Abstract
Purpose
The aim of this study was to update the compositions of biorelevant media to represent the composition and physical chemical characteristics of the gastrointestinal fluids as closely as possible while providing physical stability during dissolution runs and short-term storage.
Methods
Media were designed to reflect postprandial conditions in the stomach and proximal small intestine in the “early”, “middle”, and “late” phases of digestion. From these “snapshot” media, general media for simulating postprandial conditions were devised. Additionally, media reflecting preprandial conditions in the stomach and small intestine were revisited.
Results
A set of four media is presented. A recently published medium to represent the fasted stomach, FaSSGF, needed no further revision. To simulate the postprandial stomach, a new medium, FeSSGF, is presented. Media representing the upper small intestine in the fed and fasted states were fine-tuned according to physicochemical and biochemical characteristics in vivo. All four media proved to be stable under ambient storage conditions for at least 72 h as well as under usual dissolution test conditions.
Conclusions
The updated dissolution media can be used to predict formulation performance and food effects in vivo. These media are more physiologically relevant and show better physical stability than their corresponding predecessors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
An important application of dissolution testing is to predict in vivo performance of solid oral dosage forms (1). However, the simple aqueous buffer solutions typically used for quality control dissolution testing do not represent all aspects of physiological conditions in the gastrointestinal (GI) tract and usually offer, at best, empirical a posteriori correlations with in vivo data. Prediction of intralumenal performance in the proximal gut generally requires adequate simulation of the conditions in the stomach and the proximal part of the small intestine.
Several attempts have been made to simulate fasting conditions in the stomach, starting with the Simulated Gastric Fluid (SGF) USP, which has a pH of 1.2 and contains pepsin (2). Dressman et al. (1) and Galia et al. (3) proposed addition of synthetic surfactants like sodium lauryl sulfate or Triton-X® 100 to the fasted gastric media to reduce the surface tension of the media to physiological values. However, these media were later shown to overestimate gastric dissolution because they induce solubilization effects greater than would be physiologically relevant (4). Recently, a Fasted State Simulated Gastric Fluid (FaSSGF) containing pepsin and low amounts of bile salt and lecithin was developed by Vertzoni et al. (5). This medium appears to be more appropriate than the previously proposed media because the reduced surface tension in the medium is created by physiological concentrations of pepsin rather than synthetic surfactant (5). It has a pH of 1.6 and a surface tension of 42.6 mN/m (see Table I).
Although it has been suggested that the most suitable media representing the fed state stomach is the homogenized form of the meal used in the clinical study (6), difficulties in drug analysis limit use of this approach. Alternative media include milk and Ensure® Plus (7). Ensure® Plus and full fat (3.5%) milk both have physicochemical properties that are similar to those of the standard meal recommended by the American HHS-FDA for the effects of food in bioavailability (BA) and bioequivalence (BE) studies (7). Milk has previously been used as a dissolution medium to simulate the fed stomach in several studies (8–12). Macheras et al. (9,13,14) investigated the effect of temperature and fat content of milk on drug solubilization and drug binding. It was found that drug binding to milk components was higher when the fat content of milk was increased (14). The solubility of all investigated drugs in milk were higher than in pH 6.5 phosphate buffer and increased with drug lipophilicity, milk fat content and temperature (9,13). Milk is also known to influence disintegration of drug products. Anwar et al. (15) showed that tablet disintegration was more than five times longer in milk than in fasted state simulated gastric media. However, a major issue when trying to simulate the intragastric environment in the fed state is that the composition changes with time as digestion proceeds and emptying occurs. One way to model this is to periodically add aliquots of an acidic solution of pepsin into milk (16). An alternative approach is to develop “snapshot” media, each corresponding to a certain time-frame after ingestion of the meal.
The first medium representing conditions in the small intestine was Simulated Intestinal Fluid (SIF) USP (2). The original version had a pH of 7.5, which was revised to 6.8 in 1996 (17) to better reflect the pH in the proximal small intestine. Biorelevant media simulating preprandial and postprandial conditions in the upper small intestine, including ‘Fasted State Simulated Intestinal Fluid (FaSSIF)’ and ‘Fed State Simulated Intestinal Fluid (FeSSIF)’ were introduced about 10 years ago (1,10). These media were intended to simulate additional important aspects of the GI fluids, including bile salts and lecithin. They were designed partly on the basis of literature data available at the period for bile salt and lecithin concentrations and pH in the human GI tract and partly on the basis of buffer capacities measured in a fistulated dog model (1). The feasibility of predicting in vivo behavior of drug products using these traditional biorelevant media has been demonstrated in several in vitro–in vivo correlations (IVIVC) (11,18–23). Some adjustments to the media have since been made, with the goal of improving predictions of in vivo performance (19–21,24).
Recent studies in healthy human volunteers have revealed that conditions in the small intestine after the ingestion of a meal differ in some ways to the composition of FaSSIF and FeSSIF (25). One key concern is that the concentrations of bile salt observed in vivo are somewhat lower than in FeSSIF. On the other hand, FeSSIF contains no lipolysis products, which, together with the bile, can enhance the solubility of poorly soluble drugs (1,26). Additionally, recent data in humans indicate that the pH in the upper small intestine decreases rather slowly after meal intake. For these reasons, the composition of FeSSIF needs to be revised to better predict in vivo performance of oral dosage forms.
In this study, in vivo data recently summarized by Porter et al. (27) were applied to generate compositions of biorelevant media more representative of the fasted and fed conditions in the proximal gut. To reflect changing conditions in the postprandial stomach and upper small intestine, “snapshot” media were first developed, each corresponding to a certain time-frame after ingestion of a meal. From these, general media for the fasted and fed state in the upper GI tract were evolved. The new media were compared with ex vivo human aspirates with respect to their physicochemical and biochemical properties and additionally evaluated in terms of their stability under in vitro dissolution testing and ambient storage conditions.
MATERIALS AND METHODS
Chemicals and Reagents
Long-life, heat-treated and homogenized milk (UHT-milk) containing 3.5% fat (Milfina Hochwald, Kaiserslautern, Germany) was purchased commercially. Glyceryl monooleate (GMO, Rylo M19 Pharma®, 99.5% monoglyceride, lot 173403-2202/107) was a gift from Danisco Specialities, Brabrand, Denmark. Egg phosphatidylcholine (Lipoid E PC®, 99.1% pure, lot 108015-1/42) was donated from Lipoid GmbH, Ludwigshafen, Germany. 37% hydrochloric acid (conc. HCl), 85% ortho-phosphoric acid and pepsin (Ph. Eur., 0.51 U/mg, lot 1241256) were obtained from Fluka Chemie AG, Buchs, Switzerland. Maleic acid (99% pure, lot S33471-226), and pancreatin (8×USP specifications, lot 045K0673) were purchased from Sigma-Aldrich Chemie GmbH (Steinheim, Germany). Sodium oleate (82.7% pure, lot 51110) was obtained from Riedel-de Haën, Seelze, Germany. Sodium taurocholate (NaTC, 97% pure, lot 2006040099) was used as received from Prodotti Chimici e Alimentari SpA, Basaluzzo, Italy. Sodium hydroxide solution (0.1 N NaOH) and hydrochloric acid solution (0.1 N HCl) were purchased from VWR International GmbH (Darmstadt, Germany). Calcium chloride (CaCl2), dichloromethane, glacial acetic acid, sodium acetate trihydrate, sodium chloride (NaCl), sodium dihydrogen phosphate monohydrate and NaOH pellets were all of analytical grade and purchased from Merck KGaA (Darmstadt, Germany).
