Abstract
Objective
Evaluate the impact of reduced gastric acid secretion after administration of two acid-reducing agents on the physicochemical characteristics of contents of upper gastrointestinal lumen of fasted adults.
Materials and Methods
Eight healthy male adults, fasted from food for 12 h, participated in a three-phase crossover study. Phase 1: No drug treatment prior to aspirations. Phase 2: Oral administration of 40 mg pantoprazole at ~9 am the last 3 days prior to aspirations and at ~7 am on aspiration day. Phase 3: Oral administration of 20 mg famotidine at ~7 pm prior to aspirations and at ~7 am on aspiration day. Samples from the contents of upper gastrointestinal lumen were aspirated for 50 min, after administration of 240 ml table water at ~9 am.
Results
Reduction of gastric acid secretion was accompanied by reduced buffer capacity, chloride ion concentration, osmolality and surface tension in stomach and by increased pH (up to ~0.7 units) in upper small intestine during the first 50 min post-water administration. The mechanism of reduction of acid secretion seems to be important for the buffer capacity in stomach and for the surface tension in upper gastrointestinal lumen.
Conclusions
Apart from gastric pH, reduced acid secretion affects physicochemical characteristics of contents of upper gastrointestinal lumen which may be important for the performance of certain drugs/products in the fasted state.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reduced acid secretion in stomach has been shown to significantly impair absorption of poorly soluble, weakly alkaline drugs (1). As of the end of 2006, 38% of active pharmaceutical ingredients (APIs) approved in the U.S.A. after 1981 for oral administration were basic molecules (2). It is estimated that 50% of recently approved molecular targeted oral cancer therapies are weak bases and display solubility-limited dissolution properties that are known to impact drug absorption (3). For this reason, the effects of acid reducing agents on cancer therapeutics will be challenging for drug-development scientists, oncologists and regulatory agencies in ensuring that patients achieve safe and efficacious exposures of their cancer therapeutics and thus optimal patient outcomes (3). Indicative of the severity of the issue are recent proposals for gastric re-acidification of gastric contents in order the efficacy of certain APIs to be maintained (4).
Various strategies are being considered for mitigating the effects of increased gastric pH and for enabling the development of effective and safe pharmaceutical products of poorly soluble weak bases (5). However, to date there is no systematic study of potential changes in the characteristics of contents of upper gastrointestinal (GI) lumen that may accompany the increased gastric pH and affect the intraluminal performance of poorly soluble weak bases in the fasted state.
The primary objective of this study was to evaluate the impact of hypochlorhydria (i.e. partially impaired acid secretion) and achlorhydria (i.e. no acid secretion) in stomach on the physicochemical characteristics of contents of stomach and upper small intestine of fasted adults under conditions typically employed for conducting such drug – drug interaction (DDI) studies with stomach pH altering medications during clinical development. A secondary objective was to evaluate whether the medication class employed results in differential effect on these characteristics. More specifically, hypochlorhydria and achlorhydria were achieved by treating healthy adults with a proton pump inhibitor (PPI), pantoprazole, and a histamine-2 receptor antagonist (H2-RA), famotidine, respectively. Both agents are routinely used for typical DDI studies this study was intended to mimic. Compared with other members in their class, these acid reducing agents are much less likely to induce or inhibit metabolic processes (6). Famotidine has also been shown not to affect pancreatic exocrine or gastrointestinal motility functions (7).
Pantoprazole was selected as the PPI for this study, as it has high and not highly variable bioavailability (bioavailability is approximately 77%) which does not change on multiple dosing, so that maximum blood levels are achieved after the first dose (8,9). It has been documented that the potency of pantoprazole is similar to that of omeprazole, another commonly used PPI, on mg for mg basis (9). From 6 to 8 h after a single oral dose of 30 mg omeprazole, there is a 66% reduction in basal acid output and 71.2% reduction in pentagastrin stimulated acid output (10). In the present investigation, 40 mg pantoprazole per day for 3 days prior to and in the morning of each aspiration day were administered to each volunteer. A similar scheme has been previously used by others with esomeprazole (the S-enantiomer of omeprazole) to simulate the effects of acid reducing agents in stomach (11,12).
With famotidine, dose related suppression of basal and stimulated gastric acid output has been shown with oral doses of 5–40 mg; within this dose range the oral absorption is dose independent (7,13). In the present investigation, each volunteer was administered 20 mg famotidine on the day before and in the morning of each aspiration day.
Experimental
Materials
Pantoprazole (PNT) film-coated tablets (Controloc®, 40 mg, Takeda Hellas, Greece) and famotidine (FMT) film-coated tablets (Peptan® 20 mg, MSD/Vianex, Athens, Greece) were purchased locally. All chemicals were of analytical grade and purchased from Sigma Aldrich Chemie GmbH (Germany), except for egg phosphatidylcholine (for constructing standard curves), which was donated by Lipoid GmbH (Germany). All solvents were of HPLC grade.
Human Study
The study was held in the Red Cross Hospital of Athens after receiving approval by the Scientific and the Executive Committee of the Hospital (AP 10117–3 April 2014). The study followed the tenets of the Declarations of Helsinki for biomedical research involving human subjects.
Inclusion Criteria
Willingness to participate as indicated by subject’s signed informed consent; age 18–50 years; body weight within 20% of ideal body weight as determined by Metropolitan Life Tables; verification of suitability by a general physical examination; ability to abstain from cigarette smoking, alcohol, and over-the-counter and prescription medication(s) for 3 days prior to Aspiration Day until the end of the Aspiration Day. A blood sample was taken to assess electrolyte balance, kidney and liver function, blood morphologic characteristics and lipid levels and the subject had to be deemed healthy in all these examinations to qualify.
Exclusion Criteria
Existence of a major health problem (cardiovascular, pancreatic, hepatic, thyroid etc.); existence of any condition requiring prescription drug therapy; recent history of gastrointestinal symptom regardless of the severity (e.g. heartburn, constipation etc.); receipt of an investigational agent (new or generic) within 30 days prior to the initiation of the study; presence of antibodies indicating active acute or chronic HIV, HBV, or HCV infection; use of medication which may affect GI function (including antacids, PPIs, H2-RAs, and laxatives) within 30 days of the study.
