Abstract
Finding an effective cure for Alzheimer’s disease has eluded scientists despite intense research. The disease is a cause of suffering for millions of people worldwide and is characterized by dementia accompanied by cognitive and motor deficits, ultimately culminating in the death of the patient. The course of the disease progression has various underlying contributing pathways, with the first and foremost factor being the development and accumulation of aberrant and misfolded proteins exhibiting neurotoxic functions. The impairment of cellular clearance mechanisms adds to their accumulation, resulting in neuronal death. This is where the PROteolysis TArgeting Chimera (PROTAC) technology comes into play, bringing the UPS degradation machinery in the proximity of the target protein for initiating its degradation and clearing abnormal protein debris with unparalleled precision demonstrating an edge over traditional protein inhibitors in many respects. The technology is widely explored in cancer research and utilized in the treatment of various tumors and malignancies, and is now being applied in treating AD. This review explores the application of PROTAC technology in developing lead compounds for managing this deadly disease along with detailing the pieces of evidence justifying its utility and efficacy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dementia is the 5th leading cause of death worldwide, and Alzheimer’s disease (AD) is the most common contributor, accounting for about 60–70% of dementia cases. Estimates indicate that currently 6.5 million Americans aged 65 or older are living with Alzheimer’s and the number is likely to increase to 12.7 million by 2050 [1]. The disorder is catastrophic in terms of morbidity and mortality, characterized by primarily motor deficits, dementia, and cognitive impairments [2]. The rising number of patients with AD and the non-availability of a cure for the disease present great hardships and difficulties among patients, their caretakers and physicians, and researchers. For understanding the etiology of the disease, the interplay of many factors needs to be considered, including oxidative stress, mitochondrial dysfunction, and protein abnormalities [2, 3]. Aberrant and misfolded proteins with altered conformations are found in AD which changes their functions from physiological to neurotoxic [4, 5]. These forms aggregate and accumulate intracellularly in the case of tau proteins or α-synuclein and extracellularly in synaptic spaces in the case of Aβ proteins showing abnormal protein–protein interactions [6]. The existence of these abnormal protein clusters interfering with normal cell signaling has led to AD being qualified as proteinopathy [6]. Protein misfolding can occur due to alterations in the conformations of native proteins or improper folding of newly formed polypeptides [4, 5]. The defective folding of these proteins can result in surface exposure of hydrophobic amino acid side chains that were initially buried deeply, making them susceptible to joining additional monomers and forming oligomers and aggregates, which can turn infectious, also referred to as prions [7]. These prions can penetrate the cellular membrane and may further spread the infected proteins, aggravating the disorder [7, 8]. Therefore, the only way left for a smooth neuronal functioning is successful clearance of these abnormal protein structures. The two prominent clearing paths are through the ubiquitin–proteasome system (UPS) and autophagy involving lysosomes. Both of these pathways are found to be impaired in AD [8] and have been identified as the cause of neuronal death in AD [9,10,11,12,13]. The pivotal role of protein degradation in the pathogenesis of the disease has motivated researchers to design novel techniques for establishing efficient protein degradation, including the development of the PROteolysis TArgeting Chimera (PROTAC) technology, a trailblazing tool that efficiently degrades the target protein by bringing the UPS machinery in its proximity [51]. This review tries to connect the dots between the uses of PROTACs for targeting culprit proteins involved in multiple allied AD pathways. An effort is made here to give insights into the PROTACs developed to date for the treatment of AD and an attempt is also made to bridge the gap between said technology’s powerful strengths and future challenges in managing this deadly disorder.
Protein Degradation in AD (Autophagy and UPS)
The immortal and non-dividing nature of neuronal cells predisposes them to the toxic effects of accumulated misfolded proteins and damaged organelles and the associated cytotoxicity [8]. Neurons depend on cellular proteasomes for maintaining cellular homeostasis and clearance of these accumulated proteins [14, 15]. There are two major pathways responsible for initiating this clearance cycle which are: the Ubiquitin–Proteasome System and the Autophagy Lysosomal Pathway. The diminishment of these pathways with aging is one of the factors initiating the development of neurodegenerative disorders [9, 12].
