.5 mM GdCl3.6H2O, 6.5 mM HgCl2, 1 mM InCl3, 6.5 mM RuCl3, or 6.5 mM ScCl3.6H2O) were added to establish up to 200 μM Ga, 50 μM Gd, 5 μM Hg, 500 μM In, 100 μM Ru, and 250 μM Sc in the test solutions. The mean measured concentrations were: 0.67, 1.0, 1.7, 3.8, 15, and 52 μM Ga, 0.19, 1.2, 2.9, 6.0, 13, and 36 μM Gd, 0.32, 0.67, 1.4, 2.2, 3.2, and 3.9 μM Hg, 0.36, 1.5, 12, 80, 230, and 470 μM In, 0.60, 1.1, 2.3, 3.8, 11, and 25 μM Ru, and 0.82, 1.9, 3.3, 12, 90, and 240 μM Sc. The treatments were replicated twice over time, with each experimental unit containing seven seedlings (the average of these forming one replicate). Solution pH was not adjusted in any treatment.
After transplanting seedlings into the Perspex strips and after the 24 h acclimatisation period, digital images were captured for determination of root length (0 h). The strips were then transferred to beakers containing the treatment solutions, with further digital images captured after 24 and 48 h. After 48 h growth, roots were examined using a dissecting light microscope (see below).
PhreeqcI 2.13.04 (Parkhurst 2007), with the Minteq database, was used to estimate solution speciation using the mean measured solution pH and concentrations of Ca and each trace metal at the beginning (0 h) and completion (48 h) of the experimental period. Equilibrium constants (Table 2) were added to the database for Ga, Gd, In, and Sc (the database already contained constants for Hg). For solutions containing Gd and Hg, speciation was modeled using the pH and metal concentration of the highest treatment causing ruptures. Hydrolysis of Ga, In, and Sc salts resulted in a decrease in pH upon mixing (Table 1), hence speciation was modeled at the lowest concentration (highest pH) and the highest concentration (lowest pH) which caused ruptures. The predicted speciation should be taken as being indicative rather than definitive, particularly for Ga, In, and Sc. No constants were available for Ru(III), precluding the modeling of solutions containing Ru.
Experiment 2
Experiment 2 examined: (1) how quickly ruptures formed after exposure to each trace metal and (2) the location and nature of the ruptures that formed. Two concentrations were selected to examine the influence of concentration on the formation of ruptures by metals which cause ruptures over a wide range of concentrations (i.e. In, Ru, and Sc). A single concentration was selected for Ga and Gd corresponding to that which caused the highest frequency of rupturing. Two Hg concentrations were selected as Experiment 1 had indicated a marked difference in the morphology of the ruptures even within a narrow concentration range.
A total of 66 treatments (all without pH adjustment) was imposed: 11 metal concentrations (mean measured values) (control, 5.9 μM Ga, 11 μM Gd, 1.4 μM Hg, 2.4 μM Hg, 13 μM In, 150 μM In, 2.7 μM Ru, 13 μM Ru, 3.5 μM Sc, and 92 μM Sc) each with six harvest intervals (2, 4, 8, 12, 24, and 48 h). As in the preliminary experiments and in Experiment 1, seedlings in the 66 Perspex strips were grown for 24 h in solutions containing 1,000 μM CaCl2 and 5 μM H3BO3. Thereafter, a digital image was captured of the roots in each strip (0 h) before transfer to one of the 66 beakers (i.e. one strip per beaker). The strips were then removed (and photographed) at the appropriate time intervals. Four of the seven roots in each strip were prepared for SEM, with the remaining three roots prepared for examination using a dissecting light microscope (see below). Solution samples were collected for analysis from the 66 beakers immediately after mixing (0 h) and after each harvest.
Microscopy
For SEM, roots were freeze-substituted according to the method of Wharton (1991), dried using a Balzers critical point drier, and coated with ca. 15 nm of Pt using an Eiko IB-5 sputter coater (see Kopittke et al. (2008) for more details). The roots were examined using a field emission SEM (JEOL JSM 6300F) at 10 kV and 39 mm working distance.
Roots to be examined using a dissecting light microscope were placed in a 10% ethanol solution and refrigerated (4°C) for up to 1 day. Immediately prior to examination, the roots were removed and stained with ca. 0.025% crystal violet (a cationic dye) solution. Seven seedlings were placed in a solution containing 5.5 μM Ru. After 12 h growth, semi-thin sections (ca. 1 μm thick) were prepared for light microscopy by fixation with glutaraldehyde (Kopittke et al. 2007). The semi-thin sections were stained with 1% toluidine blue in 1% sodium borate and examined by light microscopy (Olympus BX61).
