ABSTRACT
Purpose
To evaluate precipitation in and supersaturation of intestinal contents after administration of pharmacologically relevant doses of dipyridamole and ketoconazole to 12 healthy adults.
Methods
On two separate days each subject was administered in stomach 240 ml aqueous solutions of two dipyridamole doses (30/90 mg) and two ketoconazole doses (100/300 mg). Physicochemical characteristics, total drug content, and drug concentration were measured in individual intestinal contents (≤7 ml) aspirated at specific times post-dosing. Drug concentration after incubation (37°C/48 h) and equilibrium solubility were measured. Precipitate crystallinity was evaluated by x-ray powder diffraction.
Results
Precipitated fraction was minimal (dipyridamole, ≤7%) or limited (ketoconazole, ≤16%). Ketoconazole precipitates were mostly amorphous. Depending on dose, intestinal contents with pH > 3.6 were supersaturated with dipyridamole up to 10 and 30 min and with ketoconazole up to 30 and 50 min post-administration. Intestinal contents with pH > 5 and concentration of micellar components <5 mM were supersaturated with ketoconazole or dipyridamole, but precipitated fraction was significant only for ketoconazole. After incubation, crystalline precipitates were found in almost all samples. Slow precipitation of base and/or precipitation of other phases account for this observation.
Conclusions
Intralumenal precipitation of weakly alkaline, lipophilic, high permeability drugs may not be substantial. Estimating intestinal supersaturation in regard to free base is inadequate as other phases may precipitate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Due to their ionization characteristics, bases dissolve more easily at acidic pHs. Therefore, after oral administration in the fasted state, concentrations in the gastric contents may exceed solubility in the contents of the upper intestinal lumen, and a fraction may precipitate upon entering the duodenum. Solubility varies with the compound, the composition of the medium, and the method of measurement. In most cases, it refers to the equilibrium solubility, i.e. to the concentration of the drug in a saturated solution when excess of the most stable crystalline form of the drug is present. Precipitated fraction, π (0 ≤ π ≤ 1), can be estimated by the following equation:
where C is the concentration of the drug and C t is the total amount of drug (dissolved and precipitated) per unit volume.
However, the rate of particle growth is compound- (1) and medium- (2) dependent. In vitro data in simple aqueous media, simulated intestinal fluids, and human intestinal fluids suggest that it may be comparable with the upper intestinal transit rates (1,2). Therefore, after administration in the fasted state, contents of upper small intestine may be supersaturated with the base, and the flux across the intestinal mucosa may be higher than expected from equilibrium solubility considerations. The degree of supersaturation can be expressed by the relative supersaturation index, σ (3):
where C and C s are the concentration of the drug in solution and equilibrium solubility of the drug, respectively. According to equation 2, a solution can be unsaturated (σ < 0), saturated (σ = 0), or supersaturated (σ > 0).
As of the end of 2006, 37.9% of active pharmaceutical ingredients (APIs) approved in the U.S.A. after 1981 for oral administration have been weak bases (4). Nevertheless, in vivo data relevant to precipitation are almost non-existent. It has recently been shown that intestinal precipitation of a weak base that is under development by AstraZeneca is less of a limitation for in vivo drug absorption than implied from equilibrium solubility and in vitro test assessments (5). Although that was probably the first study dealing with in vivo precipitation/supersaturation of bases, observations were made indirectly (using plasma data), and, therefore, quantitative assessment of the degree of precipitation/supersaturation in vivo was not possible.
Τhe goal of the present study was to evaluate on a quantitative basis the precipitation in and the supersaturation of contents of the upper small intestine after administration of two lipophilic weak bases to healthy fasted adults. Dipyridamole [2-({6-[bis(2-hydroxyethyl)amino]-4,8-bis(piperidin-1-yl)-[1,3]diazino[5,4-d]pyrimidin-2-yl}(2-hydroxyethyl)amino)ethan-1-ol, clogP 2.7–4.9, pK a 5.7–6.4] and ketoconazole [1-[4-(4-{[(2S,4R)-2-(2,4-dichlorophenyl)-2-(1H-imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy}phenyl)piperazin-1-yl]ethan-1-one, clogP 4.4, pK a 2.9 and 6.5] (6) were used as model compounds.
MATERIALS AND METHODS
Materials
Dipyridamole (DPD) was from Boehringer Ingelheim Espanã, S.A. (Malgrat De Mar, Spain, lot # 08342). Ketoconazole (KCZ) was from Janssen Cork, Janssen Pharmaceutical Ltd. (Little Ireland, Cork, Republic of Ireland, lot # 0712003723). Αcetonitrile and methanol of HPLC grade were from E. Merck (Darmstadt, Germany). Egg phosphatidylcholine (Lipoid E PC® 99.1% pure, lot #105019-1/14) was kindly donated by Lipoid GmbH (Ludwigshafen, Germany). Water of HPLC grade was obtained by using a Labconco® water pro ps. System (Kansas City, USA). All other chemicals were of analytical grade.