The ubiquitin proteasome system (UPS) is localized in the cytosol and nucleus of the cell and is responsible for degrading 70–80% of intracellular proteins. It comprises ubiquitin, made up of 76 amino acid residues conjugated to substrate proteins via a linker attached to the C-terminal glycine residue [16]. This commonly involves a lysine residue’s side chain or an N-terminal methionine [17, 18]. The ubiquitination process proceeds through an enzymatic cycle involving the interplay of highly specific enzymes like E1 ubiquitin-activating enzymes, E2 ubiquitin-conjugating enzyme, and E3 ubiquitin ligases [19]. E1 activates ubiquitin via an ATP-dependent reaction, forming a high-energy thioester bond between the cysteine active site of E1 and the carboxyl group of ubiquitin. After this, ubiquitin is passed onto E2, forming an identical thioester intermediate with it, followed by binding of E2 and the substrate by the E3 enzyme, and ubiquitin is transferred to the substrate [20, 21]. The 26S proteasome, a large multi-subunit complex, plays a central role in the degradation of the Ub-conjugated proteins [15] (Fig. 1). The wide range of E3-protein ligases is able to distinguish between various substrates due to its high specificity and subjectivity.
The UPS has a remarkable impact on the progression of Alzheimer’s disease. It has a direct correlation with various AD pathologies. UPS is involved in the degradation of Aβ, and its alteration in AD, aggravates the amassing of Aβ in several parts of the brain of AD patients. It also leads to increased Aβ formation by upregulating α-secretase activity in neurons of the AD brain [22]. On the other hand, it is also found that Aβ inhibits the proteolytic activities of the 26S proteasomes and significantly increases the levels of Ub-protein conjugates in neurons which is a pathological hallmark of AD [23, 24]. It also leads to the inhibition of the multivesicular sorting pathway which is known to be an important route for retrograde transportation, supplying important substrates from neuronal terminals to the cell body for signaling and degradation via lysosomes [13]. Similar to its involvement in Aβ’s pathological aspects, UPS is also involved in the degradation of tau through the 26S proteasome. The association between UPS and tau pathology r results from the recurrent building up and aggregation of Ub in paired helical filaments (PHF) and neurofibrillary tangles in order to initiate the tau degradation. The polyubiquitinated tau molecules present within the paired helical filaments is localized in the form of Lys48-linked poly-Ub form, which is the most recognized degradation signal. This clearly illustrates the role of UPS-mediated tau removal in the protection against AD pathogenesis. Hence, Aβ accumulation, tau hyperphosphorylation and neurodegeneration are all connected to the UPS dysfunction in AD [15]. Moreover, functional failure of UPS in AD pathology is also evident from the research findings of downregulation of proteasome activities in multiple sections of the AD brain which includes the inferior parietal lobe, superior and middle temporal gyri and para hippocampal gyrus [25]. Another major connection between UPS and the pathology of AD comes from the identification of frameshift mutation in the Ub transcript known as UBB+1, formed as a result of molecular misreading of Ubiquitin-B Protein (UBB) in AD patients leading to the elongation of molecule by 20 amino acids [26]. The UBB+1 is an effective polyubiquitination acceptor, however, neither it can be activated by the E1 due to the absence of crucial G76 moiety nor it can bind to a substrate or the Ub portion. The resultant chain is also difficult to be disassembled by the deubiquitinases like isopeptidases T. The aggregated poly-Ub chains also result in inhibition of proteosomal degradation leading to neuronal apoptosis [27, 28]. The UBB+1 expression considerably rises in the brain due to aging resulting in UPS suppression and accumulation of toxic protein aggravating AD [15].