Design of Updated Biorelevant Dissolution Media
Media to Simulate Postprandial Conditions in the Stomach
According to Kalantzi et al., gastric pH decreases continuously after meal ingestion from pH 6.4 to 2.7 (25). Above and below its isoelectric point (IEP) of pH 4.6, UHT-milk is physically stable. Therefore, pH values of 6.4, 5.0 and 3.0 were chosen to prepare “snapshot” media corresponding to the “early”, “middle”, and “late” phases of gastric digestion, respectively.
Milk was diluted with buffers for the “middle” and “late” media to reflect ongoing secretion of gastric juice (and hence dilution of the meal) as well as emptying of the meal components with time. For the “middle” medium the ratio of the milk to buffers was 1:1 and for the “late” medium it was 1:3. For the “middle” and “late” media, the ratio of the salt to the acid form of the buffer necessary to obtain the desired pH was calculated with the Henderson–Hasselbalch equation (28).
Buffers were chosen according to their ability to maintain the desired pH and buffer capacity without exceeding the physiological osmolality. The Van Slyke equation was used to calculate the buffer concentrations needed to obtain the target buffer capacity (29).
In Eq. 2, β is the buffer capacity, C is the total buffer concentration, i.e. the sum of the molar concentrations of acid and salt, and [H3O+] is the molar concentration of the hydronium ion.
The amount of NaCl needed to adjust the medium to the physiologic osmolality was calculated on the basis of the freezing-point depression (30) and refined experimentally.
In Eq. 3, ΔT f is the freezing-point depression, i is the van ’t Hoff factor, accounting for the number of individual ions formed by a compound in solution (e.g. i = 2 for NaCl in water), K f is the cryoscopic constant, which is −1.858 K kg/mol, and m is the concentration in moles of solute per kilogram of solvent (mol/kg) or molality of the solution.
Table II summarizes the compositions of the various fed state gastric media. Medium composition was calculated to reflect the pH value, buffer capacity and osmolality of the gastric aspirates during the first 75 min (early), from 75–165 min (middle) and after 165 min (late) following meal ingestion.
Medium to represent “early” conditions in the fed stomach: consists of UHT-milk with NaCl added to adjust the osmolality. To prepare one liter “early” medium, 148 mmol NaCl was dissolved in 980 ml milk. The pH was adjusted with 0.1 N HCl or 0.1 N NaOH as needed and then the final volume was adjusted with milk.
Medium to represent “middle” conditions in the fed stomach: consists of UHT-milk and an acetate buffer mixed in equal volumes. To prepare one liter of the “middle” medium, 500 ml of milk was mixed with approximately 480 ml of blank “middle” buffer. The mixture was stirred using a magnetic stirrer while adjusting the pH to 5.0 with 0.1 N HCl. The medium was then adjusted to one liter with blank “middle” buffer.
Medium to represent “late” conditions in the fed stomach: consists of UHT-milk and a phosphate buffer mixed at a ratio of 1:3. To prepare one liter of the “late” medium, 250 ml of milk was mixed with approximately 730 ml of blank “late” buffer. The mixture was stirred using a magnetic stirrer and the pH was adjusted to 3.0 with 0.1 N HCl. The medium was stirred for an additional 20 min on a magnetic stirrer, then adjusted to one liter with blank “late” buffer.
Since two of the most important aims of in vitro testing during development are to compare formulations and to predict food effects, it is of practical interest to designate a global fasted state and a global fed state medium for this purpose. FaSSGF is the obvious choice for the fasted state (Table I), while for the fed state the “middle” medium appears to embrace most of the physiological changes associated with meal intake. Obviously, the composition of the “middle” medium cannot represent all meal types at all points in the postprandial phase. Its choice as a “global” representative of postprandial conditions in the stomach was based on the following considerations:
-
1.
The FDA recommends dosage form intake 30 min after the meal in pharmacokinetic studies investigating food effects (31);
-
2.
The pH profile in the stomach is dependent on the composition and homogeneity of the meal (e.g. solid/liquid vs liquid) as well as the age-group of the subjects studied (25,32,33);
-
3.
The good physical stability of the medium under usual dissolution test and short-term storage conditions; and
-
4.
Ease of analyte separation from the medium.
The “middle” medium can thus be used to generally predict postprandial performance and, in conjunction with FaSSGF, to forecast food effects. Consequently, we have designated it as Fed State Simulated Gastric Fluid (FeSSGF).
Medium to Simulate Preprandial Conditions in the Upper Small Intestine
To update the simulation of fasted state conditions in the upper small intestine, only minor changes to FaSSIF were necessary (see Table III). The amount of lecithin was decreased from 0.75 mM in FaSSIF to 0.2 mM in FaSSIF-V2. The osmolality is somewhat lower in FaSSIF-V2 than in FaSSIF, in accordance with in vivo data. The pH of 6.5 was maintained, with substitution of maleate buffer for phosphate buffer in FaSSIF-V2.
Media to Simulate Postprandial Conditions in the Upper Small Intestine
As for the gastric fluids, the composition of the intestinal fluids changes over time in the fed state. Therefore, three “snapshot” media were developed to reflect conditions in the upper small intestine during the digestion process. Their compositions are indicated in Table IV.
Sodium taurocholate was used to represent the bile salts due to its comparatively low pKa value and hence good solubility at all pH values under consideration. Media containing sodium taurocholate as the sole bile salt have proven suitable for predicting the solubility of poorly soluble drugs in human aspirates (34). Pure bile salt was used to circumvent practical problems associated with use of crude salts in preparation of FeSSIF (24). Maleate buffer was used for simulated intestinal fluids in this study. With a pKa2 of 6.27 (the first pKa is at 1.92 and therefore irrelevant for buffering at intestinal pH values) (35), appropriate buffer capacities can be achieved over the required pH range of 5.4 to 6.5 (Tables III and IV) covering both fasted and fed state media without exceeding the physiologically relevant osmolality. Another advantage is that maleic acid is known to be able to retard rancidity of fats and oils (36,37). The ratios of salt/acid of the buffer species, the buffer concentrations, and the amounts of NaCl needed to obtain the required osmolalities were calculated using Eqs. 1–3.
To prepare one liter medium the following procedure was used:
-
1.
Approximately 900 ml blank buffer was prepared using amounts of NaCl, maleic acid and NaOH calculated for one liter of medium. The pH was then adjusted to the target pH.
-
2.