Results
Solution composition and speciation
In Experiment 1, the measured initial concentrations of trace metals in solution were considerably lower than the nominal concentrations. This was particularly evident with Ga and Ru, with measured concentrations on average 87% lower than the nominal concentration for Ga and 73% for Ru. Corresponding values for the other trace metal concentrations were 36% for Gd, 27% for In, 13% for Sc, and 9% for Hg (selected data are presented in Table 1). This loss from solution occurred immediately upon solution mixing, and as a gradual precipitation during the 48 h experimental period in the case of some metals. At harvest, the concentrations of Ga were an average of 51% lower than those measured upon mixing; values for the other trace metals were 11% lower for Gd, 40% lower for Hg, 32% lower for In, 4% higher for Ru, and 13% lower for Sc (selected data are presented in Table 1). These changes in solution composition make it difficult to determine the trace metal concentrations that are deleterious to plant roots.
Modeling with PhreeqcI 2.13.04 (Parkhurst 2007) predicted that Gd and Hg were present almost exclusively as Gd3+ or HgCl2 0 (Table 3). These predictions are consistent with the observation that the addition (and gradual precipitation) of these two metals had minimal influence on solution pH (Table 1). In contrast, across the pH range of importance to this study, the speciation for Ga, In, and Sc varied among treatments (Table 3). For the treatments causing rupturing, modeling predicted that the solutions were dominated by Ga(OH)2 +, Gd3+, HgCl2 0, either In3+, In(OH)2+, In(OH)2 +, In(OH)3 0, or InCl2+, and either Sc3+ or ScOH2+ (Table 3) (no data were available for Ru). For all concentrations of Ga, Gd, In, or Sc, solutions were predicted to be saturated with Ga(OH)3(s), Gd(OH)3(s), In(OH)3(s), or Sc(OH)3(s) (no solubility data were available for Ru(OH)3(s)).
The addition of Ga, In, Ru, and Sc salts decreased solution pH due to hydrolysis, the magnitude of the decrease in pH being greater at higher concentrations. Consequently, differences in growth rate between treatments of Ga, In, Ru, and Sc are possibly confounded by changes in pH (see Table 1 and Table 3). Cowpea (cv. Caloona) root growth rate at pH 4.0 was only ca. 40% of that at pH 4.6 to 6.3 (unpublished data).
Trace metal effects on root elongation and morphology
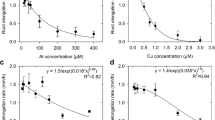
Root growth in the control treatment was good, with root length increasing an average of 1.5 mm/h in both Experiment 1 (Fig. 1) and Experiment 2 (Fig. 2). However, all six trace metals substantially reduced root elongation, confirming previous reports that Ga, Gd, Hg, In and Sc are toxic to plant roots (Clarkson 1965; Clarkson and Sanderson 1969; Kinraide 1991; Reid et al. 1996; Patra and Sharma 2000; Kataoka et al. 2002). We were not able to find a previous study demonstrating the rhizotoxicity of Ru, though ruthenium red (a polycationic dye) has been shown to reduce pollen tube growth (Zhang et al. 1999). In Experiment 1, a 50% reduction in root elongation rate was observed at an estimated 0.90 μM Ga, 1.2 μM Gd, 0.59 μM Hg, 0.72 μM In, 1.3 μM Ru, and 1.8 μM Sc (Fig. 1). In Experiment 2, the average elongation rate over the 48 h experimental period was reduced by 90% by 5.9 μM Ga, 81% by 11 μM Gd, 80% and 94% by 1.4 and 2.4 μM Hg, 77% and 96% by 13 and 150 μM In, 86% and 93% by 2.7 and 13 μM Ru, 72% and 99% by 3.5 and 92 μM Sc (Fig. 2).