Human Study
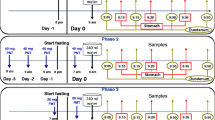
The study was held in the Red Cross Hospital of Athens after receiving approval by the Greek Ethics Committee (IS-111) and by the National (Greek) Organization for Medicines (GR 210181 - EudraCT # 2008-006178-13). It was a cross-over four-phase study. Subjects were administered 240 ml of drug solution to the antrum of their stomach as follows:
-
Phase 1:
A low dose DPD (30 mg) solution was administered (DPD-L).
-
Phase 2:
A high dose DPD (90 mg) solution was administered (DPD-H).
-
Phase 3:
A low dose KCZ (100 mg) solution was administered (KCZ-L).
-
Phase 4:
A high dose KCZ (300 mg) solution was administered (KCZ-H).
For each compound, both low dose and high dose are within the range of pharmacologically relevant single doses (7).
Samples were aspirated from a location near the ligament of Treitz at specific time points and up to 70 min post dosing.
Preparation of Drug Solutions
DPD
Commercially available non-carbonated mineral water (AVRA, Greece) was acidified with HCl so that its pH became 2.7 (Solution A). The DPD-L solution was prepared by dissolving 30 mg DPD in 240 ml of solution A (0.12 mg/ml). The DPD-H solution was prepared by dissolving 90 mg DPD in 240 ml of solution A (0.38 mg/ml). The final pH of both DPD-L and DPD-H solutions was 2.7.
KCZ
Commercially available non-carbonated mineral water was acidified with HCl so that its pH became 2.6 (Solution B) or 2.4 (Solution C). The KCZ-L solution was prepared by dissolving 100 mg KCZ in 240 ml of solution B (0.42 mg/ml). The KCZ-H solution was prepared by dissolving 300 mg KCZ in 240 ml of solution C (1.25 mg/ml). The final pH of both KCZ-L and KCZ-H solutions is 2.7.
Subjects
Twelve healthy adults (10 M/2 F) with a mean age of 26 years (range 20–38 years) gave informed consent and participated in the study. One subject was 15% heavier, and the rest were up to 20% lighter than their ideal body weights. Subjects did not have history or clinical evidence of gastrointestinal (GI) disease, did not participate in another clinical study, and did not receive medicines that influence the function of GI tract up to at least 30 days prior to the study. The health status of each subject was confirmed by physical examination and screening of blood parameters for renal and hepatic functions.
One female subject had been taking birth control pills for more than 3 months prior to her participation in the study, and she took her pill after the end of each experimental day. Both female subjects were not pregnant according to their written declaration and the results of pregnancy test in the morning of each experimental day.
Study Protocol
For each subject, the study was performed on two separate experimental days. Alcohol and any over-the-counter medication were discontinued 3 days prior to and throughout each experimental day, whereas food intake was discontinued for 12 h prior to the start of each experimental day, and water was consumed ad libitum. At about 8 a.m. on the experimental day, the subject arrived at the clinic and was briefly screened for his/her health status by the gastroenterologist.
Experimental Day A
After spraying the back of the mouth with lidocaine, the subject was intubated nasally using a sterile two-lumen duodenal tube (Freka® Trelumina, CH/FR 16/9, 150 cm, Fresenius Kabi Deutschland GmbH, Bad Hombourg, Germany).
No attempt was made to isolate the aspiration segment from the rest of GI contents. The double bore tube was 150 cm long with an external diameter of 5.3 mm and a plastic tip at its distal end. A series of holes 55–65 cm proximal to the tip were used to access the antrum of the stomach. A further series of hand-made holes 13.5–23.5 cm proximal to the tip were used to aspirate samples from the ligament of Treitz. Insertion of the tube was assisted by a guiding wire, and its position was monitored fluoroscopically. After reaching its final position and removing the wire, the subject lay semisupine, and 240 ml of DPD-L solution were administered using 60-ml (capacity) syringes to the antrum. Administration lasted for about 1 min, and samples (up to about 7 ml) were aspirated from the ligament of Treitz at 5, 10, 20, 30, 40, 50, 60 and 70 min after the administration of the solution. Immediately after aspiration, about 8 ml of air was pumped into the tube to clear its contents back into the lumen (total internal volume of this sampling tube was estimated to be about 4 ml). After aspiration of the last sample, the subject was administered 200 ml of non-carbonated mineral water and relaxed by lying semisupine on the bed for 2 h. At the end of the 2-h relaxing period, the subject was administered 240 ml of DPD-H solution, and samples were aspirated as described above. At the end of the experimental day and before removing the tube/discharging the subject, the final position of the tube was confirmed fluoroscopically.
Experimental Day B
Compared with experimental day A, the only difference on day B was that the drug in administered solution was KCZ. KCZ-L was administered first, and KCZ-H was administered second.
The administered DPD and KCZ solutions were measured to be stable at room temperature for at least 24 h. Previous experience with DPD and KCZ indicated that these model compounds are stable in incubated luminal contents (6,8,9).