Five hundred milliliters of this “blank” buffer was transferred into a one liter round-bottom flask.
-
3.
NaTC was dissolved in the blank buffer by continuous stirring, after which a freshly prepared solution of lecithin in dichloromethane (100 mg/ml) was added. This produced an emulsion, i.e. the resulting product was turbid.
-
4.
The dichloromethane was then driven off, initially using a rotary evaporator and vacuum at approximately 40°C for 15 min at 650 mbar. The pressure was decreased stepwise to the final pressure of 100 mbar, which was subsequently maintained for 15 min. This procedure resulted in a clear to slightly hazy, micellar solution, having no perceptible odor of dichloromethane.
-
5.
After incorporation of bile salt and lecithin, a freshly prepared solution of GMO in dichloromethane (50 mg/ml) was added and a second evaporation step performed.
-
6.
Next, appropriate amounts of sodium oleate were added slowly into the micellar solution under continuous stirring and, as the last step,
-
7.
The volume was adjusted to one liter after the final pH adjustment using the “blank” buffer and deionized water.
Optionally, pancreatin can be incorporated into the fed state media right before the final pH adjustment. In this case, CaCl2 (5 mM) is added to the micellar solution just before the pancreatin to facilitate lipolysis. The amount of pancreatin added is based on the lipase activity needed to digest lipids contained in the formulations as well as those present in the media per se. Allowing for an overage this comes to approximately 100 lipase units per milliliter of medium (units are USP units throughout). A concentrated suspension of pancreatin is prepared by mixing pancreatin powder (8×USP specifications) in deionized water to obtain a lipase activity of 10,000 U/ml. The suspension is then centrifuged at 5°C for 15 min at 4,000 rpm, 20 ml of the supernatant are added and the final volume of the medium is adjusted to 1 l.
As with the gastric media, it is practical to select compositions which globally reflect conditions in the upper small intestine before and after meals for the purposes of comparing formulations and/or assessing food effects. For the fasted state, FaSSIF-V2 is suitable. For the fed state, a further composition (FeSSIF-V2) was developed (Table IV). This medium combines the postprandial changes in pH, buffer capacity, osmolality and bile component concentrations, while utilizing concentrations of lipolysis products that reflect aqueous phase values in the aspirates.
Physicochemical Characterization of Media
All physicochemical measurements were performed in triplicate (n = 3), unless otherwise stated.
pH
A freshly calibrated pH-meter (model 720A, Orion Research Inc., Beverly, MA, USA) was used for the media pH determinations.
Osmolality
Osmolality values were measured with a Knauer Osmometer Automatic (Knauer, Berlin, Germany) by determining the freezing-point depression of the media.
Buffer Capacity
Buffer capacity was determined according to the USP 29 (2), in which a potentiometric titration method is described. Briefly, the buffer capacity was estimated by dropwise addition of 0.1 N HCl, measuring the volume required to change the pH by one unit.
Surface Tension
Surface tension measurements of the media were conducted at 37°C. Ten measurements were recorded with SITA online f10 bubble pressure tensiometer (SITA Messtechnik GmbH, Dresden, Germany). The principle of the bubble pressure tensiometer is to force an air bubble into a liquid sample through a capillary of radius, r. According to the Young–Laplace equation, the pressure, Δp, needed to transfer the bubble into the liquid is proportional to the surface tension, σ (38).
This method of surface tension measurement gives the values for dynamic surface tension. To be able to compare the values obtained from the bubble pressure tensiometer with the results from Du Noüy tensiometer, which gives the values for static surface tension, the measurements were conducted at the lower end of the frequency range available, i.e. 0.5 Hz (39).
Stability of the Media
The media were evaluated for their stability under usual dissolution testing as well as ambient storage conditions.
To test media stability under standard dissolution testing conditions, i.e. paddle method, 75 rpm, 37°C, experiments were allowed to run for up to 72 h, during which the medium was observed for signs of physical instability such as coacervation, floating or formation of lipid droplets. This was aided by adding a dispersion of Sudan I, a lipophilic red dye, to the media at a concentration of approximately 5 mg per liter of medium. The physicochemical properties of the media were also compared before and after the tests.
To test media stability under ambient storage conditions, 100 ml aliquots of the medium were transferred into graduated cylinders and stored for 72 h at room temperature (approximately 25°C). Evaluation of stability was carried out as for samples subjected to dissolution testing conditions.
RESULTS AND DISCUSSION
Physicochemical Properties of Media Simulating the Environment in the Stomach
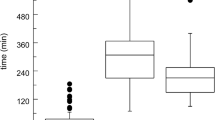
In the preprandial state, no major changes in aspirate properties are observed with time (25). Therefore, one representative medium for the fasted stomach (FaSSGF) suffices for biorelevant dissolution testing (5). The composition of FaSSGF is detailed in Table I. In contrast, several “snapshot” dissolution media were designed to correspond to changes in composition during digestion in the stomach. Thus, depending on the timing of dosage form administration relative to meal intake, appropriate media can be proposed for simulations of the in vivo dissolution. The pH values and osmolalities of the three “snapshot” media are superimposed on the pH and osmolality profiles measured in aspirated human gastric juice in Fig. 1.
Median in vivo profiles of A pH and B osmolality in postprandial stomach (25) and the corresponding values of the “snapshot” media (represented by horizontal bars).
As UHT-milk has a pH of approximately 6.7, only a small pH adjustment was needed to prepare the “early” medium. For the other “snapshot” media, it was necessary to choose buffers with appropriate pKa values to simultaneously attain target pH and buffer capacity values. A small quantity of NaCl was added to UHT-milk to match the osmolality of the “early” medium to the postprandial conditions. The amounts of NaCl calculated for preparation of the “middle” and “late” media took the buffer components of these media into account and here, too, target values were attained. Surface tension is constant after meal intake, remaining between 30 and 31 mN/m (25). The surface tension of the “snapshot” media is inversely proportional to the concentration of milk. The early “snapshot” medium had the lowest surface tension of 49.7 ± 0.3 mN/m whereas the “middle” and “late” media had surface tensions of 52.3 ± 0.3 and 58.1 ± 0.2 mN/m, respectively. As the latter values are somewhat higher than their physiological counterparts, wetting might be underestimated using the “middle” and “late” media. Even though pepsin levels range from 0.26 to 0.58 mg/ml in the fed stomach (25), pepsin was not added to the “snapshot” media because it would lead to physical and chemical changes in composition of the medium during the dissolution experiment and thus negate the “snapshot” concept. Changes in protein level with time are instead represented by dilution of the milk with buffers as one progresses from the “early” to “late” snapshot media.