Effects of Ga, Gd, Hg, In, Ru, and Sc on the elongation (closed circles) and rupturing (open squares) of cowpea roots grown for 48 h in solutions containing ca. 1,000 μM CaCl2 and 5 μM H3BO3 (Experiment 1). Solution pH was not adjusted, being ca. pH 5.4 at 0 h with Gd or Hg in solution whilst solutions containing Ga, In, Ru, or Sc had a pH ranging from 5.3 to 3.6 (Table 1). Vertical bars (where larger than the symbols) represent the standard deviations of the mean
Effects of 5.9 μM Ga, 11 μM Gd, 1.4 or 2.4 μM Hg, 13 or 150 μM In, 2.7 or 13 μM Ru, and 3.5 or 92 μM Sc on root elongation of cowpea (Experiment 2). Solution pH was not adjusted in any treatment, but decreased from pH 5.4 to 5.2–4.0 in solutions containing Ga, In, Ru, and Sc due to hydrolysis of salts of these metals (see Table 1). Each data point represents the average elongation rate between that time and 0 h. Vertical bars represent the standard deviations of the mean
Besides reducing elongation, some concentrations of each trace metal in solution caused the outer-layers of the root to rupture (Fig. 1). It is noteworthy that the formation of ruptures was dependent upon the trace metal concentration in solution, varying considerably among the metals tested. In some instances, ruptures formed at a low concentration and over only a narrow range: 1.4 to 3.2 μM Hg (corresponding to an 88% to 100% reduction in root elongation rate); 2.3 to 11 μM Ru (89% to 96% root growth reduction). Both Ga and Gd caused ruptures at low concentrations, but over a wider range, forming in solutions containing between 1.7 and 52 μM Ga (corresponding to an 82% to 100% reduction in root elongation rate), and 2.9 and 36 μM Gd (60% to 93% root growth reduction). In contrast, ruptures formed between 12 and 470 μM In (83% to 97% root elongation rate reduction), and between 1.9 and 240 μM Sc (77% to 99% root growth reduction) (Fig. 1). Interestingly, nearly all of the roots ruptured in the highest concentrations of In (470 μM) or Sc (240 μM) (Fig. 1), but no roots ruptured when exposed to the highest concentrations of Hg (3.9 μM) or Ru (25 μM), and few roots (≤25%) ruptured when exposed to the highest concentrations of Ga (52 μM) or Gd (36 μM) (Fig. 1). Whilst ruptures developed across a range of concentrations of all six metals (Fig. 1), the percentage of roots which ruptured varied within these ranges. For example, almost 100% of roots ruptured in solutions of In, Ru, and Sc (within the appropriate ranges), but <50% of roots ruptured in solutions containing Gd (Fig. 1).
The ruptures, caused by the tearing and separation of the rhizodermis and outer cortex, were similar in appearance for all six metals (Figs. 3 and 4). The ruptures were typically transverse, although several formed on a slight angle (Fig. 4). Examination of transverse sections by light microscopy revealed that the underlying cells appeared to be undamaged, as illustrated by Ru-intoxicated roots (Fig. 5). The width and depth of the individual ruptures varied among treatments. Most ruptures were <200 μm wide (Figs. 3 and 4) although some were >1,000 μm wide (e.g. Fig. 3i). Although difficult to determine quantitatively, most ruptures appeared to be <50 μm deep (Fig. 4), with those caused by Ga being shallowest and deepest for Hg, In, and Ru (Figs. 3 and 4). In addition, rupture depth tended to increase with an increase in metal concentration (e.g. Figs. 3f, g and 4h, j), with some ruptures in the high Hg, In, Ru, and Sc treatments ca. 200 μm deep (Fig. 4j).
Light micrographs of a transverse section of a cowpea root taken ca. 4 mm behind the root apex after 12 h growth in 5.5 μM Ru and stained with 1% toluidine blue in 1% sodium borate. No damage to cells of the stele or inner cortex was visible despite rupturing of rhizodermal and outer cortical cells in the transverse section of the whole root (a) or the close-up of the boxed section (b)
An increase in concentration of each trace metal resulted in a decrease in the length of the zone showing ruptures the after 48 h exposure as estimated from light micrographs of 14 roots in each treatment in Experiment 1. For example, the length of rupturing zone decreased from 10 to 3.5 mm with an increase in concentration from 12 to 470 μM In. Likewise, there was a decrease from 7.6 to 1.0 mm as Sc increased from 3.3 to 240 μM (data not presented).