The overall mean/median sample volume was 5.5/6.4 ml for the DPD experiments and 6.3/7.0 ml for the KCZ experiments; i.e. the total volume of intestinal fluid aspirated from the end of duodenum, during each phase was about 40–45 ml. At the end of each phase, the mean fluid volume in the stomach (4.3 ± 2.0 ml) was not higher than that prior to administration of the drug solution (10 ± 15 ml), whereas bile salts in the stomach after the end of aspirations were minimal (up to 0.05 mM). These data suggest that influx of duodenal contents due to aspirations was minimal (if any).
Median total-aspirated-volume/pH values for the contents from the stomach and from the upper small intestine prior to the first administration of DPD were 6.2 ml/1.7 and 1.2 ml/6.6, respectively, whereas prior to the second administration of DPD they were 9.0 ml/2.6 and 2.0 ml/6.2, respectively. The corresponding numbers prior to the first KCZ administration were 3.3 ml/1.8 and 0.8 ml/7.0, respectively, whereas prior to the second administration of KCZ they were 10.0 ml/2.0 and 2.0 ml/5.5, respectively. Median amount of drug in the stomach prior to the second administration of DPD could not be quantified, whereas prior to the second administration of KCZ it was less than 33 μg. Median drug concentration in the small intestine prior to the second administration of DPD was 2.1 μg/ml and prior to the second administration of KCZ was 4.9 μg/ml. These data indicate that the 2-h period between the two administrations performed at each experimental day was adequate for the solution after the first administration to be practically removed from the upper GI lumen.
Handling of Samples
Upon collection, each sample was immediately divided in two portions: The first was used for measuring total drug content (solid and dissolved drug), C t , in duplicate. The second was transferred in a vial that was filled with the sample and sealed (to minimize loss of bicarbonates during subsequent handling). The vial was immediately centrifuged (37°C, 12560 × g, 10 min) to remove any solid particles and the supernatant was divided in four parts: the first was used for assaying the drug concentration, C, in duplicate; the second was incubated (37°C, 48 h, 75 oscillations/min) and, after centrifugation (37°C, 12560 × g, 10 min), the drug concentration, C inc , was measured; the third was incubated (37°C, 48 h, 75 oscillations/min) in presence of excess of the compound, and, after centrifugation (37°C, 12560 × g, 10 min), equilibrium solubility, C s , was measured; equilibration times using identical experimental conditions have been reported to be much shorter than 48 h for measuring of DPD and KCZ solubility in human intestinal fluids that initially contain no drug (8); and the fourth was used for assaying individual bile salts (taurocholate (TC), glycocholate (GC), taurochenodeoxycholate (TCDC), ursodeoxycholate (USD), glycochenodeoxycholate (GCDC), glycodeoxycholate (GDC) and cholate (C)), lecithin (phospatidylocholine and lyso- phospatidylocholine), cholesterol, free fatty acids (linoleic, palmitic and stearic acids), monoglycerides, and total protein content.
After each centrifugation, as much as possible of the supernatant was carefully poured out of the tube so that any precipitated material remained in the tube and stored at −70°C. After all relevant assays, tubes that contained at least 0.1 mg drug particles were subjected to X-ray powder diffraction (XRPD) analysis.
Samples for measuring C t and C were stored in glass vials at −20°C. Samples for measuring C inc and C s were immediately incubated. Samples for measuring individual bile salts, lecithin, cholesterol, free fatty acids, monoglycerides and protein content were stored at −70°C in separate plastic vials so that each assay was performed immediately after the first thawing.
The superiority of centrifugation (37°C, 12560 × g, 10 min) over filtration through PVDF membrane filters (0.45 μm HV, Durapore® membrane filters, Millipore, Ireland) and through cellulose nitrate filters (0.45 μm, Purabind® 045, Whatman, Germany) in removing solid particles was confirmed with preliminary solubility measurements of DPD and KCZ using canine intestinal fluids collected as described previously (8). Filtrations involved the use of glass syringes and stainless steel filter holders. Both drugs required the application of excessive pressure, whereas adsorption onto the filters was substantial and variable (2.5–37% for DPD/Durapore® data, 4.5–31% for KCZ/Durapore® data and 39–97% for KCZ/Purabind® data). In addition, coefficient of variation of solubility data was 8.2% for DPD/Durapore® data and 0.9% for DPD/centrifugation data, whereas, for KCZ, centrifugation has been shown to lead to smaller CVs than filtration when measuring equilibrium solubility in colonic fluids (9).
Analysis of Samples
Measurement of C t involved the addition of acetonitrile using a volume that ensures complete dissolution of potentially existing drug particles in the vial (1:6 v/v). After vortexing and centrifugation (10°C, 12560 × g, 10 min), the supernatant was diluted with mobile phase, and a sample was injected into the HPLC system. Measurement of C, C inc and C s involved treatment with acetonitrile, centrifugation (10°C, 12560 × g, 10 min), and appropriate dilution of supernatant with mobile phase prior to injection into the HPLC system. DPD and KCZ were assayed with HPLC-UV methods that were based on previously published methods (6). Individual bile salts, lecithin, cholesterol, free fatty acids and monoglycerides were assayed with HPLC-CAD methods (10,11). Total protein content was determined using a commercially available kit (BCATM Protein Assay Kit, Thermo SCIENTIFIC, Rockford, USA).