Subjects
Eight healthy male adults with a mean age of 27 years (range 23–34 years) gave informed consent and participated in the study. Body weights of all subjects deviated from the ideal weights by less than 12%. Health status of each subject was confirmed by physical examination and screening of blood parameters for renal and hepatic functions, as described above.
Study Protocol
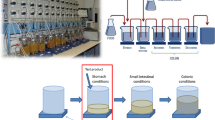
The study consisted of three phases and was performed on a crossover basis (Fig. 1).
-
Phase 1:
Subject was fasted from food (water ad libitum) for at least 12 h prior to the initiation of aspirations.
-
Phase 2:
Subject was administered 40 mg of pantoprazole (Controloc®, 40 mg/tab) per os at about 9 am for 3 days prior to the Aspiration Day. On the Aspiration Day at about 7 am subject was administered 40 mg of pantoprazole. Subject was fasted from food (water ad libitum) for at least 12 h prior to the initiation of aspirations.
-
Phase 3:
Subject was administered 20 mg of famotidine (Peptan®, 20 mg/tab) per os at 7 pm on the day before the Aspiration Day. On the Aspiration Day at 7 am subject was administered 20 mg of famotidine. Subject was fasted from food (water ad libitum) for at least 12 h prior to the initiation of aspirations.
At about 8 am on each Aspiration Day, the subject arrived at the clinic and received about 150–200 mL of table water. At about 8:30 am the upper throat was sprayed with lidocaine and the subject was intubated nasally using a sterile two-lumen tube (Freka® Trelumina, CH/FR 16/9, 150 cm, Fresenius Kabi Deutschland GmBH, Bad Hombourg, Germany). The double bore tube was 150 cm long with an external diameter of 5.3 mm in stomach, an external diameter of 2.9 mm at the pylorus and in the small intestine, and a plastic tip at its distal end. A series of holes 55–65 cm proximal to the tip were used to access the antrum of the stomach. A further series of hand-made holes 13.5–23.5 cm proximal to the tip were used to aspirate samples from the ligament of Treitz (14). Insertion of the tube was assisted by a guiding wire, and its position was monitored fluoroscopically. After reaching its final position and removing the wire, the subject laid semisupine, secretions accumulated in stomach during the intubation process were removed and a sample was aspirated to measure baseline gastric pH. After collecting a sample from the upper small intestine and baseline pH in upper small intestine was recorded, 240 mL of table water were administered via the tube through the gastric series of holes. The administration lasted for about a minute. Samples (up to 8 mL) were aspirated from the stomach at 10, 20, 35 min and from the end of duodenum at 5, 15, 30, 50 min, after the administration of water. At the end of each aspiration and before removing the tube / discharging the subject, the final position of the tube was confirmed fluoroscopically.
Handling of Aspirated Samples
pH and buffer capacity were measured immediately upon sample aspiration.
The remaining of each aspirated sample was immediately divided into eight subsamples for measuring chloride ion concentration, osmolality, total protein content, six individual bile acids, lipids, buffer capacity, surface tension and kinematic viscosity of aspirated samples, after one freeze-thaw cycle. All measurements, apart from surface tension and kinematic viscosity, were performed in individual subsamples. Each subsample was kept at −70°C until a relevant measurement was performed. Surface tension and kinematic viscosity were performed in pooled aspirates. Pools were created by using all subsamples collected during a specific phase from the antrum and from the end of duodenum, i.e. 2 pools per phase were created. Each pool was created just prior to measuring the surface tension and viscosity.
Analysis of Samples
pH values were measured with a pH electrode (Schott, model CG842, Mainz, Germany). Buffer capacities that were measured upon sample collection were measured in just one pH direction by dropwise addition of either NaOH (for samples aspirated from the stomach) or HCl (for samples aspirated from duodenum). Buffer capacities that were measured after storage at −70°C and thawing were measured in both pH directions by dropwise addition of NaOH and HCl solutions. Concentration of chloride ions was measured using a Chloride Ion-Selective Electrode with built in reference electrode (LIS-146CLCM Micro Chloride Ion Electrode, Lazaar Research Laboratories, Inc., Los Angeles, USA). Osmolality was measured by using the freezing point depression technique (semimicro osmometer Typ Dig L; Knauer, Berlin, Germany). Total protein content was determined using a commercially available kit (BCA®, Protein Assay Reagent Kit, Thermo SCIENTIFIC, Rockford, IL, USA) and bovine serum albumin as standard whereas samples were pretreated with Compact-AbleTM Protein Assay Preparation Reagent set (Pierce, Rockford, IL, USA). Taurocholic acid (TC), glycocholic acid (GC), taurochenodeoxycholic acid (TCDC), ursodeoxycholic acid (UDC), glycochenodeoxycholic acid (GCDC) and glycodeoxyholic acid (GDC) were quantified by HPLC using a Charged Aerosol Detector (15). Monoglycerides (MGs), free fatty acids (linoleic, palmitic and stearic acids), phosphatidylocholine (PC), lyso-phosphatidylocholine (Lyso-PC) and cholesterol were assayed by using a previously developed gradient HPLC method using a Charged Aerosol Detector (16). Data are presented on molar basis. Surface tension was measured according to the Du Nouy Ring method (KSV Sigma 70, KSV Instruments, U.S.A) at 37°C; mean(SD) values of 10–12 measurements that the instrument performs for each sample were reported. Kinematic viscosity was measured using a direct-flow gravimetric capillary (Cannon-Fenske type) at 37°C, using as reference the kinematic viscosity of water at 37°C (0.6959 mm2/s, www.viscopedia.com), in order to calibrate the capillary and calculate the capillary factor.
Data Analysis
Mean(SD) values are reported in the text, after confirming normality. Alternatively, median(range) values are reported. For certain parameters, overall mean or median values were also estimated by using all individual data collected within each Phase in stomach or in upper small intestine.
Data are graphically presented as Box-Whisker plots showing the median value, the 10th, 25th, 75th and 90th percentiles, and the individual outlying data points, with dotted line indicating the mean value.