Experiment 2 studied the time taken for the metals to cause ruptures. This varied among the trace metals, and were first evident after 2 h in 13 or 150 μM In, 13 μM Ru, and 3.5 μM Sc, after 4 h in 2.7 μM Ru and 92 μM Sc, after 12 h in 5.9 μM Ga and 11 μM Gd, and only after 24 h in 1.4 or 2.4 μM Hg (see examples presented in Fig. 4). The trace metals also differed in the location of the initial ruptures which formed at a distance of between ca. 1 to 4 mm from the root apex. Mercury caused rupturing closest to the apex, 1.5 and 1.2 mm for 1.4 and 2.4 μM Hg. Interestingly, Hg intoxicated roots also took the longest to develop ruptures (24 h). Exposure to the other trace metals resulted in ruptures forming 2.8 mm behind the apex for 5.9 μM Ga, 3.8 mm for 11 μM Gd, 3.4 and 3.5 mm for 13 and 150 μM In, 3.7 and 2.7 mm for 2.7 and 13 μM Ru, 3.2 and 3.0 mm for 3.5 and 92 μM Sc. There were decreases over time in the distance of the ruptures from root apex (data not presented), but interpretation of these changes was confounded by decreases in metal concentration (Table 1).
The tips of many roots exposed to trace metals developed kinks ca. 2 mm from the root apex (Fig. 3b, e, h, i), with the frequency of kinking in the order of: In > Ru > Sc > Ga ~ Gd ~ Hg. After 48 h growth, 50% of roots exposed to In developed kinks, decreasing to 40% with Ru, 30% with Sc, and 20% with Ga, Gd, or Hg (data not presented). For each metal, the proportion of roots developing kinks appeared to be directly related to the proportion of roots rupturing (data not presented). Possibly as an extreme manifestation of kinking, many of the roots grown in solutions containing 3.8 and 11 μM Ru formed loops (Fig. 3f). Furthermore, several of the roots exposed to Ga and Ru appeared to expand or swell ca. 1 to 3 mm behind the root apex; new growth emanating from the thickened sections was also evident as being appreciably thinner (Fig. 3a, f, g).
Discussion
Toxicities of Ga, Gd, Hg, In, Ru, and Sc included rupturing of the outer layers of cowpea roots (Figs. 3 and 4) whilst the underlying cells remained intact (Fig. 5). These ruptures were markedly similar to those previously reported in cowpea roots exposed to Al, Cu, and La by Kopittke et al. (2008). As with Al, Cu, and La, the ruptures caused by Ga, Gd, Hg, In, Ru, and Sc developed either within the transition zone or the elongation zone, the closest rupture to the root apex being between 1.2 mm with 2.4 μM Hg (24 h) and 3.8 mm with 11 μM Gd (12 h). Rupturing of the outer layers of the root in this study and in that of Kopittke et al. (2008) suggests a common, non-specific mechanism of toxicity for Al, Cu, Ga, Gd, Hg, In, La, Ru, and Sc by binding to the walls of cells in the rhizodermis and outer cortex. The resulting increase in cell wall rigidity in the zone of elongation reduces root growth, with ruptures forming due to the presence of rigid (slowly expanding) outer cells that overly cells of the stele and inner cortex that are expanding at a faster rate. This hypothesis is in agreement with that of Reid et al. (1996), who proposed an extracellular mechanism for the toxicity of Sc. Trace metals expected to bind strongly to the cell wall, either through ionic bonding (i.e. non-specific adsorption) (viz. Al, Ga, Gd, In, La, Ru, and Sc) or through covalent bonding (i.e. specific adsorption) (viz. Cu and Hg), caused ruptures. This did not occur with metals not expected to bind as strongly (viz. Cd, Co, Ni, Pb, Tl, and Zn). For ionic bonding to the cell wall (especially pectin which is the component of the cell wall largely responsible for adsorption of cations), selectivity increases with valency and decreases with hydrated ionic radius of the metal (Crist et al. 1994; Messiaen et al. 1997), often approximated by the ‘ionic index’, Z 2/r, where ‘Z’ is the valency and ‘r’ is the radius of the ion. Thus, metals with a high valency (and a comparatively small hydrated ionic radius) are more likely to bind ionically to the cell wall and cause rupturing than are low-valency metals. Trace metals that caused ruptures in the current study (i.e. Ga, Gd, Hg, In, Ru, and Sc) plus Al and La (Kopittke et al. 2008) have an ionic index >8 (Nieboer and Richardson 1980). We propose that the binding of metals increases cell wall rigidity through covalent bonding (specific adsorption) also; lower pKa values favouring specific adsorption for cations (McKenzie 1980; Feng et al. 2007). Of the nine monovalent or divalent cations examined, Hg and Cu had the lowest pKa values, and hence would be expected to bind the strongest to pectin in the cell wall (pKa values: Hg = 3.4, Cu = 7.6, Pb = 7.7, Zn = 9.0, Co = 9.6, Ni = 9.9, Cd = 10.1, Ca = 12.8, Tl = 13.2; Baes and Mesmer 1986; Wulfsberg 2000). Indeed, Cu has been reported to bind more strongly to pectin than other divalent metals such as Zn, Cd, and Ni (Dronnet et al. 1996), presumably due to its ability to specifically adsorb to the carboxylic exchange sites (van Cutsem and Gillet 1983).