Crystallinity of solid contents was assessed by XRPD in a PANalytical X’Pert Pro diffractometer Model PW3040/60 equipped with an X’Celerator detector (PANalytical B.V., Almelo, The Netherlands) using a Cu Kα radiation at 40 kV and 45 mA. Samples were scanned from 2.0° to 40.0° (2θ) using a 0.0167° 2θ step size and 31.75 s time count. The sample was prepared by mounting a few milligrams of sample on a Si wafer (zero background) plates, resulting in a thin layer of powder. In order to confirm that the obtained diffractogram referred to the drug and not to some other solid material in the lumen, samples were analyzed on a random basis with FT-IR and NMR.
Data Analysis
Raw data are presented as Box-Whisker plots showing the median value; the 10th, 25th, 75th, and 90th percentiles; and the individual outlying data points. The mean values are indicated with a solid white line. The concentration of micellar components was calculated as the sum of the molar concentration of individual bile salts, lecithin (sum of lyso-phosphatidylocholine and phosphatidylocholine), free fatty acids, monoglycerides, and cholesterol.
Precipitated fractions and relative supersaturation indices in the individual aspirates, π lumen and σ lumen , were estimated by using equations 1 and 2, respectively. Precipitated fractions after incubation of supernatants of individual aspirates for 48 h at 37°C, \( \pi_{{lumen}}^{{inc}} \), were estimated by using equation 1, concentration in lumen as C t and C inc as C. Relative supersaturation indices after incubation of supernatant for 48 h at 37°C, \( \sigma_{{lumen}}^{{inc}} \), were estimated by using equation 2 and C inc as C.
The significance of the precipitated fraction (i.e. assessing whether π > 0) was evaluated with one sample one-tailed t-test or one sample one-tailed Wilcoxon test, depending on the results of normality tests. The significance of the difference of supersaturation index from zero was evaluated with one sample two-tailed t-test or one sample two-tailed Wilcoxon test, depending on the results of normality tests. Type I error was set to 0.05. The normality tests were performed using SigmaStat 3.5 (SPSS Science Inc., New York, USA). The t-tests and the Wilcoxon tests were performed using S-Plus 4.5 (Insightful Corporation, Seattle, USA).
RESULTS
Environment in the Upper Small Intestine after DPD and KCZ Administrations
Within each phase, distribution of pH values in the upper small intestine was skewed as aspirates with low pH were repeatedly collected. Median pH values (Table I) were similar to those previously measured after administration of 250 ml water (12), and, therefore, administration of acidic solutions (necessary to dissolve the dose) did not seem to affect the pH in the upper small intestine substantially. This is in line with the fact that the resulting pH after the addition of 240 ml of AVRA water in 15 ml of pH 1.8 HCl solution (the conditions that generally exist in the fasted stomach) is 3.0, not much different from pH 2.6 that results after mixing 240 ml of pH 2.7 HCl solution (administered in this study) with 15 ml of pH 1.8 HCl solution.
Distribution of total bile salts levels was also skewed as high total bile salt levels were repeatedly observed (Table I). Mean total bile salts were somewhat higher than those determined by Persson et al. (2 mM) (13) and lower than the values reported by Bevernage et al. (5.4 mM) (2). The difference may be related to the smaller number of volunteers and/or the differences in aspirating/sample treatment protocols, applied in the previous studies. Median levels (Table I) were also consistent with previous reported median values (14). The overall % mean (SD) relative bile salts composition in aspirated intestinal fluids after the administration of the four solutions was 11.9 (5.7) % TC, 36 (12) % GC, 9.7 (5.4) % TCDC, 5.0 (5.8) % UDC, 24.1 (8.8) % GCDC and 12.5 (9.4) % GDC. In two volunteers, traces of C were also detected. Therefore, primary bile salts (cholate, chenodeoxycholate) conjugated with glycine dominate, in accordance with previous findings (2,13,15).
Most lecithin in the aspirates was in form of lyso-phosphatidylocholine, and mean values (Table I) were similar with those reported previously by Persson et al. (0.2 mM) (13). Median values (Table I) were lower than values reported previously by Clarysse et al. (0.6 mM) (16). The latter difference may be related to the smaller number of samples and the analytical method applied in the previous study (a commercially available kit had been used).
Mean free fatty acids values (Table I) were similar to previously reported values (0.6 mM) (17). Median values were slightly lower than previously reported values that were estimated from fewer number of samples (0.5 mM) (16).
Mean values of monoglycerides and cholesterol (Table I) were higher than previously reported mean values: according to Persson et al. (13), the mean monoglycerides concentration was about 0.001 mM, and the mean cholesterol concentration was about 0.006 mM.
Mean total protein content (Table I) was somewhat lower than previously reported values (3.1 mg/ml (12), 7.5 mg/ml (13) and 2.1 mg/ml (18)).