For each parameter, data from different treatments and time points were obtained from the same subject and, therefore, were assumed to be correlated. The MIXED procedure of SPSS v 17.0.0 was used to model the correlation between treatments and/or between aspiration time points. This was accomplished by including random effects based on subjects, on location (stomach-duodenum) within subject, on treatments within subject or on the interaction of treatment and location within subject. The choice of the best model for the covariance was based on the lowest value of the Schwarz’s Bayesian Information Criterion (BIC). Fixed effects variables like treatments, locations, and their interactions with aspiration time were included and chosen using the F-test. Type I error was set at 0.05. For bile acids in stomach, differences between treatments were analyzed using Wilcoxon test as they presented an excess of zeros. In this case, type I error was set at 0.025. For bile acids in upper small intestine, mean values are typically reported in literature but, since distribution of values is skewed (14), in this paper both mean and median values are presented.
In order to evaluate the effect of one freeze-thaw cycle on buffer capacity, differences between values obtained upon collections and after one freeze-thaw cycle were evaluated with the paired t-test or the Wilcoxon test, depending on the results of normality and equal variance tests; type I error was set to 0.05.
Results
Effects of Pantoprazole and Famotidine Treatments on Fasting pH Values in Upper Gastrointestinal Lumen
Fasting gastric pH values in the morning prior to water administration in Phase 2 (pantoprazole) and in Phase 3 (famotidine) were significantly higher than in Phase 1 (control); median(range) values were 1.4(1.2–2.7) in Phase 1, 2.4(1.5–7.3) in Phase 2, and 7.0(2.6–7.6) in Phase 3 (p ≤ 0.009). These data confirm that the famotidine treatment regimen selected resulted in maximal effect of increasing basal gastric pH. Pantoprazole under the regimen selected resulted in hypochlorhydric conditions. The hypochlorhydric conditions achieved with the pantoprazole treatment resemble the values which are observed in certain populations groups, such as the Japanese (17), the elderly (18) and occasional users of proton pump inhibitors.
Fasting pH values in upper small intestine in the morning prior to water administration in Phase 2 and in Phase 3 were not significantly different from those in Phase 1 (p = 0.120 for the Phase 1 vs. Phase 2 comparison and p = 0.101 for the Phase 1 vs. Phase 3 comparison); median(range) values were 6.4(2.6–7.7) in Phase 1, 7.2(6.5–7.6) in Phase 2, and 7.3(7.0–7.6) in Phase 3.
Effect of Reduced Acid Secretion in Stomach on the Characteristics of Gastric Contents in the Fasted State After Water Administration
pH
During the first 35 min post water administration, intragastric pH values in Phase 1 (control) were significantly lower than in Phase 2 (pantoprazole) or in Phase 3 (famotidine) at all sampling time points (p ≤ 0.001). In Phase 1, median(range) pH values of samples aspirated 10, 25 and 35 min post water administration were 2.9(1.8–3.9), 1.7(1.2–2.0) and 1.6(1.1–2.4), respectively (Fig. 2). pH values post water administration in Phase 1 are in line with previous data (19). Corresponding values in Phase 2 and in Phase 3 were 6.4(2.0–7.2), 5.2(1.4–7.3) and 2.4(1.3–7.6), and 7.2(6.9–7.3), 7.1(6.0–7.2) and 7.1(5.0–7.3), respectively (Fig. 2). Unlike with Phase 3, in Phase 1 and in Phase 2 intragastric pH decreased significantly as a function of time post water administration (p ≤ 0.014). The decreasing intragastric pH with time in Phase 2 is in contrast with the steady and higher intragastric pH values, after similar treatment of healthy adults with esomeprazole (12). Overall median values for gastric pH during the first 35 min post water administration in Phase 1, in Phase 2 and in Phase 3 were 1.8, 5.1, and 7.1, respectively.
Buffer Capacity
During the first 35 min post water administration, buffer capacity measured immediately upon aspiration with NaOH in Phase 1 was higher than in Phase 2 or Phase 3 but the difference reached significance only versus Phase 3 (Fig. 3, empty boxes, p < 0.001). In Phase 1, mean(SD) buffer capacity values of samples aspirated 10, 25 and 35 min after water administration were 4.7(4.6), 21.3(11.4) and 27.6(15.7) mmol/L/ΔpH, respectively, i.e. in line with previous data (Kalantzi et al. 2006). Corresponding values in Phase 2 and Phase 3 were 1.7(2.3), 6.3(10.6), 12.4(15.5) and 0.49(0.21), 0.69(0.21), 1.29(0.65) mmol/L/ΔpH, respectively. Buffer capacity values increase between 10 and 35 min post water administration in all Phases, but the trend reached significance only in Phase 1 (Fig. 3, empty boxes, p = 0.002).
Buffer capacity of contents of the stomach of fasted healthy adults as a function of time, after administration of 240 ml table water into the antrum of the stomach measured in Phase 1 (White boxes, n = 6–8), in Phase 2 (Light pink boxes, n = 4–8), and in Phase 3 (Dark blue boxes, n = 6–8) immediately upon collection using NaOH (empty boxes), after one freeze-thaw cycle using NaOH (lined boxes) and after one freeze-thaw cycle using HCl (dotted boxes). Due to limited available volumes, data after one freeze-thaw cycle in samples aspirated 35 min post water administration could not be collected.
One freeze-thaw cycle affected buffer capacity measured with NaOH in Phase 1 and in Phase 3 significantly (Fig. 3, empty vs. lined boxes within Phase 1 and within Phase 3, p < 0.02) but not in Phase 2 (Fig. 3, empty vs. lined boxes within Phase 2, p > 0.06). Also, compared with the values estimated after titration with NaOH, buffer capacities estimated with HCl were significantly higher in Phase 1 and in Phase 3 (Fig. 3, lined vs. dotted boxes within Phase 1 and within Phase 3, p < 0.03) but not in Phase 2 (Fig. 3, lined vs. dotted boxes within each Phase, p > 0.05). These data suggest that the smaller decrease of buffer capacity in the pantoprazole Phase (Phase 2) may not be entirely due to the incomplete reduction of gastric acid secretion.