The hypothesis that the ability of these trace metals to cause ruptures is related to the strength to which they bind to the cell wall is in general agreement with Kinraide and Yermiyahu (2007) and Kinraide (2009) who proposed that the toxic effects of metal ions may be related to their binding strength. Indeed, examination of the LogKPM values (plasma membrane binding constants) from Kinraide (2009) using an analysis of variance (GenStat 2003) indicated that rupturing was significantly correlated to the strength of binding (P < 0.001); the nine metals observed to cause ruptures had higher binding strengths (ranging from 2.84 for Cu to 5.47 for Ru) than the six metals which didn’t cause ruptures (ranging from 0.38 for Tl to 2.65 for Pb) (the value of 5.47 for Ru was obtained from Dr T.B. Kinraide).
In the present study and in that of Kopittke et al. (2008), all trace metals that form trivalent cations, and are likely to be bound non-specifically, decreased root growth and caused ruptures. However, modeling indicated that, in the current study, only the bulk solutions of Gd were dominated by the free metal ion (Gd3+) (Table 3) (no speciation data were available for Ru). This was also evident for Al3+ or La3+ in modeling the data of Kopittke et al. (2008) (data not presented). Other solutions with potentially trivalent trace metal cations were dominated by Ga(OH)2 +, either In3+, In(OH)2+, In(OH)2 +, In(OH)3 0, or InCl2+, and Sc3+ or ScOH2+ (Table 3) (solutions of Hg and Cu were dominated by HgCl2 0 or Cu2+). Whilst the free ion was not the dominant species for most metals, this does not preclude the possibility that the free ion is the most toxic species (see Kinraide (1997) for a discussion of the comparative toxicities of the various hydroxy-Al species), but further work is required in this regard.
The metal concentration in solution influenced the formation of ruptures. At low metal concentrations, elongation was reduced but no rupturing was observed (Fig. 1). In these treatments, it is possible that growth was reduced by an increase in cell wall rigidity but not to such an extent that ruptures were caused. For those treatments causing ruptures, an increase in metal concentration tended to increase the depth of the individual ruptures (e.g. see Figs. 3f, h and 4h, j). It is proposed that at these higher concentrations, the amount of metal which accumulated within the root (at any given depth) increased, thereby increasing the depth of rigidification and hence the depth of rupturing. For these treatments, it was also noted that, due to the lower rate of root elongation, the length of elongating root in which metals accumulated (and caused a rigidification) decreased, thereby reducing the length of the zone which displayed ruptures.
Some ruptures were up to 1 mm wide (Fig. 3i). This observation suggests that the underlying cells continued to elongate (at least for a while) even when exposed directly to the metal-containing bulk solution. Three possibilities exist for the apparent continued growth of the underlying cells: (1) the walls of underlying cells may be less susceptible to rigidification and rupturing, perhaps due to a higher degree of methylation of the pectin (which would decrease metal binding to the cell wall), (2) only small quantities of metal accumulate within the walls of cells in the inner layers prior to rupturing of the outer layers (for Al, see Stass et al. (2006)), or (3) the elasticity of the cell wall results in the release of accumulated strain once rupturing commences (causing an increase in rupture width). Further studies are required to test these possibilities. It was also noted that several of the roots (particularly those exposed to Ga and Ru) expanded ca. 1 to 3 mm behind the root apex (Fig. 3a, f, g) but the cause of this symptom is unclear.