Physicochemical Properties of Media Simulating the Environment in the Upper Small Intestine
In the preprandial state, no major changes in aspirate properties are expected to occur with time (25). Therefore, one representative medium for the fasted intestine (FaSSIF-V2) suffices for biorelevant dissolution testing. In contrast, several “snapshot” dissolution media were needed to reflect changes in composition during digestion in the upper small intestine. The pH values and osmolalities of the three “snapshot” media are superimposed on the pH and osmolality profiles measured in human duodenal aspirates in Fig. 2, with analogous data for bile salts, monoglycerides and free fatty acids shown in Fig. 3. To achieve constant and reproducible compositions, only certain products of lipolysis, i.e. monoglycerides and free fatty acids, were added to the dissolution media. Since glycerides based on oleate are the single most prevalent group of lipids in the Western diet and in BA/BE studies (7), oleate-based components (GMO and sodium oleate) were chosen to reflect lipolysis products in the media. Tri- and diglycerides were not included as they would form emulsions, making analysis unnecessarily difficult. Current evidence suggests that drugs are absorbed from the aqueous/micellar phase of the intestinal contents (27,40). Since triglyceride and diglyceride levels in the aqueous/micellar phase are severely limited by their poor solubility (41), they are unlikely to contribute substantially to drug solubility in this phase.
Median in vivo profiles of A pH and B osmolality in the postprandial upper small intestine (25) and the corresponding values of the “snapshot” media (represented by horizontal bars).
Median in vivo profiles of A bile salts, B monoglycerides and C free fatty acids in the postprandial upper small intestine (25) and the corresponding values of the “snapshot” media (represented by horizontal bars).
The pH in the distal duodenum decreases from 6.6 to 5.2 over the first 210 min following administration of Ensure® Plus (25). This trend is reflected by the pH values of the three intestinal “snapshot” media. Note that the pH value of the “late” medium represents a balance between the average pH value at 210 min after meal intake and the value in the fasted state, since the low pH at the end of the digestive phase is short-lived (25).
The buffer capacity in the duodenum changed little postprandially (25). The biorelevant media were therefore designed to have a buffer capacity of 25 mmol l−1 ΔpH−1 in the “early” and the “middle” phases of the small intestinal digestion. Similar to pH, the buffer capacity of the “late” medium was designed to be a balance between the late digestive phase and the fasted state conditions and was thus set at 15 mmol l−1 ΔpH−1 [the buffer capacity in the fasted duodenum is only 5.6 mmol l−1 ΔpH−1 (25)].
Osmolality in the distal duodenum increases slightly during the first 120 min after meal intake, after which it gradually re-equilibrates to approximately iso-osmolal (290 mOsm kg−1). Osmolalities of the media reflect these trends.
In contrast to FeSSIF, which has a surface tension of 46.3 ± 0.1 mN/m (39), the “early” and “middle” media have surface tensions of approximately 30 mN/m (Table V), which is closer to the surface tension of the aspirates (25), due to the presence of GMO and high concentrations of sodium oleate. For the “late” medium, surface tension is higher than the value observed in the corresponding aspirates, due to the much lower concentration (0.8 mM) of sodium oleate. Adding CaCl2 and pancreatin to the media decreases the surface tension (Table V). Indeed, the formation of soaps between one divalent calcium ion and two fatty acids is expected to result in a lower surface tension (40). Moreover, it has been observed that surface tension can be significantly decreased by the addition of enzyme to water and buffer solutions (42).
The benefits of working with a one-phase system in terms of reproducibility and ease of analyte separation from the medium outweigh by far the drawback of the slightly higher surface tension (40 mN/m vs. aspirate value of 30 mN/m).
Stability of “Snapshot” Media
In Fig. 4, the physical appearance of the three gastric “snapshot” media is illustrated after 72 h storage at room temperature. In Figs. 5 and 6, the physical appearance of the various media simulating the upper small intestine immediately after preparation and after 72 h storage at room temperature is illustrated.
Table VI summarizes the physicochemical parameters of the media simulating the fed gastric and upper small intestinal conditions observed initially, after being subjected to usual dissolution test conditions and after storage under ambient conditions.
Gastric Media
After 72 h under ambient conditions, phase separation was observed in the “late” gastric medium (Fig. 4). A supernatant phase free of milk solids was formed and its volume increased with storage time, due to continuing agglomeration of milk in the buffer. However, after re-mixing of the medium by shaking, the physicochemical properties of the medium were not different from the initial values. The “early” and “middle” media showed more satisfactory stability. Of these two, the “middle” medium (FeSSGF) demonstrated the best results, in terms of both visual observation and physicochemical characterizations.
Under the standard dissolution test conditions, paddle method, 75 rpm, at 37°C, all three media were stable in terms of pH, buffer capacity and osmolality for at least 8 h. After being subjected to dissolution test conditions for 72 h the osmolality values of all three “snapshot” media increased and the “early” medium showed a deviation of pH from the initial values. Some precipitation of the proteins was also observed.
Small Intestinal Media
Owing to the high concentrations of oleate used in the “early” and “middle” snapshot media, milky emulsions were obtained. As sodium oleate is poorly soluble at pH < 6, a lower concentration of 0.8 mM (near its solubility in this pH range) had to be used to prepare FeSSIF-V2 and the “late” medium. In both cases a slightly hazy, micellar solution resulted. After 72 h under ambient conditions, all intestinal media showed acceptable stability in terms of pH, osmolality and buffer capacity. Only the “middle” FeSSIF showed phase separation of the lipid components, most likely because levels of oleate in the medium exceed its aqueous solubility at pH 5.8, leading to formation of a poorly stable emulsion at this pH (Fig. 6). If “middle” FeSSIF is prepared at elevated pH of 6.5, no phase separation occurs (Fig. 6). FeSSIF-V2, a composite of the three intestinal “snapshot” media, is recommended for general appraisal of dosage form performance in the fed state, since it is single-phase, easy to prepare and facilitates analyte quantification. Due to the low concentration of sodium oleate, FeSSIF-V2 is stable even at pH values lower than 5 and due to its only slightly hazy appearance, visual inspection of the process during dissolution testing is possible.
Stability of all intestinal media under usual dissolution test conditions (paddle method, 75 rpm, at 37°C) was observed with respect to pH, buffer capacity, osmolality and appearance for at least 8 h.
Drug Analysis in Fed “Snapshot” Media
Due to the complexity of the biorelevant media compositions, selection of appropriate drug separation and analytical methods are necessary to obtain meaningful results.
Designing an analytical procedure to reproducibly and accurately quantify the amounts of drug released in milk-based media is particularly difficult. Drugs can distribute into several phases of milk: they can bind to the milk proteins or dissolve in the oily or the aqueous phase of the emulsion. The centrifugation and protein precipitation method, as proposed by Fotaki et al. (16), appears to be suitable for most analyses in FeSSGF, since it requires neither special equipment nor a high quantity of organic solvent. In our laboratories, undissolved drug could be separated from the early- and middle gastric “snapshot” media using the centrifugation method (43). Proteins remained stable and did not precipitate during the sample preparation steps.
For the “late” gastric medium, the preprandial gastric medium, FaSSGF, the fasted intestinal medium, FaSSIF-V2, and the fed intestinal medium, FeSSIF-V2, a simple filtration step is generally sufficient to separate undissolved drug from the medium. However, in the special case of lipid-based formulations, centrifugation is required in all media to remove the non-emulsified lipid droplets in order to assay the aqueous phase.