The autophagy mode of degradation is another major degradation pathway for clearing abnormal proteins from cytoplasm, triggered by cellular insults like starvation. It is also pivotal for capturing and degrading damaged or disrupted cytoplasmic structures like mitochondria (mitophagy) or invading microbes (Xenophagy), or protein aggregates (aggrephagy). The pathway involves the ubiquitin-dependent degradation of cargo via lysosomes in place of proteasomes involved in above mentioned pathways, and the cycle is modulated by autophagy-related genes [14, 29]. Protein quality control maintained by autophagy is imperative for the removal of aggregated pathogenic protein forms in neurodegenerative disorders like tau and Aβ in AD, α-synuclein in Parkinson’s disease, and polyQ-Htt in Huntington’s disease [29]. Any dysfunction in this autophagy process can give rise to neurodegeneration even in the absence of any disease-associated mutant, as shown in a research study with mice models lacking the Atg5 (autophagy-related 5) gene responsible for autolysosomal formation in their neural cells; such mice were found to develop motor defects along with the aggravated build-up of cytoplasmic inclusion bodies in neurons [30]. The cycle employs ubiquitin binding autophagy adaptors like p62/sequestosome 1, optineurin, Nuclear Domain 10 Protein 52 and Tax1 Binding Protein 1 (TAX1BP1) [31, 32]. The p62 adaptor is associated with the ubiquitin domain which interacts with the polyubiquitin chains of misfolded proteins and a PB1 domain that modulates the self-aggregation forming condensed cargo p62 complexes [33]. These cargo-loaded p62 and other joined complexes are delivered to autophagic vacuoles by the interaction of p62 with light chain 3 II (LC3-II) on the surface of autophagic double membrane structures [34]. This process subsequently decreases the toxicity of the free forms or oligomeric species of misfolded proteins [29]. After the delivery of misfolded proteins to phagophores, the membranes fuse together and proliferate further to form autophagosomes which fuse with lysosomes to form autolysosomes in which these cargoes are degraded by lysosomal hydrolases (Fig. 2) [35].
The degradation of misfolded protein structures via autophagy and subsequent cell cleansing determines proper neuronal functioning and survival. However, similar to the impairment of the UPS pathway, autophagy is also known to be impaired in AD brains. The examination of AD brains shows reduced levels of autophagosomes [36], autophagy regulatory proteins like beclin-1 [37], autophagy marker ATG5 and mitophagy marker RBR E3 ubiquitin-protein ligase (PRKN) [38, 39] signaling the impairment of autophagy in AD [40]. The impaired autophagy dampens the clearance of abnormal proteins and influences their accumulation in the synapse leading to AD.
Thus, protein degradation is imperative for proper neuronal functioning. The processes involved in cellular quality control have interconnected links with AD pathology and can be a wonderful target for its therapeutic management. Hijacking the ubiquitin-dependent degradation has been one of approaches gaining attention of researchers to treat a variety of neurodegenerative disorders.
PROTACS as the Emerging Technology for Protein Degradation
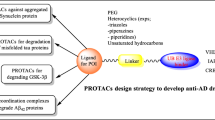
The PROTAC technology was first reported in 2001 to target the ubiquitin ligase complex SKF (Skp1-Cullin-F-box-HRT1) [43,44,45], and since then it has been a focus of interest for attacking otherwise undruggable protein targets; it is increasingly being employed in biological research and therapeutic development [41, 48]. PROTACS are bifunctional molecules that hijack the ubiquitin proteasome system to perform target protein degradation. The PROTAC entity is made up of a ligand that binds selectively to an E3 ligase connected by a linker to a ligand that binds the protein of interest. PROTACS brings the E3 ligase close to the protein of interest (POI) to trigger the ubiquitination by the E3 ligases and consequently lead to proteosomal degradation [42,43,44,45,46,47] (Fig. 3). Thus, the PROTAC molecule must possess an adequate affinity for both the E3 ligase and the protein of interest as its substrates. As compared to conventional small molecule inhibitors that operate by blocking the catalytic activity of the druggable protein via occupancy-driven pharmacology, PROTACs function by the event-driven mode of action to eliminate the protein and put an end to all its possible functions, be it enzymatic, scaffolding, regulatory or other activities [48,49,50,51,52,53].
There are several advantages associated with PROTACs. First is their potential to target undruggable targets or proteasomes that comprise about 85% of all the human proteins. PROTACs are able to target proteins that do not display any well-defined active sites or possess flat protein interfaces. Thus, these are appropriate for targeting transcription factors and scaffolding proteins that do not possess specific binding sites [54, 55]. PROTACs are known to exert isoform-selective degradation, provided that each isoform-PROTAC-E3 complex can modulate differential degradation outcomes [56]. The selectivity depends on the protein–protein interactions between the E3 ubiquitin ligase and the target protein. The proteins that are not degraded result from the formation of unstable ternary complexes with PROTAC-recruited E3 ligases. The weak PROTAC:target protein affinity can stabilize the high-affinity target:PROTAC:ligase trimer interactions facilitating efficient degradation [56]. Another major plus point comes from the ability of PROTACs to overcome drug resistance due to mutations. The nonsynonymous mutations occurring at the active site can develop resistance to small molecule inhibitors. In contrast, the degradation induced by PROTACs only depends on the transient and the reversible association with substrate, making it capable of degrading the mutant proteins [57]. Prime examples showing their useful implementation in treating AD come from PROTACs used for initiating tau protein degradation or for targeting Bromodomain and extraterminal (BET) family proteins. PROTACs are known to possess high-target specificity [55, 58] employed for achieving degradation of resistant targets and exerting rapid and sustained depletion of the target proteins.