Interestingly, many of the roots exposed to toxic concentrations of metals developed kinks (Fig. 3) similar to those caused by Al and La (Kopittke et al. 2008). The frequency of kinking tended to follow that of rupturing, with the rate of kinking typically higher in treatments where the rate of rupturing was also high (data not presented). Indeed, it was noted that root kinks were often in close proximity to ruptures (e.g. see Fig. 3c, i). Several of the roots exposed to 2.7 μM Ru developed loops (Fig. 3f). These were typically associated with ruptured portions of the root. Whilst both kinks and loops appeared to be related to the development of ruptures, it is unclear as to whether these symptoms form due to a physical process (e.g. through a rupture forming on one side of the root that causes a change in root direction) or a chemical process (e.g. a disruption of auxin transport within the root that disrupts gravitropism). Other toxic effects not associated with cell wall rigidity also need attention.
Conclusions
Soluble Ga, Gd, Hg, In, Ru, and Sc reduced the elongation of cowpea roots within 2 h of exposure. Roots in solutions containing various concentrations of these six trace metals developed transverse ruptures within the transition or elongation zone caused by the tearing and separation of the rhizodermis and outer cortex. The decrease in root growth and the nature of the ruptures suggest that these trace metals bind strongly, either non-specifically or specifically, to the walls of cells in the rhizodermis and outer cortex, increasing cell wall rigidity thereby reducing the ability of the outer cells to elongate. The ruptures were markedly similar to those caused by Al, Cu, and La, suggesting a common, non-specific mechanism of toxicity for these nine trace metals. This does not preclude other toxic effects of trace metals, specifically effects involved in the uptake and translocation of essential nutrients.
Abbreviations
- FIMS-AAS:
-
Flow injection mercury system-atomic absorption spectroscopy
- ICP-OES/MS:
-
Inductively coupled plasma-optical emission spectrometry/mass spectrometry
- SEM:
-
Scanning electron microscopy
References
Babula P, Adam V, Opatrilova R, Zehnalek J, Havel L, Kizek R (2008) Uncommon heavy metals, metalloids and their plant toxicity: a review. Environ Chem Lett 6:189–213. doi:10.1007/s10311-008-0159-9
Baes CF, Mesmer RE (1986) The Hydrolysis of Cations. R.E. Krieger, Malabar, Fla, p 489
Clarkson DT (1965) Effect of aluminium and some other trivalent metal cations on cell division in root apices of Allium cepa. Ann Bot (Lond) 29:309–315
Clarkson DT, Sanderson J (1969) The uptake of a polyvalent cation and its distribution in the root apices of Allium cepa: Tracer and autoradiographic studies. Planta 89:136–154. doi:10.1007/BF00386981
Crist DR, Grist RH, Martin JR, Watson JR (1994) Ion exchange systems in proton–metal reactions with algal cell walls. FEMS Microbiol Rev 14:309–313. doi:10.1111/j.1574-6976.1994.tb00104.x
Diakonov II, Ragnarsdottir KV, Tagirov BR (1998) Standard thermodynamic properties and heat capacity equations of rare earth hydroxides: II. Ce(III)-, Pr-, Sm-, Eu(III)-, Gd-, Tb-, Dy-, Ho-, Er-, Tm-, Yb-, and Y-hydroxides. Comparison of thermochemical and solubility data. Chem Geol 151:327–347. doi:10.1016/S0009-2541(98)00088-6
Dronnet VM, Renard C, Axelos MAV, Thibault JF (1996) Characterisation and selectivity of divalent metal ions binding by citrus and sugar beet pectins. Carbohydr Polym 30:253–263. doi:10.1016/S0144-8617(96)00107-5
Feng XH, Zhai LM, Tan WF, Liu F, He JZ (2007) Adsorption and redox reactions of heavy metals on synthesized Mn oxide minerals. Environ Pollut 147:366–373. doi:10.1016/j.envpol.2006.05.028
GenStat (2003) GenStat for Windows. Release 7.2, 7th edn. VSN International Ltd, Oxford
Hummel W (2004) The influence of cyanide complexation on the speciation and solubility of radionuclides in a geological repository. Environ Geol 45:633–646. doi:10.1007/s00254-003-0928-5
Kataoka T, Stekelenburg A, Nakanishi TM, Delhaize E, Ryan PR (2002) Several lanthanides activate malate efflux from roots of aluminium-tolerant wheat. Plant Cell Environ 25:453–460. doi:10.1046/j.0016-8025.2001.00821.x