Chloride ion Concentration
During the first 35 min post water administration, concentration of chloride ions in Phase 2 and in Phase 3 was lower than in Phase 1 (Fig. 4a) but the difference reached significance only at 20 and 35 min post water administration (p ≤ 0.003). In Phase 1, mean(SD) values in samples aspirated 10, 20 and 35 min post water administration were 41.0(25.3), 110.1(43.8), and 176.3(84.9) mM, respectively, i.e. in line with previous data (20). Corresponding values in Phase 2 and in Phase 3 were 18.2(10.5), 54.2(49.0), 88.2(86.1) and 10.1(3.2), 20.3(7.7), 47.9(28.5) mM, respectively. Chloride ion concentration increased between 10 and 35 min post water administration in all three phases but the trend reached significance only in Phase 1 and in Phase 2 (p < 0.001). These data are in line with the gradual emptying of gastric contents and the minimal hydrochloric acid secretion in Phase 3.
Concentration of chloride ions (a), osmolality (b), total protein content (c), and total bile salt content (d) in the stomach of fasted healthy adults as a function of time, after administration of 240 ml table water into the antrum of the stomach. Key: White boxes, Phase 1; Light pink boxes, Phase 2; Dark blue boxes, Phase 3. Each box was constructed by using 4–8 individual values.
Osmolality
During the first 35 min post water administration, osmolality in Phase 2 and in Phase 3 was lower than in Phase 1 (Fig. 4b) but the difference reached significance only versus Phase 3 (p ≤ 0.002 at 20 min and 35 min). In Phase 1, mean(SD) osmolality values of samples aspirated 10, 25 and 35 min after water administration were 44.9(22.6), 103.6(41.5) and 144.0(44.0) mOsmol/kg, respectively, i.e. in line with previous data (19). Corresponding values in Phase 2 and Phase 3 were 29.1(14.1), 61.9(32.8), 98.2(53.3) and 22.9(4.1), 39.6(15.7), 91.2(54.0) mOsmol/kg, respectively. Osmolality values increased significantly between 10 and 35 min post water administration but remained hypo-osmotic in all Phases (Fig. 4b).
Total Protein Content
Total protein content was not significantly different among the three Phases (Fig. 4c). This is in line with previous data in humans showing that pepsin is not affected by long term administration of omeprazole (10,21). The trend for increased protein content with time post water administration did not reach significance, in all Phases. Mean(SD) values for protein content at 10, 20 and 35 min post water administration were 0.27(0.14), 0.53(0.18) and 0.71(0.35) mg/ml in Phase 1, 0.40(0.15), 0.87(0.66) and 0.88(0.61) mg/ml Phase 2 and 0.45(0.32), 0.60(0.21) and 0.92(0.32) mg/ml Phase 3, respectively (Fig. 4c). Values are in line with previous data in healthy adults (19).
Bile Acids
Median(range) values for total bile salt content in Phase 1 (Fig. 4d) 10, 20 and 35 min post water administration were 0(0–145), 0(0–114) and 54.0(0–620) μM, respectively. Values are in line with previous data (15). Similar median(range) values were estimated for total bile acid content in Phase 2 [4.8(0–262), 0(0–604) and 0(0–1166) μM, respectively] and in Phase 3 [0(0–83.0), 15.0(0–971) and 256(0–1975) μM, respectively]. In all three Phases, glycocholate and glycochenodeoxycholate were the most prevalent bile acids (Table I), in line with previous studies (22). The trend for increased total bile acids content under reduced gastric acid conditions at late times post water administration could be related with the small basal intragastric volumes under such conditions (23).
Surface Tension
Mean(SD) values for the surface tension of gastric contents after administration of 240 ml water to fasted males in Phase 1, Phase 2 and Phase 3 were 43.22(0.74), 34.29(0.51) and 37.35(0.99) mN/m, respectively. Data in Phase 1 are in line with previously published data in healthy adults using individual aspirates (19). The lowest surface tension was observed in Phase 2, i.e. under conditions moderate reduction of gastric acid reduction, indicating that the mechanism for reducing gastric acid secretion is important for the surface tension of gastric contents.
Effect of Reduced Acid Secretion in Stomach on the Characteristics of Contents of Upper Small Intestine in the Fasted State After Water Administration
pH
During the first 50 min post water administration, pH values in upper small intestine in Phase 1 were lower than in Phase 2 or in Phase 3 (Fig. 5), but the difference reached significance only in Phase 3 for the period 15–30 min post water administration (p ≤ 0.014). In Phase 1, median pH values of samples aspirated 5, 15, 30 and 50 min post water administration were 6.8, 6.2, 6.3, and 6.5, respectively (Fig. 5), in line with previous data (19). Corresponding median values in Phase 2 and in Phase 3 were 6.9, 6.9, 6.4, 7.3 and 7.2, 7.2, 7.2, 7.3, respectively (Fig. 5). Increased pH in upper small intestine when achlorhydric conditions prevail in stomach has been also observed previously when achlorhydric conditions were achieved in stomach by administering repeated doses of esomeprazole (12). In all Phases, pH did not change significantly with time after water administration. Similar observations have also been made previously (12,24). However, unlike with Phase 1, in Phases 2 and 3, occasional excursions to highly acidic pH values were not observed (Fig. 5), i.e. pH in upper small intestine was much less variable. Overall median values for pH during the first 50 min. post water administration in Phase 1, Phase 2 and Phase 3 were 6.5, 7.0, and 7.2, respectively.
Buffer Capacity
During the first 50 min. post water administration, buffer capacity was not statistically different among the three Phases (Fig. 6, empty boxes). Mean(SD) buffer capacity values of samples aspirated 5, 15, 30 and 50 min after water administration were 8.4(2.9), 19.2(33.7), 9.0(3.8), and 14.2(10.5) mmol/L/ΔpH in Phase 1, 6.6(3.2), 9.4(4.2), 8.2(3.7), and 35.7(57.2) mmol/L/ΔpH in Phase 2, and 6.1(0.8), 9.0(3.8), 7.7(2.8), and 6.9(2.7) mmol/L/ΔpH in Phase 3, respectively, i.e. in line with previous data collected in healthy adults (19).