Due to the immense potential of PROTACs to degrade aberrant proteins, it has been implemented to treat AD, by inducing the degradation of misfolded and aggregated proteins like tau, BET and GSK-3β, as discussed in detail in the next section.
Potential AD Targets for PROTACs
PROTACs Targeting Tau Proteins
The primary culprit in AD pathology is the tau protein that primarily binds to microtubules in healthy neurons. Microtubules form the backbone of the neuronal structure and aid in transporting proteins and organelles through the cytoplasm. As the onset of changes occurs in AD patients, tau proteins become hyperphosphorylated and start forming threads and tangles, disrupting microtubules and destroying neuronal transportation and communication [59]. The cell may also become deprived of nutrition due to this disruption, ultimately culminating in cell death. Tau proteinss have been observed as the most viable target for AD treatment and have been immensely explored for curative therapies [60,61,62]. A significant PROTAC intervention advanced by Lu et al. was the formulation of Keap-1-dependant PROTAC (Fig. 4) for tau degradation by employing UPS [63]. The Keap-1 protein (Kelch-like ECH-associated protein-1) was chosen as the target for employing UPS and initiating the degradation of tau proteins as it functioned as the substrate adaptor protein for the Cullin-3 (Cul3)/Ring-Box1 (Rbx1)-dependant E3 ubiquitin ligase complex. NF-E2-related factor-2 (Nrf2) functions as the prevalent substrate of the complex and plays a role in the regulation of oxidative stress [64, 65]. The PROTAC includes Ac-LDPETGEYL-OH peptide for recognition and binding with Keap-1, and peptide YQQYQDATADEQG for tau recognition. A short peptide was incorporated to increase flexibility and added with poly-d-arginine (RRRRRRRR) for cell penetration. The formed PROTAC was found to show strong binding in vitro with both Keap1 and tau proteins, coimmunoprecipitating with both the proteins. Further analysis using flow cytometry and other assays demonstrated a reduction in intracellular tau concentration in a time- and concentration-dependent manner [63].
In another research study, Chu et al. developed a series of PROTACs consisting of varying motifs for binding with E3 and tau [71]. These molecules comprised 3 parts which included a moiety for selective recognition of tau, for which 2 peptides from α- and β-tubulin were chosen that were known to interact with the tau proteins: α (430–441): KDYEEVGVDSVE and β (422–434): YQQYQDATADEQG [66, 67]. It also included a moiety for selective E3 recognition, for which 2 peptides based on the substrates of the two E3 ligases were chosen. These are DRHDS(p) GLDS(p)M, procured from IкBα, bound to Skp1-cullin-F box (SCF) E3 Ligase [68, 69] and the other one was ALAPYIP, procured from the substrate of E3 ligase, von Hippel-Lindau tumour suppressor protein (VHL) [43]. The tau recognizing moieties were linked to the E3 recognition moieties using short peptides like GSGS or GGSGG to enhance flexibility. For facilitating penetration, poly-arginine (D-Arg)8 was fused to the C-terminus of the peptides [70]. Out of the 12 developed entities, PROTAC TH006, which included YQQYQDATADEQG peptide for recognizing tau, GSGS peptide as the linker, ALAPYIP for recruiting UPS for degradation and poly-d-arginine (RRRRRRRR) for penetrating the cell, was reported to be most effective in initiating the degradation of tau proteins and increasing its polyubiquitination depending on VHL-E3 ligase. It also normalized the unevenly distributed mitochondria in cells with a high concentration of tau proteins and it decreased the toxicity of Aβ plaques [71]. A small-molecule PROTAC, C004019, designed by Wang et al., consisted of a triazole-based tau binder moiety and a VHL (E3-ligase) to aid in tau degradation by E3-Ubiquitin ligase. The formed compound was found to initiate vigorous tau clearance in HEK293 and SH-SY5Y cells expressing human tau. Intracerebral ventricular infusion of C004019 led to significant tau clearance in vivo, and single and multiple doses administered subcutaneously downregulated the tau concentration in brains of wild-type, hTau-transgenic and 3xTg-AD mice along with enhancement of synaptic and cognitive functions [72].