In addition to the aforementioned details, some general concerns regarding drug analysis have to be kept in mind. These include possible changes in drug solubility with change in temperature during sample handling (the need to pre-warm all sampling and filtration devices), and, where applicable, stopping the activity of pancreatin in samples.
General Comments
pH
pH effects on release from drug products are well-documented in the literature. Among the myriad of examples, a few that come to mind include release of weakly acidic and basic drugs from immediate release dosage forms (44–46), release from enteric coated products (47–49) and from slow release dosage forms (50,51). Especially for drugs and excipients that have a pKa value within the physiological range, it is most important to adjust the pH of the test medium to an appropriate value.
Buffer Capacity
Buffer capacity is especially important to the performance of ionizable compounds and excipients. Mooney et al. (52,53) analyzed the dissolution of ionizable drugs mathematically and concluded that the buffer capacity of the media can have a large impact on drug dissolution. On the excipient side, enteric coating performance depends on buffer capacity as well as pH (54). Further, free fatty acids resulting from digestion of lipid dosage forms can induce a pH shift in the media (55). Shifts in pH can in turn affect both the solubility and dissolution of drugs and excipients as well as the activity of any enzymes present in the medium. To achieve representative pH and buffer capacity, suitable buffers must be selected for media preparation.
Although bicarbonate is the most prevalent buffer in the upper GI tract in the fasted state, it is difficult to reproduce in the laboratory at a constant buffer capacity (56). Therefore buffers were selected primarily according to their ability to achieve the desired combination of pH, osmolality and buffer capacity, with less importance placed on selecting buffers according to their physiological relevance. In the fed state, buffers present in the meal will dominate those secreted by the gastric mucosa, and will therefore vary with the composition of the meal. During digestion in the stomach the buffer capacity remains relatively constant (25). To ensure a consistent buffer capacity at each of the “snapshot” media pH values, different buffers had to be used.
Medium Volume
Media compositions are all tabulated on the basis of a volume of one liter, to facilitate comparison of the compositions as well as the calculations for media preparation. The actual media volume used would preferably be tailored to the physiology being simulated, taking into account the constraints of the test apparatus available. Physiological factors to be considered include pre- vs. postprandial state and the region in the GI tract. The volumes of GI fluids in different states in the GI tract have been summarized in several publications, e.g. (1,57,58). For example, the volume of fluids in the fasted stomach is usually only on the order of 30–50 ml. Adding a contribution from the co-administered fluid (250 ml), one reaches a total volume of about 300 ml. In the fed stomach a volume of 500 ml or more would be more suitable. To simulate fasted conditions in the small intestine, a volume of up to 200 ml would be reasonably consistent with the values reported in the literature. By contrast, volumes of up to one liter would be appropriate for the fed state small intestine.
When a small medium volume is appropriate, e.g. 200–250 ml for the fasted stomach, a test apparatus which can function properly with a small volume should be chosen e.g. USP Apparatus 3 (reciprocating cylinder, Bio-Dis) or the mini-paddle apparatus. A practical alternative for a laboratory without these specialized apparatus would be to use a slightly larger volume (e.g. 300–500 ml in a standard USP 2 apparatus), in the knowledge that the percent dissolved might be overestimated with this combination. For larger volumes e.g. 500–1,000 ml standard compendial apparatus can accommodate the volume easily.
Osmolality
As both osmolality and ionic strength can have an impact on the release of drugs as well as on excipient performance, choice of these parameters should also be based on physiological values. For example, Li et al. (59) investigated the dissolution rate of different haloperidol salts as a function of chloride ion concentration and concluded that conversion of the mesylate and phosphate salts to the chloride salt slowed dissolution, with a common ion effect observed at higher chloride ion concentrations. Other studies have shown that ionic strength influences water uptake and the swelling properties of coated beads prepared by cationic polymer dispersions [Eudragit® RS and Eudragit® RL 30D (60), and Eudragit® RS 30D (61)] and that these effects can be correlated with drug release from the beads. For example, Rudolph et al. (62) demonstrated delayed dissolution of 5-aminosalicylic acid from Eudragit® L coated tablets at higher osmolality. Similarly, the solubility and swelling of lambda carrageenan–drug complex (63) are affected by the ionic strength and osmolality of the media, leading to faster dissolution rate of the drug.
Bile Secretions
Drug solubility and dissolution can be considerably enhanced in the mixed micelles formed by bile secretions in the small intestine, and, in the fed state, the products of lipolysis additionally come into play (19,27,64–67). The effects are especially apparent for poorly soluble lipophilic drugs (68). In addition to solubilization, an improvement in wetting characteristics has been demonstrated (42,69,70). Bile salts and lecithin are major components of the currently used media simulating the conditions in the small intestine. Several studies have shown that dissolution results in these media can be used to predict in vivo performance of poorly soluble, lipophilic drugs (11,23,64). As a result of enhanced solubility and dissolution, bioavailability of poorly soluble drugs is improved when administered with meals (20,68,71,72). This is partly attributable to the effects of the fat content in the meal and partly due to the products of fat digestion, principally monoglycerides and fatty acids, on drug solubility and dissolution. The updated media therefore incorporate these components. The concentrations of oleate in the media were based on concentrations observed in vivo for pooled free fatty acids and glyceryl monoesters.
Enzymes and Lipid-Based Formulations
Digestion can be very important to the dissolution of lipid-based drug products. Pancreatic lipase, the main enzyme responsible for lipid digestion, can optionally be included in the composition of the medium. Armand et al. (73–75) reported that triglyceride hydrolysis occurs mainly in the duodenum, through synergistic actions of gastric and colipase-dependent pancreatic lipases with bile at a pH of around 6.0, leading to the formation of free fatty acids and 2-monoglycerides. Comparing literature data on pancreatin levels (73,76–78), expressed as USP lipase units, three sources (73,77,78) reported lipase levels ranging from about 500–1,000 U/ml in the fed state. Lipid concentrations in the micellar phase generally do not exceed more than about 70 mM postprandially (Fig. 3). This 70 mM would, in fact, require a maximum of only 70 U/ml of lipase to effect complete lipolysis (corresponding to 35,000–70,000 U in total if an intralumenal fluid volume of 500 to 1,000 ml is assumed).
For a lipid formulation in a capsule, a volume of about 1 ml of triglycerides can be assumed, which would require a total of about 1,000 U of lipase for digestion. Assuming a media volume of 500 to 1,000 ml, this would correspond to 1–2 U/ml. Thus, lipase levels in the normal human small intestine exceed by far the concentration actually required to complete lipolysis of a lipid dosage form. For the purposes of dissolution testing of lipid formulations, it is only necessary to add enough pancreatin (about 100 lipase units per milliliter) to digest the modest concentrations of lipid in the medium and in the dosage form to be tested.