Buffer capacity of contents of upper small intestine of fasted healthy adults as a function of time, after administration of 240 ml table water into the antrum of the stomach measured in Phase 1 (White boxes, n = 6–8), in Phase 2 (Light pink boxes, n = 6–8), and in Phase 3 (Dark blue boxes, n = 7–8) immediately upon collection using HCl (empty boxes), after one freeze-thaw cycle using HCl (lined boxes) and after one freeze-thaw cycle using NaOH (dotted boxes). Due to limited available volumes, data after one freeze-thaw cycle in samples aspirated 30 and 50 min post water administration could not be collected.
One freeze-thaw cycle affected buffer capacity measured with HCl in all Phases significantly (Fig. 6, empty vs. lined boxes within each Phase, p < 0.02). Compared with the values estimated after titration with NaOH, buffer capacities estimated with HCl were significantly higher in all Phases (Fig. 6, lined vs dotted boxes within each Phase, p < 0.01).
Chloride ion Concentration
During the first 50 min post water administration, concentration of chloride ions was not statistically different among the three Phases (Fig. 7a). Mean(SD) values in samples aspirated 5, 15, 30 and 50 min. post water administration were 42.1(11.7), 70.3(33.0), 106.6(30.0), and 121.6(16.1) mM in Phase 1, 44.0(16.1), 58.1(34.4), 91.5(30.7), and 135.9(35.7) mM in Phase 2, and 30.7(6.5), 55.1(29.1), 84.3(46.7), and 96.7(36.8) mM in Phase 3, respectively, i.e. in line with previous data collected in healthy adults (20). In all Phases, chloride ion concentration increased significantly during the 5 to 50 min period post water administration (Fig. 7a).
Concentration of chloride ions (a), osmolality (b), total protein content (c), and total bile salt content (d) in the upper small intestine of fasted healthy adults as a function of time, after administration of 240 ml table water into the antrum of the stomach. Key: White boxes, Phase 1; Light pink boxes, Phase 2; Dark blue boxes, Phase 3. Each box was constructed by using 4–8 individual values.
Osmolality
During the first 50 min post water administration, osmolality was not statistically different among the three Phases (Fig. 7b). Mean(SD) osmolality values of samples aspirated 5, 15, 30 and 50 min after water administration were 92.5(17.9), 126.9(49.2), 207.4(31.6), and 217.4(44.5) mOsmol/kg in Phase 1, 78.4(27.1), 96.5(30.7), 170.0(57.6), and 222.8(27.2) mOsmol/kg in Phase 2, and 65.0(15.7), 111.3(51.6), 154.5(77.0), and 187.4(56.7) mOsmol/kg in Phase 3, respectively, i.e. in line with previous data (19). In all Phases, osmolality increased significantly during the 5 to 50 min period post water administration (Fig. 7b).
Total Protein Content
During the first 50 min post water administration, total protein content was not significantly different among the three Phases (Fig. 7c). In all Phases, total protein content increases significantly during the 5 to 50 min period post water administration. Mean(SD) values for total protein content 5, 15, 30 and 50 min post water administration were 1.00(0.37), 1.8(1.2), 2.7(1.7) and 3.7(1.1) mg/ml in Phase 1, 1.5(1.4), 1.6(1.1), 2.3(1.3) and 3.2(1.1) mg/ml in Phase 2, and 0.90(0.52), 2.1(1.3), 2.6(1.5), 3.3(2.0) mg/ml in Phase 3. Data are in line with previous values in healthy adults (19).
Bile Acids
During the first 50 min post water administration, total bile salt content was not significantly different among the three Phases (Fig. 7d). In all Phases, total bile acids content did not change significantly with time, after water administration (Fig. 7d). Mean(Median) values for total bile salt content in samples aspirated 5, 15, 30 and 50 min. after water administration were 1.1(1.1), 4.8(2.3), 7.7(5.0), and 3.3(1.9) mM in Phase 1, 3.4(1.3), 6.5(6.7), 7.0(6.2), and 3.2(1.7) mM in Phase 2, and 0.8(0.5), 4.6(3.4), 6.2(3.7), and 5.5(5.4) mM in Phase 3, respectively, i.e. in line with previous data (14,22,24,25). As in stomach, in all three Phases, glycocholate and glycochenodeoxycholate were the most prevalent bile acids (Table I), in line with previous studies (24,25).
Free Fatty Acids
During the first 50 min post water administration, free fatty acid content was not significantly different among the three Phases (Fig. 8a). In all Phases, free fatty acid content did not change significantly with time, after water administration (Fig. 8a). Mean(SD) values for the free acid content 5, 15, 30 and 50 min post water administration were 401(273), 576(559), 946(1105), and 280(260) μM in Phase 1, 617(671), 832(474), 994(837), and 531(356) μM in Phase 2, and 425(409), 800(527), 709(565) and 625(614) μM in Phase 3. These data are in line with previous data (22,24,25).
Free fatty acid content (a), total phosphatidylcholine content (b) and cholesterol content (c) in the upper small intestine of fasted healthy adults as a function of time after administration of 240 ml table water into the antrum of the stomach. Key: White boxes, Phase 1; Light pink boxes, Phase 2; Dark blue boxes, Phase 3. Each box was constructed by using 4–8 individual values.
Total Phosphatidylocholine
During the first 50 min post water administration, total phosphatidylcholine content was not significantly different among the three Phases (Fig. 8b). In all Phases, total phosphatidylcholine content did not change significantly with time, after water administration (Fig. 8b). Mean(SD) values at 5, 15, 30 and 50 min post water administration were 116(109), 574(564), 605(857), and 314(300) μM in Phase 1, 340(640), 679(552), 752(654), and 314(236) μM in Phase 2, and 181(287), 701(961), 570(568) and 314(319) μM in Phase 3. These data are slightly higher than previously reported data (14,22,24). These data suggest that the ratio [total bile acid content/total phosphatidylcholine content] in the fasted upper small intestinal lumen after administration of glass of water is about 10/1 in all Phases, i.e. close to the ratio recently suggested to be used in fasted state simulating intestinal fluids [FaSSIF-V3, (26)]. Previously suggested simulated intestinal fluids had lower ratios (to balance the lack of simulation of cholesterol, fatty acids and monoglycerides, FaSSIF, 4/1) or simply because the lack of simulation of cholesterol, fatty acids and monoglycerides were not taken into account, FaSSIF-V2, 15/1) (27,28).