PROTACs Targeting Epigenetic Processes
Epigenetic mechanisms like DNA methylation, chromatin remodeling and histone post-translational modifications, histone protein variants, and non-coding RNA, influence the course of brain development and proper brain functioning. Alterations in DNA structure can lead to the initiation of various pathological conditions. Mutations in chromatin-associated factors can lead to neurological disorders like AD [73]. The most remarkable factor that plays a role in major neurodegenerative disorders is age accompanied by a diminishment in cognitive capabilities. The process of aging is associated with impaired levels of histone acetylation and methylation [73, 74]. The acetylation and deacetylation of histone proteins are catalyzed by histone acetyltransferases (HATs) and histone deacetylases (HDACs), respectively. It regulates the condensation of chromatin and the transcription of genes [75]. Inhibition of HDAC may serve as a wonderful target for improving memory and cognition and thus may augment other therapeutic aids in treating AD. There is evidence that blockade of HDAC2 reduces memory impairment accompanying neurodegeneration accompanied by reinforcement of structural and synaptic plasticity [76]. Some HDAC inhibitors alleviated the memory impairment in 3xTg AD mouse models. Examples of some of these inhibitors are RGFP-966, a selective HDAC3 inhibitor [77], and M344 for class I and IIB histone deacetylases [78].
With the potential of HDAC inhibition for memory enhancement in mind, researchers designed novel PROTACS targeting HDAC. The first PROTAC targeting HDAC proteins aimed at selective degradation of the SIRT2 (sirtuins) which constitute class-III HDAC proteins employing NAD + as a cofactor for exerting their action [79]. The newly developed triazole-based SIRT2-selective PROTAC (1) (Fig. 5) employed thalidomide which is a bonafide cereblon ligand. This PROTAC induced up to 90% isotype-selective Sirt2 degradation at 5 μM concentration in HeLa cells, leading to the microtubule network’s hyperacetylation and improved process elongation [80].
PROTACs Targeting BET Proteins
The BET proteins commence the transcription of inflammatory genes on the activation of the immune system. These comprise four proteins which are BRD2 (Bromodomain-containing protein 2), BRD3 (Bromodomain-containing protein 3), BRD4 (Bromodomain-containing protein 4), and BRDT (Bromodomain-testis associated protein), which are expressed in most cells and tissues of the body except BRDT which is expressed in testis [81, 82]. BET proteins contain 2 tandem bromodomains-BD1 and BD2 that form bonds with acetyl lysine histone residues like H3K27ac, H4K5ac, H4K12ac and non-histone acetylated proteins like NF-кB (Nuclear Factor-кB), Twist and GATA1. Gene transcription of NF-кB was modulated by Brd2 and Brd4, which can in turn aggravate the transcription of various genes involved in neuroinflammation following various types of brain injury [82]. BRD4 serves as the chromatin reader that binds lysine’s in histones and tunes the neurons’ transcription in response to neuronal activation. It is imperative for proper brain functioning and is linked to memory function and neurological disorders [83]. Thus, BET inhibitors were visualized as potential targets for the treatment of AD.
BET inhibitors like JQ1 (2) (Fig. 5) were incorporated with E3 ligands to form PROTACs that target BET bromodomains. The efficacy of JQ1 in AD mice models is controversial in terms of its effects. Some researchers advance the fact that JQ1 administered at a dose of 50 mg/kg downregulated brain inflammation and phosphorylation of tau at Ser396 in 3xTg mouse models of AD. The concentration of pro-inflammatory mediators like IL-1β, IL-6, TNF-α, Ccl2, Nos2 and Ptgs2 was found to be drastically reduced. But the inhibitor was found to be ineffective in improving learning and cognitive deficits in these mice models [84]. In contrast, a different study demonstrated its potential efficacy in aiding memory improvement and synaptic plasticity along with enhancement in hippocampal LTP in a dose-dependent manner which contradicts the results of the previous studies [85]. Another study reported JQ1 to have excellent blood brain barrier permeability and good tolerance in mice at a dose of 50 mg/kg daily for 1 week or 3 weeks. Administration of JQ1 did not cause anxiety or mobility problems but it was found to not exert any effects on short-term memory; instead, long-term memory was found to be hampered causing memory deficits [83]. It was conjugated with the phthalimide moiety which was the ligand for the E3 ubiquitin ligase CRBN. JQ1 recognizes BET protein and then the proteasomes degrade it. It displaced BET proteins from chromatin and the phthalimide employed E3 ubiquitin ligases resulting in polyubiquitylation of BET proteins and proteasome-dependent degradation. Thus, the PROTAC compound led to rapid and selective BET protein degradation causing impairment of BRD4 and transcriptional factors interaction and lipopolysaccharide-induced transcription of pro-inflammatory genes in microglia of SIM-A9 mice. dBET1 administration also reduced the deleterious effects of neuroinflammatory disease-activated microglia [81, 82].