Kinraide TB (1991) Identity of the rhizotoxic aluminum species. Plant Soil 134:167–178
Kinraide TB (1997) Reconsidering the rhizotoxicity of hydroxyl, sulphate, and fluoride complexes of aluminium. J Exp Bot 48:1115–1124. doi:10.1093/jxb/48.5.1115
Kinraide TB (2009) Improved scales for metal ion softness and toxicity. Environ Toxicol Chem.. doi:10.1897/08-208.1
Kinraide TB, Yermiyahu U (2007) A scale of metal ion binding strengths correlating with ionic charge, Pauling electronegativity, toxicity, and other physiological effects. J Inorg Biochem 101:1201–1213. doi:10.1016/j.jinorgbio.2007.06.003
Kopittke PM, Asher CJ, Kopittke RA, Menzies NW (2007) Toxic effects of Pb2+ on growth of cowpea (Vigna unguiculata). Environ Pollut 150:280–287. doi:10.1016/j.envpol.2007.01.011
Kopittke PM, Blamey FPC, Menzies NW (2008) Toxicities of soluble Al, Cu, and La include ruptures to rhizodermal and root cortical cells of cowpea. Plant Soil 303:217–227. doi:10.1007/s11104-007-9500-5
McKenzie RM (1980) The adsorption of lead and other heavy metals on oxides of manganese and iron. Aust J Soil Res 18:61–73. doi:10.1071/SR9800061
Messiaen J, Cambier P, Van Cutsem P (1997) Polyamines and pectins. I. Ion exchange and selectivity. Plant Physiol 113:387–395
Nieboer E, Richardson DHS (1980) The replacement of the nondescript term ‘heavy metals’ by a biologically and chemically significant classification of metal ions. Environ Pollut Series B. Chem Phys 1:3–26
Parkhurst D 2007 PhreeqcI v2.13.04. United States Geological Survey. http://water.usgs.gov/owq/software.html (Accessed March 2007)
Patra M, Sharma A (2000) Mercury toxicity in plants. Bot Rev 66:379–422. doi:10.1007/BF02868923
Reid RJ, Rengel Z, Smith FA (1996) Membrane fluxes and comparative toxicities of aluminium, scandium and gallium. J Exp Bot 47:1881–1888. doi:10.1093/jxb/47.12.1881
Stass A, Wang Y, Eticha D, Horst W (2006) Aluminium rhizotoxicity in maize grown in solutions with Al3+ or Al(OH)4 − as predominant solution Al species. J Exp Bot 57:4033–4042. doi:10.1093/jxb/erl174
Thomas GS, Kamath PV (2006) The layered double hydroxide (LDH) of Zn with Ga: Synthesis and reversible thermal behaviour. Solid State Sci 8:1181–1186. doi:10.1016/j.solidstatesciences.2006.03.006
Thornton I (1995) Metals in the Global Environment: Facts and Misconceptions. International Council on Metals and their Environment, Ottawa, p 103
van Cutsem P, Gillet C (1983) Proton–metal cation exchange in the cell wall of Nitella flexilis. Plant Physiol 73:865–867. doi:10.1104/pp.73.3.865
Wharton DA (1991) Freeze-substitution techniques for preparing nematodes for scanning electron microscopy. J Microscopy-Oxford 164:187–196
Wood SA, Samson IM (2006) The aqueous geochemistry of gallium, germanium, indium and scandium. Ore Geol Rev 28:57–102. doi:10.1016/j.oregeorev.2003.06.002
Wulfsberg G (2000) Inorganic chemistry. University Science Books, Sausalito, California, p 978
Zhang WH, Rengel Z, Kuo J, Yan G (1999) Aluminium effects on pollen germination and tube growth of Chamelaucium uncinatum. A comparison with other Ca2+ antagonists. Ann Bot (Lond) 84:559–564. doi:10.1006/anbo.1999.0952
Acknowledgments
The authors thank Jason Phipps for his assistance with the preliminary experiments, Associate Professor Stephen Adkins for the use of the dissecting microscope, and Dr Kim Sewell and Rick Webb from the Centre for Microscopy and Microanalysis at The University of Queensland for their assistance with the electron microscopy. This research was funded through the Cooperative Research Centre for Contamination Assessment and Remediation of the Environment (CRC-CARE) Project 3-3-01-05/6 and through the Australian Research Council's (ARC) Discovery funding scheme (DP0665467).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Shao Jian Zheng.
Rights and permissions
About this article
Cite this article
Kopittke, P.M., McKenna, B.A., Blamey, F.P.C. et al. Metal-induced cell rupture in elongating roots is associated with metal ion binding strengths. Plant Soil 322, 303–315 (2009). https://doi.org/10.1007/s11104-009-9917-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-009-9917-0