Equilibrium Solubility of DPD and KCZ in Aspirates from the Fasted Upper Small Intestine
Measurement of equilibrium solubility in samples with pH ≤ 3.6 (54 out of 336 individual aspirates in total) was associated with substantial pH change during the solubility experiments (equilibrium pH was 0.8–1.6 units higher than initial pH). Since such pH changes at this pH range affect equilibrium solubility values dramatically, the use of measured solubility values for estimating the relative supersaturation index in the lumen becomes problematic. In contrast, at pH ≥ 3.6, changes of pH at equilibrium are much smaller, and, even though they still exist, their impact on solubility values is limited; other factors (such as bile salts) contribute substantially to the equilibrium solubility value. Therefore, relative supersaturation index was estimated only for aspirates with pH ≥ 3.6. However, since the bias in estimation of a mean or median solubility value would be much higher if the relevant solubility data were excluded, estimation of average solubility and comparisons with previously published values were made after including all available data (n = 336). Mean equilibrium solubilities of DPD in intestinal aspirates after DPD-L and DPD-H administrations were 469 μg/ml and 614 μg/ml, respectively, whereas median values were 51 μg/ml and 83 μg/ml, respectively. For KCZ, mean equilibrium solubility after low and high dose administration was 449 μg/ml and 404 μg/ml, respectively, whereas the corresponding median values were 36 μg/ml and 73 μg/ml, respectively. Mean KCZ solubilities were close to previously reported means (19), and median DPD and KCZ solubilities were close to solubilities previously reported in pooled intestinal aspirates (8). Since distributions of DPD and KCZ solubility data were skewed, it may be concluded that mean solubilities of the two compounds may not be representative of the average solubility.
For both DPD and KCZ, equilibrium solubilities in intestinal aspirates with pH ≥ pKa correlated linearly with the total bile salt concentrations, (R2 = 0.74 for DPD and 0.78 for KCZ). Correlations were only slightly improved when total micellar concentrations were used.
Precipitation in and Supersaturation of Lumenal Contents after Administration of DPD and KCZ Solutions
Total amounts of drug per ml, C t , and corresponding drug concentrations, C, in the upper small intestine at specific times after drug administration to fasted healthy adults are shown in Fig. 1 (DPD data) and in Fig. 2 (KCZ data). Precipitated fractions and supersaturation indices are presented in Table II (DPD data) and Table III (KCZ data). Precipitation fraction was estimated for every individual sample, whereas supersaturation index was estimated only if the sample had pH > 3.6.
Total amount of DPD per ml, C t (grey boxes), and concentration of DPD, C (lined grey boxes), in the upper small intestine after the administration of DPD-L (a) and DPD-H (b) solutions to fasted healthy adults. The number of adults that contributed to the construction of the specific pair of boxes is shown above each pair. Solid line connects mean C t data, and dotted line connects mean C data.
Total amount of KCZ per ml, C t (grey boxes), and concentration of KCZ, C (lined grey boxes), in the upper small intestine after the administration of KCZ-L (a) and KCZ-H (b) solutions to fasted healthy adults. The number of adults that contributed to the construction of the specific pair of boxes is shown above each pair. Solid line connects mean C t data, and dotted line connects mean C data.
For DPD, at both dose levels lumenal precipitation was minimal, and mean precipitated fraction, \( \overline {{\pi_{{lumen}}}} \), was up to 7% (Table II). Significant supersaturation was observed up to 10 min post administration of DPD-L and up to 30 min post administration of DPD-H. Interestingly, significant precipitation in the lumen was observed even when the lumenal contents were not saturated with the administered DPD phase (DPD-L/50 min, Table II, first two columns). A similar observation can be made for some incubated supernatants of lumenal contents (DPD-L/40 min, DPD-L/60 min and DPD-H/60 min, Table II, last two columns). X-ray diffractograms could not be studied in detail, because the available amounts of precipitated DPD in the lumen were tiny. However, the presence of generally amorphous materials could be claimed (data not shown). Amounts in incubated samples were also tiny, because, despite the substantial precipitated fractions (Table II), they refer to very small total amount of DPD in the incubated vials.
For KCZ, significant lumenal precipitation was observed at both dose levels. After KCZ-L, precipitated fraction in the upper small intestine was up to 11% (at 30 min post administration). After KCZ-H, precipitated fraction was up to 16% (at 10 and 30 min post administration). Lumenal contents were supersaturated up to 30 min and up to 50 min post dosing of KCZ-L and KCZ-H, respectively. As with DPD, precipitation was observed even when lumenal contents were not saturated with the administered KCZ phase (KCZ-L/60 min and KCZ-L/70 min, Table III, first two columns). A similar observation was made for the KCZ-L/60 min sample after incubation (Table III, last two columns). X-ray diffractograms revealed that, after administration of KCZ solutions, three different solid states could exist in the small intestine: a crystalline state consistent with KCZ reference material (used for preparing the administered solutions) (Fig. 3a and b); a crystalline state that, based on the intense peak at low angle, it may be argued that is likely to be a hydrated form (Fig. 3c) (20); and an amorphous state (the most frequently observed state in the samples upon aspiration) (Fig. 3d). After incubation of supernatants for 48 h at 37°C, precipitated KCZ was always crystalline and had one of the two crystalline forms that had been observed also in the small intestine (Fig. 3b and c).