PROTACs Targeting GSK-3β
Glycogen synthase kinase-3 is a protein serine/threonine kinase that plays a key part in a multitude of cellular processes (catabolic and anabolic) and in monitoring cell growth and signaling [86]. It has been observed to be directly involved in the pathogenesis of AD and forms a connecting link between senile plaques and neurofibrillary tangles [87, 88]. The GSK-3 promotes tau hyperphosphorylation and is actively involved in multiple neuronal pathologies that are dysregulated in AD like the production of amyloid-β (Aβ) peptides or Aβ-induced cell death, axonal transport, adult neurogenesis, synaptic function, and cholinergic function. GSK-3β overexpression initiates tau-dependent AD pathology [89]. Pro-inflammatory activity of the kinase can result in loss of neurons [90]. Due to the intense interplay of GSK-3β in various AD pathologies, it has been employed as a prime target for therapeutic strategies. Various studies also demonstrated the effectiveness of GSK-3 inhibitors in alleviating AD symptoms. These have been demonstrated to downregulate tau phosphorylation in cells and preclinical studies in mice. One of such inhibitors that have reached phase-2 clinical trials is tideglusib, a non-ATP competitive GSK-3 inhibitor acting as an allosteric inhibitor. It exerts neuroprotective action by reducing the deposition of amyloid-β, gliosis, tau phosphorylation, and loss of neurons, and it reverses the memory deficits in transgenic mice [89, 91]. Jiang et al. recently explored a pyridinethiazole-based PROTAC (3) (Fig. 5) to degrade GSK-3β. The pyridinethiazole-based inhibitor G1 was conjugated to thalidomide to form the PROTAC PG21, a potent protein degrader which displayed dose-dependent degradation of GSK-3β. It was found to cause about 44.2% protein degradation at 2.8 μM. Further research demonstrated the PROTAC to protect against glutamate-induced cell death in HT-22 cells signaling the efficacy of PG21 in impairing the inflammatory response and cell damage in nerve cells, indicating its neuroprotective action [92].
Patented PROTAC Formulations
Several potential PROTACs have been patented. One of the PROTACs targeting tau proteins was patented by Gray et al. in 2019 (WO 2019/014429 A1). The investigator reported various forms including hydrates, solvates, cocrystals, and polymorphs of PROTACs consisting of one tau binding moiety conjugated with an E3 ubiquitin ligase binding moiety like lenalidomide or thalidomide joined via a linker which was substituted and unsubstituted alkylene, alkenylene, arylene, heterocyclylene, heteroalkylene, or other similar moieties. PROTACs were analyzed using tau degradation assays to evaluate their tau degrading efficiency in human cells and were reported to degrade hyperphosphorylated tau and total tau proteins in human tau-A152T neurons and tau-P301L neurons after a 24-h treatment [93, 94].
Similarly, Crew et al., in 2020, reported a series of PROTACs (WO 2020/041331 A1) developed with alpha-synuclein modulators which can be advantageous for the treatment of neurodegenerative disorders, mainly Alzheimer’s disease and Parkinson’s disease. The study disclosed that the bifunctional compounds consist of a Von-Hippel-Lindau (VHL), cereblon (CRBN), inhibitors of apoptosis proteins or mouse double-minute homologue 2 ligand at one end that binds to the E3 ubiquitin ligase, with the other end binding with the target moiety bringing the target protein in close proximity to the ubiquitin ligase and inducing protein degradation. The ELISA technique was used to evaluate the α-synuclein protein degradation activity in HEK293 TREX α-syn A53T cells. Out of the series of compounds synthesized, compound 4, 5, 6 and 7 displayed significant α-synuclein degrading activity with less than 35% protein remaining relative to DMSO control; compounds 8 and 9 (Fig. 6) also displayed significant α-synuclein degrading activity with 35–70% protein remaining relative to DMSO control [95, 96].