Cholesterol
Cholesterol concentration in the upper small intestine is not affected by the reduced gastric acid secretion (Fig. 8c). In all Phases cholesterol concentration did not change significantly with time post water administration (Fig. 8c). Mean(SD) values from data collected during the first 50 min. post water administration 117(119), 273(225), 435(408), and 169(103) μM in Phase 1, 225(291), 363(256), 478(379) and 269(234) μM in Phase 2, and 117(100), 334(219), 380(309), and 200(228) μM in Phase 3, in line with previously reported values (14).
Temporal trends of molar concentrations of bile acids, free fatty acids, total PC and cholesterol are similar, with an apparent peak at 30 min in all cases. This is in line with the fact that all these components are present in the same colloidal species.
Surface Tension
Mean(SD) values for the surface tension of contents in upper small intestine, after administration of 240 ml water to the stomach of fasted males in Phase 1 (control phase), Phase 2 (pantoprazole phase) and Phase 3 (famotidine phase) were 32.70(0.51), 29.81(0.65) and 35.20(1.28) mN/m, respectively. Data in Phase 1 are in line with previously published data in healthy adults (19). The lowest surface tension in Phase 2 is in line with the lowest surface tension in stomach in the same Phase.
Kinematic Viscosity
Mean(SD) values for the kinematic viscosity of contents in upper small intestine, after administration of 240 ml water to the stomach of fasted males in Phase 1, Phase 2, and Phase 3 were 0.7602(0.0129), 0.7919(0.0021) and 0.7816(0.0011) mm2/s, respectively. These data suggest that viscosity of contents in upper small intestine in the fasting state is not affected by the reduced gastric acid secretion and values are slightly higher than that of water (0.6959 mm2/s, www.viscopedia.com).
Discussion
Data from this study show that, apart from the pH change in stomach, additional physiological changes should be considered when evaluating the luminal performance of APIs (especially of weak bases) and dosage forms in individuals on gastric acid reducing treatment. The focus of this study was to understand these changes that occur after administration of the agents in a typical DDI study design. However, more data are needed for justifying the usefulness of all data collected in the present investigation when evaluating performance of APIs/drug products in individuals with chronic hypochlorhydria. For example, it has been documented that long term hypochlorhydria results to bacterial overgrowth in gastric and jejunal fluids and some of the relevant species promote deconjugation of bile acids (29). Deconjugation may have an impact on surface tension (wettability), micellar structure and solubilization capacity in the upper small intestine.
With the focus on a DDI study setting in mind, based on the data from the present study, physiological changes in stomach that accompany the reduced gastric acid secretion include the reduced buffer capacity, chloride ion concentration, osmolality and surface tension. These changes are or can be related to the altered gastric pH. For surface tension, however, other mechanisms could also be involved. Intravenous administration of famotidine (4 mg/kg) results in increased thickness of the adherent mucus gel in rats (30). The impaired quality of mucus and the weakened mucus – bicarbonate barrier, after prolonged administration of famotidine has been observed also in the human stomach (31,32). As far as PPIs are concerned, there are no published data on the effect of pantoprazole on mucosal function in humans. Rabeprazole enhances gastrin mucin content (33) within 1 week of administration. Composition of gastric contents is also affected by the enhanced esophageal mucin secretion, after rabeprazole administration (34). Based on these data part of the increased mucus may be released into the bulk of the gastric contents and decrease surface tension.
Based on data from the present study, physiological changes in upper small intestine which accompany the reduced gastric acid secretion include the increased pH in upper small intestine by about 0.7 units (from ~6.5 in Phase 1 to ~7.2 in Phase 3), despite the unaltered buffer capacity. Since in Phase 2 hypo- and not a-chlorhydric conditions were established, it is not clear from this study if the increased pH in upper small intestine is due to the a-chlorhydric conditions induced in Phase 3 or it is related also to the mechanism of inhibition of gastric acid secretion. The relevance of this change to absorption of compounds requires further investigation. In principle compounds with solubility sensitive to pH in that region may be affected (for example lipophilic weak bases with pKa values between 6 and 7 and high doses that stomach solubilization may not be sufficient) although the net effect will need to be evaluated on a compound basis.
Two findings of the present investigation that are valid regardless of the extent of gastric acid secretion and are reported for first time in the literature deserve to be briefly highlighted. The first relates to the ability of contents of stomach and of upper small intestine to resist in changes to their pH in the fasted state. Specifically, buffer capacity in both locations and regardless of the extent of acid secretion in stomach is much higher when changes to more acidic values are attempted, i.e. contents are more powerful in resisting to lowering the pH than to increasing the pH in upper GI lumen. The second relates to the viscosity of contents of the upper small intestine. Contents are more viscous than de-ionized water. To date the impact of viscosity on drug/drug product performance has been considered only for events in the stomach (35,36). The impact of increased viscosity in upper small intestine on various events relating to drug/drug product performance remains to be evaluated.