Representative X-ray powder diffraction patterns of KCZ precipates. a KCZ reference material (used for preparing the administered solutions) and measuring equilibrium solubilities); b Crystalline pattern that refers to the precipitate collected after the incubating (37°C, 48 h, 75 oscillations/min) the supernatant from the sample aspirated 20 min. after KCZ-H administration to volunteer #4; c Crystalline pattern that refers to precipitate collected after incubating (37°C, 48 h, 75 oscillations/min) the supernatant from the sample aspirated 5 min after KCZ-H administration to volunteer #1; d Amorphous pattern that refers to the precipitate in the aspirated sample collected 30 min after KCZ-H administration to volunteer #10.
DISCUSSION
Based on this study, precipitation of two lipophilic, high permeability weak bases in the upper small intestine, after oral administration of pharmacologically relevant doses in the fasted state is, at most, limited. Using DPD solubility in pH 7.0 citrate-phosphate buffer, the dose-to-solubility ratios of DPD for the DPD-L and DPD-H doses are 9 l and 27 l, respectively. Using KCZ solubility in pH 7.0 citrate-phosphate buffer, the dose-to-solubility ratios of KCZ for the KCZ-L and KCZ-H doses are 55 l and 164 l, respectively. It may be argued, therefore, that if the dose of a highly permeable base is (almost) completely dissolved during gastric residence and the dose-to-solubility of the non-ionized form is up to about 50 l, one should expect minimal precipitation in the lumen. Significant precipitation should be expected when dose/solubility ratio of the non-ionized form is about 160 l or higher. Such finding seems to be in agreement with recent work predicting fasted state intestinal precipitation of nelfinavir (pKa 6.0) intralumenally at a dose-to-solubility ratio of 417 l (at pH 7) (21). It is interesting, however, that precipitation can occur even at low intestinal pHs (seven aspirates with KCZ concentration 67.2–932.5 μg/ml and 2.5 < pH < 4.4 had π lumen > 0.1). Also, in this study, in order to facilitate differentiation between precipitates and undissolved particles, gastric contents entering the small intestine were in dissolved state. After administration of a solid dosage form of a weak base, it is possible that few particles may escape dissolution during gastric residence and may act as nuclei for precipitation in the small instestine; i.e., precipitation in that case may be more pronounced.
Precipitation was observed in almost all DPD and KCZ aspirates after their incubation for 48 h at 37°C, indicating that lumenal contents are at least saturated with the administered phase and precipitation occurs at slow rates. However, precipitation was measured to be significant even in cases where lumenal contents and/or incubated lumenal contents were unsaturated in regard to the administered phase (Tables II and III). For example, the sample aspirated from subject #3 60 min after DPD-L administration had the following characteristics: C t = 20.4 μg/ml, C = 16.8 μg/ml, C inc = 11.7 μg/ml and C s = 378.5 μg/ml (pH of the media in which these concentrations were measured was 4.5–4.8). Since equilibrium solubility of the most stable crystalline phase was measured, drug precipitation in lumenal contents and/or incubated lumenal contents that are unsaturated with the free base could happen if the precipitated phase is different from that used for measuring equilibrium solubility, i.e. different from the free base. This is possible if, for example, a hydrated form of the drug is formed, as postulated for the KCZ-H/5 min precipitate after incubation of the aspirated sample of volunteer #1 (Fig. 3c). Hydrates are known to have lower solubities than anhydrous forms (e.g. 22), and the crystalline state of most KCZ precipitates after their incubation at 37°C for 48 h was that shown in Fig. 3c and not that of the reference material (Fig. 3a) (data not shown). Precipitated phase can also be different from the free base when the pH of the aspirated sample is acidic, as precipitation of salt(s) of the base may occur. We attempted to confirm the presence of salt(s) in precipitates with highly acidic pHs using NMR, but the unavailability of reference salt(s) and the complexity of composition of the aspirated samples did not allow for clear-cut conclusions. Although there are no reports on solubility of KCZ salt(s), it has been shown that solubility of KCZ varies with the identity of the anion of buffer at pH 3. Specifically, solubility of KCZ in phosphate, citrate, D-tartrate, L-tartrate, and D, L-tartrate buffers with pH 3 varied from 2.07 mg/ml (in phosphate buffer) to 8.5 mg/ml (in L-tartrate buffer) at 23°C (23).
With the problems associated with the reliability of measurement of equilibrium solubility of the base at acidic pH values (due to change of pH at equilibrium and/or the formation of insoluble complexes) and the possibility of precipitation of a different phase even at pH values close to neutral, one should be cautious when considering precipitation vs. supersaturation. Supersaturation should refer to a specific phase, and equilibrium solubility must be measured while the characteristics of the medium remain unchanged.