A series of PROTACs targeting tau proteins were designed and patented by Crew et al. under patent application number WO 2018/102067 A2 and US 2018/0125821/A1 [97, 98]. These consisted of bifunctional molecules with at one end of cereblon or VHL ligand and at the other end a tau-binding moiety. The formulated PROTACs were effective in degrading tau protein in SK-N-SH cells at the dose of 3 μl of 1 mg/ml solution [97, 98].
Gray et al. patented a series of PROTAC molecules targeting EGFR (epidermal growth factor receptor) under patent application number US 2019/0106417/ A1. These compounds have the potential to be employed in the treatment of kinase-mediated disorders which are modulated by EGFR [99]. Key structures of some significant PROTACs are depicted in the figures. The EC50 values of compounds 10, 11, 12 and 13 (Fig. 6) against T790M/L858R transformed Ba/F3 cells were found to be < 500Nm.
Conclusion and Future Prospects
Development of drugs, including those for Alzheimer’s, depends on the ability to design compounds that can penetrate the blood–brain barrier. Gene silencing techniques likewise run into problems due to the inability of nucleic acids to penetrate the blood–brain barrier [100]. In contrast, PROTACs do not suffer from this problem. The advent of PROTACs technology tackled various issues associated with the traditional small molecule inhibitors, which possessed poor selectivity leading to adverse effects and drug resistance. However, the application of this technology is still in its infancy due to the paucity of knowledge and evidences needed for safely treating neurodegenerative disorders. As of now, fewer than 10 of more than 600 E3 ubiquitin ligases have been employed for targeted protein degradation. Many more E3 ligases are yet to be explored and developed, which can be a major area of focus [101, 102]. Exploring additional E3 ligases can help in the prevention of off-target effects [103]. Identification of factors governing effective target-ligase pairings can lead to the development of additional E3 ligase tools that can validate further progress in this arena [102]. Direct binding assays can also be an approach for evaluating the binding and hit identification strategies for E3 ligases [102]. Furthermore, optimizing the design, synthesis, and evaluation of PROTACs is necessary to develop useful decision trees to be used in future synthesis programs and to establish a concrete evaluation platform [82, 101, 103]. Novel drug targets should be elucidated for targeting by PROTACs. As of now, PROTACs have only been used to target druggable proteins, but the technology has the potential to target non-druggable proteins too. Many such potential targets for managing Alzheimer’s disease are yet too to be found for PROTACs like Sirt2, which is dysregulated in AD. Other potential targets and scaffolding proteins that can be tapped for the AD treatment are BCL proteins, β-arrestins, and β-catenins. Another issue with the development of PROTACs is their molecular size which can sometimes be bulky enough to majorly impact their suitability to act as drugs as well as to cause a problem with their penetration into the blood–brain barrier, which is imperative for its efficacy in AD [104]. Applying crystallography techniques can also help understand the structural mechanism of PROTACs [102]. Until now, the developed PROTACs are not being evaluated in full-fledged clinical trials for proper elucidation of their pharmacokinetics, dosing, and toxicity, which are much required key points to be considered prior to their clinical application in the treatment of AD. Another limitation associated with PROTACs is that these cannot be used to tackle the genetic mutations associated with the familiar forms of AD. Since PROTACs are not able to treat the root cause (genetic mutations), their lifetime administration to the patient would be required, which could be thought to result in potential adverse effects in patients. Another limitation is that although PROTACs can halt the further progression of the disease, they cannot reverse the damage that has already occurred. Since there is a lack of diagnostical techniques to map the exact extent of disease progression, using PROTACs may not be effective in patients in advanced stages. Yet another limitation associated with its use is that since AD is limited to only certain parts of the brain in the initial stages of the disease, there remains an inadequacy to evaluate the exact concentration of PROTACs reaching the affected portions of the brain. Hence, successful therapeutic application of PROTACs in treating AD requires a thorough consideration of these limitations.