The data from this study can help to better design such studies during clinical development of new chemical entities. As discussed previously, the design employed in this study represents a typical study design for studying DDIs with PPIs or H2-RAs during clinical development for compounds where solubility changes as a function of stomach pH are anticipated (most typically weak bases). Under current development paradigms, typically compounds would need to be tested with both regimens for the interactions against both agents to be described in the drug label. In this study, both of the treatment regimens selected proved effective in achieving the intended effect of shifting the stomach pH, supporting the use of such design for DDI studies. The significantly higher pH achieved with the famotidine treatment, relative to the pantoprazole treatment, raises the possibility that, at least for weak bases, testing with famotidine could serve as a worst case scenario in terms of solubilization capacity. Thus, if no effect on pharmacokinetics is seen after the famotidine treatment, perhaps testing with a PPI, which effect on solubilization would be less pronounced, does not need to be pursued for weak bases, thus reducing the number for unnecessary additional clinical trials Recently, a preliminary conceptual framework has been recommended that the worst case scenario should be tested by using a PPI (37). Such recommendation was based on the fact that PPIs generally have a longer duration of suppression effect on gastric acid secretion than do H2-RAs and antacids, and are expected to interfere with the intestinal absorption of weak bases to greater extent (37). Such recommendation may be, therefore, logical to make under regular clinical practice. However, data from this study clearly show that, if the objective is to evaluate the effect of reduced gastric acid secretion on drug absorption, under a controlled study, the protocol applied in the present study with the H2-RA agent (including the spacing of doses) is perfectly adequate for achieving the maximal effect; if a positive DDI is observed, potential new DDI study to explore staggered dosing for mitigating the pH effect could be considered with a PPI, following the protocol (and the spacing of doses) applied in the present study.
Finally, although the focus of attention of this manuscript is free base compounds, the higher intestinal pH achieved by famotidine may also translate into a larger difference in absorption for weak acids with pKas between 6 and 7.
Concluding Remarks
Based on data from this study, reduction of gastric acid secretion in humans is accompanied by additional physicochemical changes which could additionally impact the luminal performance of orally administered drugs/drug products in the fasted state, especially of lipophilic weak bases. Relevant changes include the reduced buffer capacity, chloride ion concentration, osmolality and surface tension in stomach and the increased pH in upper small intestine. The mechanism of reduction of gastric acid secretion (PPIs vs. H2-RAs) seems to be important for the buffer capacity in stomach and surface tension of contents in upper gastrointestinal lumen. The effect of famotidine on stomach pH was much more pronounced relative to pantoprazole, raising the possibility that famotidine can be used as the worst case scenario in assessing interactions of weak bases with stomach pH altering medications.
The impact of these changes in upper gastrointestinal lumen on intraluminal performance of model active pharmaceutical ingredients is currently being evaluated in pooled aspirates collected in the present study and in biorelevant media prepared based on data from the present study.
Abbreviations
- API:
-
Active pharmaceutical ingredient
- BIC:
-
Bayesian information criterion
- DDI:
-
Drug-drug interaction
- FaSSIF:
-
Fasted state simulating intestinal fluid
- FMT:
-
Famotidine
- GC:
-
Glycocholic acid
- GCDC:
-
Glycochenodeoxycholic acid
- GDC:
-
Glycodeoxycholic acid
- GI:
-
Gastrointestinal
- H2-RA:
-
Histamine-2 receptor antagonist
- HBV:
-
Hepatitis B virus
- HCV:
-
Hepatitis C virus
- HIV:
-
Human immunodeficiency virus
- HPLC:
-
High performance liquid chromatography
- Lyso-PC:
-
Lyso-phosphatidylcholine
- MG:
-
Monoglyceride
- PC:
-
Phosphatidylcholine
- PNT:
-
Pantoprazole
- PPI:
-
Proton pump inhibitor
- SD:
-
Standard deviation
- TC:
-
Taurocholic acid
- TCDC:
-
Taurochenodeoxycholic acid
References
Lahner E, Annibale B, Delle FG. Systematic review: impaired drug absorption related to the co-administration of antisecretory therapy. Aliment Pharmacol Ther. 2009;29(12):1219–29.
Paulekuhn GS, Dressman JB, Saal C. Trends in active pharmaceutical ingredient salt selection based on analysis of the orange book database. J Med Chem. 2007;50:6665–72.
Smelick GS, Heffron TP, Chu L, Dean B, West DA, Duvall SL, et al. Prevalence of acid-reducing agents (ARA) in cancer populations and ARA drug-drug interaction potential for molecular targeted agents in clinical development. Mol Pharm. 2013;10:4055–62.
Yago MR, Frymoyer AR, Smelick GS, Frassetto LA, Budha NR, Dresser MJ, et al. Gastric reacidification with betaine HCl in healthy volunteers with rabeprazole-induced hypochlorhydria. Mol Pharm. 2013;10:4032–7.
Mitra A, Kesisoglou F. Impaired drug absorption due to high stomach pH: a review of strategies for mitigation of such effect to enable pharmaceutical product development. Mol Pharm. 2013;10:3970–9.
Humphries TJ, Merritt GJ. Review article: drug interactions with agents used to treat acid-related diseases. Aliment Pharmacol Ther. 1999;13 Suppl 3:18–26.
Chremos AN. Clinical pharmacology of famotidine: a summary. J Clin Gastroenterol. 1987;9 Suppl 2:7–12.
Parsons ME. Pantoprazole, a new proton-pump inhibitor, has a precise and predictable profile of activity. Eur J Gastroenterol Hepatol. 1996;8 Suppl 1:S15–20.
Stedman CA, Barclay ML. Review article: comparison of the pharmacokinetics, acid suppression and efficacy of proton pump inhibitors. Aliment Pharmacol Ther. 2000;14:963–78.
Howden CW, Forrest JAH, Reid JL. Effects of single and repeated doses of omeprazole on gastric acid and pepsin secretion in man. Gut. 1984;25:707–10.
Krishna G, Moton A, Ma L, Medlock MM, McLeod J. Pharmacokinetics and absorption of posaconazole oral suspension under various gastric conditions in healthy volunteers. Antimicrob Agents Chemother. 2009;53:958–66.
Walravens J, Brouwers J, Spriet I, Tack J, Annaert P, Augustijns P. Effect of pH and comedication on gastrointestinal absorption of posaconazole. Clin Pharmacokinet. 2011;50:725–34.
Echizen H, Ishizaki T. Clinical pharmacokinetics of famotidine. Clin Pharmacokinet. 1991;21(3):178–94.
Psachoulias D, Vertzoni M, Goumas K, Kalioras V, Beato S, Butler J, et al. Precipitation in and supersaturation of contents of the upper small intestine after administration of two weak bases to fasted adults. Pharm Res. 2011;28:3145–58.