Figures 4, 5 and 6 contain data from all available individual aspirates that do not have highly acidic pH (pH > 3.6), and, if precipitation has been observed, drug concentration in the aspirated sample is not lower than equilibrium solubility of the base. Due to lack of detailed information, it was decided to include data from aspirates in which the precipitate had different crystalline state than that of the solid material used for measuring equilibrium solubility. It must be acknowledged that equilibrium solubility in those samples may be slightly different than the measured equilibrium solubility of the base. If indeed the different crystalline state refers to hydrate form, it should have lower equilibrium solubility than the anhydrous form, and, therefore, values of supersaturated indices in Figs. 4, 5 and 6 may be slightly underestimated.
The relative supersaturation index of DPD base, σ lumen , as a function of lumenal DPD concentration and pH [n = 46 aspirated samples] (upper graph) and as function of lumenal DPD concentration and concentration of total micellar components [n = 38 aspirated samples] (lower graph). Aspirates with pH < 3.6 and aspirates in which precipitation was observed but the supernatants were not saturated with the base are not included.
The precipitated fraction of KCZ base, π lumen , (upper graph) and the relative supersaturation index of KCZ base, σ lumen , (lower graph) as a function of lumenal KCZ concentration and pH. Both upper and lower graphs contain data from the same individual aspirates (n = 83). Aspirates with pH < 3.6 and aspirates in which precipitation was observed but the supernatants were not saturated with the base are not included.
The precipitated fraction of KCZ base, π lumen , (upper graph) and the relative supersaturation index of KCZ base, σ lumen , (lower graph) as a function of lumenal KCZ concentration and concentration of total micellar components. Both upper and lower graphs contain data from the same individual aspirates (n = 62). Aspirates with pH < 3.6 and aspirates in which precipitation was observed but the supernatants were not saturated with the base are not included.
Due to minimal precipitation, the relationship of DPD precipitation with the pH and/or the concentration of micellar components cannot be investigated with certainty. However, at lumenal DPD concentrations between 19.4 and 275.6 μg/ml, contents of upper intestinal lumen are supersaturated with the base when pH is greater than about 5 and the concentration of micellar components is less than about 10 mM (Fig. 4a and b, respectively). Based on Fig. 5, at lumenal KCZ concentrations between 19.6 and 924.8 μg/ml, the base precipitates when pH in upper intestinal lumen is greater than about 5, whereas supersaturation with the base is also observed at the same pH range. Based on Fig. 6, at concentrations between 19.6 and 924.8 μg/ml, the base precipitates if the concentration of micellar components is less than about 5 mM, whereas intestinal contents are supersaturated with the base when concentration of micellar components is less than about 10 mM. Therefore, for concentration of micellar components between 5 and 10 mM, it seems that contents can be supersaturated without significant precipitation. In summary, at lumenal concentrations observed after administration of pharmacologically relevant DPD or KCZ doses, the average lumenal contents (pH > 5 and concentration of micellar components <5 mM) are supersaturated with the base.
Finally, it is interesting to note that by using DPD and an in vitro transfer model, Kostewicz et al. (24) observed higher precipitation in and supersaturation of fasted state simulating intestinal fluid than that measured in the contents of the upper small intestine of healthy adults (this study). Reasons include the higher concentrations of DPD employed in the donor (gastric) compartment of the in vitro setup and, perhaps, differences between the precipitated phase and the phase for which equilibrium solubility is measured. Using concentrations closer to those measured in this study and a different in vitro transfer model, Gu et al. (25) also overestimated the lumenal precipitation of DPD observed in the present study, although to a lesser extent.
In conclusion, extensive intralumenal precipitation of lipophilic, high permeability, weak bases may be relatively rare in the human intestine, restricted to compounds with a significantly worse risk profile than DPD and KCZ. Intralumenal precipitation for explaining changes on plasma levels of BCS Class II weak bases in pharmacokinetic studies should be used with caution. Evaluation of precipitation in vs. supersaturation of lumenal contents after administration of weak bases in the fasted state becomes complicated by the possibility of precipitation of various drug phases and also by the difficulty in measuring equilibrium solubility at intralumenal acidic pHs. These issues in addition to using biorelevant drug concentrations are important for the predictability of in vitro setups.
REFERENCES
Box KJ, Comer JE. Using measured pKa, LogP and solubility to investigate supersaturation and predict BCS class. Curr Drug Metab. 2008;9(9):869–78.
Bevernage J, Brouwers J, Clarysse S, Vertzoni M, Tack J, Annaert P, et al. Drug supersaturation in simulated and human intestinal fluids representing different nutritional states. J Pharm Sci. 2010;99(11):4525–34.
Brouwers J, Brewster ME, Augustijns P. Supersaturating drug delivery systems: the answer to solubility-limited oral bioavailability? J Pharm Sci. 2009;98(8):2549–72.
Paulekuhn GS, Dressman JB, Saal C. Trends in active pharmaceutical ingredient salt selection base on analysis of the orange book database. J Med Chem. 2007;50(26):6665–72.
Carlert S, Pålsson A, Hanisch G, von Corswant C, Nilsson C, Lindfors L, et al. Predicting intestinal precipitation-a case example for a basic BCS class II drug. Pharm Res. 2010;27(10):2119–30.
Vertzoni M, Pastelli E, Psachoulias D, Kalantzi L, Reppas C. Estimation of intragastric solubility of drugs: in what medium? Pharm Res. 2007;24(5):909–17.