Calcium was included into the medium composition to facilitate the activity of pancreatic lipase. The amounts of calcium used in this study (5 mM) are just sufficient to saponify both the fatty acid component in the medium per se as well as the fatty acids liberated from the lipid dosage forms during digestion, thereby preventing any inhibitory effects of fatty acids on pancreatic lipase activity.
Pouton has introduced a lipid formulation classification system in combination with the in vitro tests for these formulations (79). The tests emphasize the possibility of drug precipitation that may occur after the dispersion of dosage forms in the GI tract (79). For lipid formulations, dispersibility and lipolysis of the formulation itself can have a great impact on drug release. Recently an in vitro lipolysis model has been proposed and applied to simulate dynamic lipid digestion in the GI tract (80–83). Nevertheless, more evaluation will be required to determine the scope of application of the lipolysis model.
Use of “Snapshot” Media
Assessment of dissolution in the fed state can be carried out using “snapshot” media if this is necessary to answer specific questions. For example, during the extended residence of a monolithic controlled release dosage form in the fed stomach, how will the release be affected by the changing composition of the gastric fluids? Successively exposing the dosage form to the “early”, “middle” and “late” gastric media in the Bio-Dis or flow-through tester would provide a means of answering this question. Similarly, the gastric and intestinal “snapshot” media can be combined to predict changes in dosage form performance related to whether the dosage form empties from the stomach “early”, in the “middle” or “late” in the gastric emptying process. The advantage of using the “snapshot” media for these purposes is their physical stability over an extended period of time.
CONCLUDING REMARKS
Updated compositions of biorelevant dissolution media, based on physiological parameters recently summarized in the literature, are presented. FeSSGF and FeSSIF-V2 are recommended for the prediction of drug dissolution in the postprandial stomach and upper small intestine, respectively. Similarly, FaSSGF and FaSSIF-V2 are recommended for experiments reflecting the fasted state. The media reflect the influence of digestion processes better than media typically used for quality control purposes and previous biorelevant media. For more specific questions about performance of dosage forms in the fed state, the “snapshot” media, representing conditions in the “early”, “middle” and “late” phases of digestion, can be used. All media were found to be stable for at least 72 h under ambient storage conditions and at least 8 h under usual in vitro dissolution testing conditions, making them practical in most laboratories where formulation development is the key task.
References
J. B. Dressman, G. L. Amidon, C. Reppas, and V. P. Shah. Dissolution testing as a prognostic tool for oral drug absorption: immediate release dosage forms. Pharm. Res. 15:11–22 (1998).
The United States Pharmacopeia. USP 29. United States Pharmacopeial Convention Inc., Rockville, MD (2006).
E. Galia, J. Horton, and J. B. Dressman. Albendazole generics—a comparative in vitro study. Pharm. Res. 16:1871–1875 (1999).
M. Vertzoni, E. Pastelli, D. Psachoulias, L. Kalantzi, and C. Reppas. Estimation of intragastric solubility of drugs: in what medium? Pharm. Res. 24:909–917 (2007).
M. Vertzoni, J. Dressman, J. Butler, J. Hempenstall, and C. Reppas. Simulation of fasting gastric conditions and its importance for the in vivo dissolution of lipophilic compounds. Eur. J. Pharm. Biopharm. 60:413–417 (2005).
J. Krämer. Korrelation biopharmazeutischer in vivo und in vitro Daten von Theophyllin und Verapamil Retardpräparaten. Doctoral thesis, Ruprecht-Karls-University, Heidelberg, Germany, 1995.
S. Klein, J. Butler, J. M. Hempenstall, C. Reppas, and J. B. Dressman. Media to simulate the postprandial stomach I. Matching the physicochemical characteristics of standard breakfasts. J. Pharm. Pharmacol. 56:605–610 (2004).
P. Macheras, M. Koupparis, and C. Tsaprounis. Drug dissolution studies in milk using the automated flow injection serial dynamic dialysis technique. Int. J. Pharm. 33:125–136 (1986).
P. E. Macheras, M. A. Koupparis, and S. G. Antimisiaris. Drug binding and solubility in milk. Pharm. Res. 7:537–541 (1990).
E. Galia, E. Nicolaides, D. Hörter, R. Löbenberg, C. Reppas, and J. B. Dressman. Evaluation of various dissolution media for predicting in vivo performance of class I and II drugs. Pharm. Res. 15:698–705 (1998).
E. Nicolaides, E. Galia, C. Efthymiopoulos, J. B. Dressman, and C. Reppas. Forecasting the in vivo performance of four low solubility drugs from their in vitro dissolution data. Pharm. Res. 16:1876–1882 (1999).
G. Buckton, A. E. Beezer, S. M. Chatham, and K. K. Patel. In vitro dissolution testing of oral controlled release preparations in the presence of artificial foodstuffs. II. Probing drug food interactions using microcalorimetry. Int. J. Pharm. 56:151–157 (1989).
P. E. Macheras, M. A. Koupparis, and S. G. Antimisiaris. Effect of temperature and fat content on the solubility of hydrochlorothiazide and chlorothiazide in milk. J. Pharm. Sci. 78:933–936 (1989).
P. E. Macheras, M. A. Koupparis, and S. G. Antimisiaris. Effect of temperature and fat content on the binding of hydrochlorothiazide and chlorothiazide to milk. J. Pharm. Sci. 77:334–336 (1988).
S. Anwar, J. T. Fell, and P. A. Dickinson. An investigation of the disintegration of tablets in biorelevant media. Int. J. Pharm. 290:121–127 (2005).
N. Fotaki, M. Symillides, and C. Reppas. Canine versus in vitro data for predicting input profiles of l-sulpiride after oral administration. Eur. J. Pharm. Sci. 26:324–333 (2005).
V. A. Gray, and J. B. Dressman. Change of pH requirements for simulated intestinal fluid TS. Pharmacop. Forum. 22:1943–1945 (1996).
J. B. Dressman, and C. Reppas. In vitro-in vivo correlations for lipophilic, poorly water-soluble drugs. Eur. J. Pharm. Sci. 11(2):S73–S80 (2000).
H. Wei, and R. Löbenberg. Biorelevant dissolution media as a predictive tool for glyburide a class II drug. Eur. J. Pharm. Sci. 29:45–52 (2006).
V. H. Sunesen, B. L. Pedersen, H. G. Kristensen, and A. Müllertz. In vivo in vitro correlations for a poorly soluble drug, danazol, using the flow-through dissolution method with biorelevant dissolution media. Eur. J. Pharm. Sci. 24:305–313 (2005).
J. Parojcic, Z. Ethuric, M. Jovanovic, S. Ibric, and D. Jovanovic. Influence of dissolution media composition on drug release and in vitro/in vivo correlation for paracetamol matrix tablets prepared with novel carbomer polymers. J. Pharm. Pharmacol. 56:735–741 (2004).
K. Schamp, S. A. Schreder, and J. Dressman. Development of an in vitro/in vivo correlation for lipid formulations of EMD 50733, a poorly soluble, lipophilic drug substance. Eur. J. Pharm. Biopharm. 62:227–234 (2006).