Vertzoni M, Archontaki H, Reppas C. Determination of intralumenal individual bile acids by HPLC with charged aerosol detection. J Lipid Res. 2008;49:2690–5.
Diakidou A, Vertzoni M, Goumas K, Söderlind E, Abrahamsson B, Dressman J, et al. Characterization of the contents of ascending colon to which drugs are exposed after oral administration to healthy adults. Pharm Res. 2009;26(9):2141–51.
Morihara M, Aoyagi N, Kaniwa N, Kojima S, Ogata H. Assessment of gastric acidity of Japanese subjects over the last 15 years. Biol Pharm Bull. 2001;24:313–5.
Russell TL, Berardi RR, Barnett JL, Dermentzoglou LC, Jarvenpaa KM, Schmaltz SP, et al. Upper gastrointestinal pH in seventy-nine healthy, elderly, North American men and women. Pharm Res. 1993;10:187–96.
Kalantzi L, Goumas K, Kalioras V, Abrahamsson B, Dressman JB, Reppas C. Characterization of the human upper gastrointestinal contents under conditions simulating bioavailability/bioequivalence studies. Pharm Res. 2006;23:165–76.
Lindahl A, Ungell AL, Knutson L, Lennernäs H. Characterization of fluids from the stomach and proximal jejunum in men and women. Pharm Res. 1997;14:497–502.
Howden CW, Forrest JAH, Meredith PA, Reid JL. Antisecretory effect and oral pharmacokinetics following low dose omeprazole in man. Br J Clin Pharmacol. 1985;20:137–9.
Bergström CA, Holm R, Jørgensen SA, Andersson SB, Artursson P, Beato S, et al. Early pharmaceutical profiling to predict oral drug absorption: current status and unmet needs. Eur J Pharm Sci. 2014;57:173–99.
Tack J. Review article: the role of bile and pepsin in the pathophysiology and treatment of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2006;24 Suppl 2:10–6.
Clarysse S, Tack J, Lammert F, Duchateau G, Reppas C, Augustijns P. Postprandial evolution in composition and characteristics of human duodenal fluids in different nutritional states. J Pharm Sci. 2009;98(3):1177–92.
Petrakis O, Vertzoni M, Angelou A, Kesisoglou F, Bentz K, Goumas K, et al. Identification of key factors affecting the oral absorption of salts of lipophilic weak acids: a case example. J Pharm Pharmacol. 2015;67:56–67.
Fuchs A, Leigh M, Kloefer B, Dressman JB. Advances in the design of fasted state simulating intestinal fluids: FaSSIF-V3. Eur J Pharm Biopharm. 2015;94:229–40.
Jantratid E, Janssen N, Reppas C, Dressman JB. Dissolution media simulating conditions in the proximal human gastrointestinal tract: an update. Pharm Res. 2008;25(7):1663–76.
Galia E, Nicolaides E, Hörter D, Löbenberg R, Reppas C, Dressman JB. Evaluation of various dissolution media for predicting in vivo performance of class I and II drugs. Pharm Res. 1998;15(5):698–705.
Shindo K, Machida M, Fukumura M, Koide K, Yamazaki R. Omeprazole induces altered bile acid metabolism. Gut. 1998;42:266–71.
Eleftheriadis E, Kotzampassi K, Tzioufa V, Karamouzis M, Aletras H. Effects of famotidine on gastric mucus of the rat. Res Exp Med. 1990;190:219–22.
Guslandi M, Ballarin E, Tittobello A. Gastric mucus secretion in ranitidine-treated patients. Br Med J. 1981;283:699.
Guslandi M, Battaglia A, Pamparana F, Passaretti S, Pellegrini A, Tittobello A. Weaking effect of famotidine but not of nizatidine on the mucus-bicarbonate barrier of the human stomach. Drugs Exp Clin Res. 1990;16:481–5.
Skoczylas T, Sarosiek I, Sostarich S, McElhinney C, Durham S, Sarosiek J. Significant enhancement of gastric mucin content after rabeprazole administration. Its potential clinical significance in acid-related disorders. Dig Dis Sci. 2003;48:322–8.
Sarosiek I, Olyaee M, Majewski M, Sidorenko E, Roeser K, Sostarich S, et al. Significant increase of esophageal mucin secretion in patients with reflux esophagitis after healing with rabeprazole: its esophagoprotective potential. Dig Dis Sci. 2009;54:2137–42.
Pedersen PB, Vilmann P, Bar-Shalom D, Muellerz A, Baldursdottir S. Characterization of fasted human gastric fluid for relevant rheological parameters and gastric lipase activities. Eur J Pharm Sci. 2013;85:958–65.
Cvijic S, Parojcic J, Langguth P. Viscosity-mediated negative food effect on oral absorption of poorly-permeable drugs with an absorption window in the proximal intestine: In vitro experimental simulation and computational verification. Eur J Pharm Sci. 2014;61:40–53.
Zhang L, Wu F, Lee SC, Zhao H, Zhang L. pH-dependent drug-drug interactions for weak base drugs: potential implications for new drug development. Clin Pharmacol Ther. 2014;96:266–77.
ACKNOWLEDGMENTS AND DISCLOSURES
This work would not have been possible without the participation of reliable volunteers and authors would like to express their sincere appreciation. Authors would like to thank Dr Aikaterini Lourbakou and Ms Mirofora Kotoglou for their excellent assistance during intubations and Prof. Michael Koupparis, Department of Chemistry, NKUA, for his assistance in the viscosity measurements. Part of the present work was presented as a poster at AAPS Annual Meeting, October 25–29, 2015, Orlando, Florida, USA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Chara Litou and Maria Vertzoni contributed equally to this work.
Rights and permissions
About this article
Cite this article
Litou, C., Vertzoni, M., Goumas, C. et al. Characteristics of the Human Upper Gastrointestinal Contents in the Fasted State Under Hypo- and A-chlorhydric Gastric Conditions Under Conditions of Typical Drug – Drug Interaction Studies. Pharm Res 33, 1399–1412 (2016). https://doi.org/10.1007/s11095-016-1882-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-016-1882-8