National Formulary (Greek), Dipyridamole, page 138, Ketoconazole, page 330, ISBN 978-960-86876-8-4, Athens, Greece, National Drug Organization; 2007.
Kalantzi L, Persson E, Polentarutti B, Abrahamsson B, Goumas K, Dressman JB, et al. Canine intestinal contents vs. simulated media for the assessment of solubility of two weak bases in the human small intestinal contents. Pharm Res. 2006;23(6):1373–81.
Vertzoni M, Diakidou A, Chatzilias M, Söderlind E, Abrahamsson B, Dressman JB, et al. Biorelevant media to simulate fluids in the ascending colon of humans and their usefulness in predicting intracolonic drug solubility. Pharm Res. 2010;27(10):2187–96.
Vertzoni M, Archontaki H, Reppas C. Determination of intralumenal individual bile acids by HPLC with charged aerosol detection. J Lipid Res. 2008;49(12):2690–5.
Diakidou A, Vertzoni M, Goumas K, Söderlind E, Abrahamsson B, Dressman J, et al. Characterization of the contents of ascending colon to which drugs are exposed after oral administration to healthy adults. Pharm Res. 2009;26(9):2141–51.
Kalantzi L, Goumas K, Kalioras V, Abrahamsson B, Dressman JB, Reppas C. Characterization of the human upper gastrointestinal contents under conditions simulating bioavailability/bioequivalence studies. Pharm Res. 2006;23(1):165–76.
Persson EM, Gustafsson AS, Carlsson AS, Nilsson RG, Knutson L, Forsell P, et al. The effects of food on the dissolution of poorly soluble drugs in human and in model small intestinal fluids. Pharm Res. 2005;22(12):2141–51.
Mudie DM, Amidon GL, Amidon GE. Physiological parameters for oral delivery and in vitro testing. Mol Pharmaceutics. 2010;7(5):1388–405.
Brouwers J, Tack J, Lammert F, Augustijns P. Intraluminal drug and formulation behavior and integration in in vitro permeability estimation: A case study with Amprenavir. J Pharm Sci. 2006;95(2):372–83.
Clarysse S, Tack J, Lammert F, Duchateau G, Reppas C, Augustijns P. Postprandial evolution in composition and characteristics of human duodenal fluids in different nutritional states. J Pharm Sci. 2009;98(3):1177–92.
Armand M, Borel P, Pasquier B, Dubois C, Senft M, Andre M, et al. Physicochemical characteristics of emulsions during fat digestion in human stomach and duodenum. Am J Physiol. 1996;271(1 Pt 1):G172–83.
Lindahl A, Ungell AL, Knutson L, Lennernäs H. Characterization of fluids from the stomach and proximal jejunum in men and women. Pharm Res. 1997;14(4):497–502.
Clarysse S, Psachoulias D, Brouwers J, Tack J, Annaert P, Duchateau G, et al. Postprandial changes in solubilizing capacity of human intestinal fluids for BCS class II drugs. Pharm Res. 2009;26(6):1456–66.
Tian F, Zhang F, Sandler N, Gordon KC, McGoverin CM, Strachan CJ, et al. Influence of sample characteristics on quantification of carbamazepine hydrate formation by X-ray powder diffraction and Raman spectroscopy. Eur J Pharm Biopharm. 2007;66(3):466–74.
Shono Y, Jantratid E, Dressman JB. Precipitation in the small intestine may play a more important role in the in vivo performance of poorly soluble weak bases in the fasted state: case example nelfinavir. Eur J Pharm Biopharm. 2011. doi:10.1016/j.ejpb.2011.04.005.
Petrova RI, Peresypkin A, Mortko CJ, McKeown AE, Lee J, Williams JM. Rapid conversion of API hydrates to anhydrous forms in aqueous media. J Pharm Sci. 2009;98(11):4111–8.
Buchanan CM, Buchanan NL, Edgar KJ, Ramsey MG. Solubility and dissolution studies of antifungal drug: hydroxybutenyl-β-cyclodextrin complexes. Cellulose. 2007;14(1):35–47.
Kostewicz ES, Wunderlich M, Brauns U, Becker R, Bock T, Dressman JB. Predicting the precipitation of poorly soluble weak bases upon entry in the small intestine. J Pharm Pharmacol. 2004;56(1):43–51.
Gu CH, Rao D, Gandhi RB, Hilden J, Raghavan K. Using a novel multicompartment dissolution system to predict the effect of gastric pH on the oral absorption of weak bases with poor intrinsic solubility. J Pharm Sci. 2005;94(1):199–208.
ACKNOWLEDGMENTS & DISCLOSURES
Part of this work was presented at the AAPS Annual Meeting in New Orleans, 2010.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Psachoulias, D., Vertzoni, M., Goumas, K. et al. Precipitation in and Supersaturation of Contents of the Upper Small Intestine After Administration of Two Weak Bases to Fasted Adults. Pharm Res 28, 3145–3158 (2011). https://doi.org/10.1007/s11095-011-0506-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-011-0506-6