E. Nicolaides, M. Symillides, J. B. Dressman, and C. Reppas. Biorelevant dissolution testing to predict the plasma profile of lipophilic drugs after oral administration. Pharm. Res. 18:380–388 (2001).
M. Vertzoni, N. Fotaki, E. Kostewicz, E. Stippler, C. Leuner, E. Nicolaides, J. Dressman, and C. Reppas. Dissolution media simulating the intralumenal composition of the small intestine: physiological issues and practical aspects. J. Pharm. Pharmacol. 56:453–462 (2004).
L. Kalantzi, K. Goumas, V. Kalioras, B. Abrahamsson, J. B. Dressman, and C. Reppas. Characterization of the human upper gastrointestinal contents under conditions simulating bioavailability/bioequivalence studies. Pharm. Res. 23:165–176 (2006).
M. Grove, G. P. Pedersen, J. L. Nielsen, and A. Müllertz. Bioavailability of seocalcitol I: relating solubility in biorelevant media with oral bioavailability in rats-effect of medium and long chain triglycerides. J. Pharm. Sci. 94:1830–1838 (2005).
C. J. Porter, N. L. Trevaskis, and W. N. Charman. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat. Rev. Drug Discov. 6:231–248 (2007).
A. G. Hills. pH and Henderson–Hasselbalch equation. Am. J. Med. 55:131–133 (1973).
D. D. Van Slyke. On the measurement of buffer values on the relationship of buffer values to the dissociation constant of the buffer and the concentration and reaction of the buffer solution. J. Biol. Chem. 52:525–570 (1922).
R. Dolder. Ophthalmika. Wissenschaftliche Velagsgesellschaft mbH Stuttgart (1990).
US FDA, US Department of Health and Human Services, Center for Drug Evaluation and Research, Guidance for Industry: Food-Effect Bioavailability and Fed Bioequivalence Studies, December 2002.
J. B. Dressman, R. R. Berardi, L. C. Dermentzoglou, T. L. Russell, S. P. Schmaltz, J. L. Barnett, and K. M. Jarvenpaa. Upper gastrointestinal (GI) pH in young, healthy men and women. Pharm. Res. 7:756–761 (1990).
T. L. Russell, R. R. Berardi, J. L. Barnett, L. C. Dermentzoglou, K. M. Jarvenpaa, S. P. Schmaltz, and J. B. Dressman. Upper gastrointestinal pH in seventy-nine healthy, elderly, North American men and women. Pharm. Res. 10:187–196 (1993).
L. Kalantzi, E. Persson, B. Polentarutti, B. Abrahamsson, K. Goumas, J. B. Dressman, and C. Reppas. Canine intestinal contents vs. simulated media for the assessment of solubility of two weak bases in the human small intestinal contents. Pharm. Res. 23:1373–1381 (2006).
A. Albert, and E. Sargent. Ionization constants of acids and bases (Russian translation). Khimiya, Moscow, p. 139, 1964.
H. P. Fiedler. Lexikon der Hilfsstoffe, OVR Oberschwäbische Verlagsanstalt Ravensburg. Ravensburg, Germany, 1989.
S. Budavari. The Merck index 12. Merck Research Laboratories, Whitehouse Station, NJ, 1996.
J. Pellicer, V. García-Morales, and M. J. Hernández. On the demonstration of the Young–Laplace equation in introductory physics courses. Phys. Educ. 35:126–129 (2000).
E. Galia. Physiologically based dissolution tests. Doctoral thesis, Johann Wolfgang Goethe University, Frankfurt am Main, Germany, 1999.
K. J. MacGregor, J. K. Embleton, J. E. Lacy, E. A. Perry, L. J. Solomon, H. Seager, and C. W. Pouton. Influence of lipolysis on drug absorption from the gastro-intestinal tract. Adv. Drug Deliv. Rev. 25:33–46 (1997).
O. Hernell, J. E. Staggers, and M. C. Carey. Physical-chemical behavior of dietary and biliary lipids during intestinal digestion and absorption. 2. Phase analysis and aggregation states of luminal lipids during duodenal fat digestion in healthy adult human beings. Biochemistry. 29:2041–2056 (1990).
P. E. Luner. Wetting properties of bile salt solutions and dissolution media. J. Pharm. Sci. 89:382–395 (2000).
E. Jantratid, N. Janssen, H. Chokshi, K. Tang, and J. B. Dressman. Designing biorelevant dissolution tests for lipid formulations: case example—lipid suspension of RZ-50. Eur. J. Pharm. Biopharm. in press (2008), DOI 10.1016/j.ejpb.2007.12.010.
J. J. Sheng, N. A. Kasim, R. Chandrasekharan, and G. L. Amidon. Solubilization and dissolution of insoluble weak acid, ketoprofen: effects of pH combined with surfactant. Eur. J. Pharm. Sci. 29:306–314 (2006).
J. Jinno, D. Oh, J. R. Crison, and G. L. Amidon. Dissolution of ionizable water-insoluble drugs: the combined effect of pH and surfactant. J. Pharm. Sci. 89:268–274 (2000).
S. Li, S. Wong, S. Sethia, H. Almoazen, Y. M. Joshi, and A. T. Serajuddin. Investigation of solubility and dissolution of a free base and two different salt forms as a function of pH. Pharm. Res. 22:628–635 (2005).
C. Wu, and J. W. McGinity. Influence of an enteric polymer on drug release rates of theophylline from pellets coated with Eudragit RS 30D. Pharm. Dev. Technol. 8:103–110 (2003).
E. T. Cole, R. A. Scott, A. L. Connor, I. R. Wilding, H. U. Petereit, C. Schminke, T. Beckert, and D. Cade. Enteric coated HPMC capsules designed to achieve intestinal targeting. Int. J. Pharm. 231:83–95 (2002).
O. S. Silva, C. R. Souza, W. P. Oliveira, and S. C. Rocha. In vitro dissolution studies of sodium diclofenac granules coated with Eudragit L-30D-55 by fluidized-bed system. Drug Dev. Ind. Pharm. 32:661–667 (2006).
A. Trapani, V. Laquintana, N. Denora, A. Lopedota, A. Cutrignelli, M. Franco, G. Trapani, and G. Liso. Eudragit RS 100 microparticles containing 2-hydroxypropyl-beta-cyclodextrin and glutathione: physicochemical characterization, drug release and transport studies. Eur. J. Pharm. Sci. 30:64–74 (2007).
T. Phaechamud, and G. C. Ritthidej. Sustained-release from layered matrix system comprising chitosan and xanthan gum. Drug Dev. Ind. Pharm. 33:595–605 (2007).
K. G. Mooney, M. A. Mintun, K. J. Himmelstein, and V. J. Stella. Dissolution kinetics of carboxylic acids I: effect of pH under unbuffered conditions. J. Pharm. Sci. 70:13–22 (1981).
K. G. Mooney, M. A. Mintun, K. J. Himmelstein, and V. J. Stella. Dissolution kinetics of carboxylic acids II: effect of buffers. J. Pharm. Sci. 70:22–32 (1981).
S. S. Ozturk, B. O. Palsson, B. Donohoe, and J. B. Dressman. Kinetics of release from enteric-coated tablets. Pharm. Res. 5:550–565 (1988).
C. J. Porter, and W. N. Charman. In vitro assessment of oral lipid based formulations. Adv. Drug Deliv. Rev. 50(1):S127–S147 (2001).
J. E. Boni, R. S. Brickl, and J. Dressman. Is bicarbonate buffer suitable as a dissolution medium? J. Pharm. Pharmacol. 59:1375–1382 (2007).
P. G. Welling. Effects of food on drug absorption. Annu. Rev. Nutr. 16:383–415 (1996).
J. M. Custodio, C.-Y. Wu, and L. Z. Benet. Predicting drug disposition, absorption/elimination/transporter interplay and the role of food on drug absorption. Adv. Drug Deliv. Rev. 60:717–733 (2008).
S. Li, P. Doyle, S. Metz, A. E. Royce, and A. T. Serajuddin. Effect of chloride ion on dissolution of different salt forms of haloperidol, a model basic drug. J. Pharm. Sci. 94:2224–2231 (2005).
R. Bodmeier, X. Guo, R. E. Sarabia, and P. F. Skultety. The influence of buffer species and strength on diltiazem HCl release from beads coated with the aqueous cationic polymer dispersions, Eudragit RS, RL 30D. Pharm. Res. 13:52–56 (1996).
K. Knop. Influence of buffer solution composition on drug release from pellets coated with neutral and quaternary acrylic polymers and on swelling of free polymer films. Eur. J. Pharm. Sci. 4:293–300 (1996).
M. W. Rudolph, S. Klein, T. E. Beckert, H. Petereit, and J. B. Dressman. A new 5-aminosalicylic acid multi-unit dosage form for the therapy of ulcerative colitis. Eur. J. Pharm. Biopharm. 51:183–190 (2001).
M. C. Bonferoni, S. Rossi, F. Ferrari, E. Stavik, A. Pena-Romero, and C. Caramella. Factorial analysis of the influence of dissolution medium on drug release from carrageenan–diltiazem complexes. AAPS PharmSciTech. 1:E15 (2000).
E. S. Kostewicz, U. Brauns, R. Becker, and J. B. Dressman. Forecasting the oral absorption behavior of poorly soluble weak bases using solubility and dissolution studies in biorelevant media. Pharm. Res. 19:345–349 (2002).
X. Cai, D. J. Grant, and T. S. Wiedmann. Analysis of the solubilization of steroids by bile salt micelles. J. Pharm. Sci. 86:372–377 (1997).
S. D. Mithani, V. Bakatselou, C. N. TenHoor, and J. B. Dressman. Estimation of the increase in solubility of drugs as a function of bile salt concentration. Pharm. Res. 13:163–167 (1996).
M. Rosoff, and A. T. M. Serajuddin. Solubilization of diazepam in bile-salts and in sodium cholate–lecithin–water phases. Int. J. Pharm. 6:137–146 (1980).
H. M. Jones, N. Parrott, G. Ohlenbusch, and T. Lave. Predicting pharmacokinetic food effects using biorelevant solubility media and physiologically based modelling. Clin. Pharmacokinet. 45:1213–1226 (2006).
P. E. Luner, and D. Vander Kamp. Wetting behavior of bile salt-lipid dispersions and dissolution media patterned after intestinal fluids. J. Pharm. Sci. 90:348–359 (2001).
P. E. Luner, and D. VanDer Kamp. Wetting characteristics of media emulating gastric fluids. Int. J. Pharm. 212:81–91 (2001).
D. Fleisher, C. Li, Y. Zhou, L. H. Pao, and A. Karim. Drug, meal and formulation interactions influencing drug absorption after oral administration. Clinical implications. Clin. Pharmacokinet. 36:233–254 (1999).
W. N. Charman, C. J. Porter, S. Mithani, and J. B. Dressman. Physicochemical and physiological mechanisms for the effects of food on drug absorption: the role of lipids and pH. J. Pharm. Sci. 86:269–282 (1997).
M. Armand, P. Borel, B. Pasquier, C. Dubois, M. Senft, M. Andre, J. Peyrot, J. Salducci, and D. Lairon. Physicochemical characteristics of emulsions during fat digestion in human stomach and duodenum. Am. J. Physiol. 271:G172–G183 (1996).
M. Armand, B. Pasquier, M. Andre, P. Borel, M. Senft, J. Peyrot, J. Salducci, H. Portugal, V. Jaussan, and D. Lairon. Digestion and absorption of 2 fat emulsions with different droplet sizes in the human digestive tract. Am. J. Clin. Nutr. 70:1096–1106 (1999).
M. Armand. Lipases and lipolysis in the human digestive tract: where do we stand? Curr. Opin. Clin. Nutr. Metab. Care. 10:156–164 (2007).
K. Gyr, N. M. Agrawal, O. Felsenfeld, and R. G. Font. Comparative study of secretin and Lundh tests. Am. J. Dig. Dis. 20:506–512 (1975).
B. Lurie, B. Brom, S. Bank, B. Novis, and I. N. Marks. Comparative response of exocrine pancreatic secretion following a test meal and secretin-pancreozymin stimulation. Scand. J. Gastroenterol. 8:27–32 (1973).
F. Carriere, C. Renou, V. Lopez, J. De Caro, F. Ferrato, H. Lengsfeld, A. De Caro, R. Laugier, and R. Verger. The specific activities of human digestive lipases measured from the in vivo and in vitro lipolysis of test meals. Gastroenterology. 119:949–960 (2000).
C. W. Pouton. Formulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification system. Eur. J. Pharm. Sci. 29:278–287 (2006).
N. H. Zangenberg, A. Müllertz, H. G. Kristensen, and L. Hovgaard. A dynamic in vitro lipolysis model. I. Controlling the rate of lipolysis by continuous addition of calcium. Eur. J. Pharm. Sci. 14:115–122 (2001).
N. H. Zangenberg, A. Müllertz, H. G. Kristensen, and L. Hovgaard. A dynamic in vitro lipolysis model. II: Evaluation of the model. Eur. J. Pharm. Sci. 14:237–244 (2001).
A. M. Kaukonen, B. J. Boyd, C. J. Porter, and W. N. Charman. Drug solubilization behavior during in vitro digestion of simple triglyceride lipid solution formulations. Pharm. Res. 21:245–253 (2004).
A. M. Kaukonen, B. J. Boyd, W. N. Charman, and C. J. Porter. Drug solubilization behavior during in vitro digestion of suspension formulations of poorly water-soluble drugs in triglyceride lipids. Pharm. Res. 21:254–260 (2004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jantratid, E., Janssen, N., Reppas, C. et al. Dissolution Media Simulating Conditions in the Proximal Human Gastrointestinal Tract: An Update. Pharm Res 25, 1663–1676 (2008). https://doi.org/10.1007/s11095-008-9569-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-008-9569-4