Abbreviations
- AD:
-
Alzheimer’s disease
- PROTAC:
-
PROteolysis TArgeting Chimera
- UPS:
-
Ubiquitin–proteasome system
- PHF:
-
Paired helical filaments
- UBB:
-
Ubiquitin-B protein
- BET:
-
Bromodomain and extraterminal
References
Alzheimer Association (2022) 2022 Alzheimer’s disease facts and figures. Alzheimer’s Dement 15:321–387
Ganguly G, Chakrabarti S, Chatterjee U, Saso L (2017) Proteinopathy, oxidative stress and mitochondrial dysfunction: cross talk in Alzheimer’s disease and Parkinson’s disease. Drug Des Devel Ther 11:797–810
Bhatia S, Rawal R, Sharma P, Singh T, Singh M, Singh V (2021) Mitochondrial dysfunction in Alzheimer’s disease: opportunities for drug development. Curr Neuropharmacol. https://doi.org/10.2174/1570159X19666210517114016
Sharma C, Kim SR (2021) Linking oxidative stress and proteinopathy in Alzheimer’s disease. Antioxidants 10:1231
Golde TE, Miller VM (2009) Proteinopathy-induced neuronal senescence: a hypothesis for brain failure in Alzheimer’s and other neurodegenerative diseases. Alzheimers Res Ther 1:5. https://doi.org/10.1186/alzrt5
Boland B, Yu WH, Corti O, Mollereau B, Henriques A, Bezard E et al (2018) Promoting the clearance of neurotoxic proteins in neurodegenerative disorders of ageing. Nat Rev Drug Discov 17:660–688. https://doi.org/10.1038/nrd.2018.109
Brundin P, Melki R, Kopito R (2010) Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat Rev Mol Cell Biol 11:301–307. https://doi.org/10.1038/nrm2873
Tecalco-Cruz AC, Pedraza-Chaverri J, Briones-Herrera A, Cruz-Ramos E, López-Canovas L, Zepeda-Cervantes J (2022) Protein degradation-associated mechanisms that are affected in Alzheimer’s disease. Mol Cell Biochem 477:915–925. https://doi.org/10.1007/s11010-021-04334-8
Orr ME, Oddo S (2013) Autophagic/lysosomal dysfunction in Alzheimer’s disease. Alzheimers Res Ther BioMed Central 5:1–9
Zhang Y, Chen X, Zhao Y, Ponnusamy M, Liu Y (2017) The role of ubiquitin proteasomal system and autophagy-lysosome pathway in Alzheimer’s disease. Rev Neurosci 28:861–868. https://doi.org/10.1515/revneuro-2017-0013
Al Mamun A, Rahman MM, Zaman S, Munira MS, Uddin M, Rauf A et al (2020) Molecular insight into the crosstalk of UPS components and Alzheimer’s disease. Curr Protein Pept Sci 21:1193–1201
Oddo S (2008) The ubiquitin-proteasome system in Alzheimer’s disease. J Cell Mol Med 12:363–373
Almeida CG, Takahashi RH, Gouras GK (2006) β-Amyloid accumulation impairs multivesicular body sorting by inhibiting the ubiquitin-proteasome system. J Neurosci 26:4277–4288
Schmidt MF, Gan ZY, Komander D, Dewson G (2021) Ubiquitin signalling in neurodegeneration: mechanisms and therapeutic opportunities. Cell Death Differ 28:570–590. https://doi.org/10.1038/s41418-020-00706-7
Al Mamun A, Uddin MS, Kabir MT, Khanum S, Sarwar MS, Mathew B et al (2020) Exploring the promise of targeting ubiquitin-proteasome system to combat Alzheimer’s disease. Neurotox Res 38:8–17. https://doi.org/10.1007/s12640-020-00185-1
Kleiger G, Mayor T (2014) Perilous journey: a tour of the ubiquitin–proteasome system. Trends Cell Biol 24:352–359
McClellan AJ, Laugesen SH, Ellgaard L (2019) Cellular functions and molecular mechanisms of non-lysine ubiquitination. Open Biol 9:190147
Nandi D, Tahiliani P, Kumar A, Chandu D (2006) The ubiquitin-proteasome system. J Biosci 31:137–155
Komander D, Rape M (2012) The ubiquitin code. Annu Rev Biochem 81:203–229
Grimm S, Höhn A, Grune T (2012) Oxidative protein damage and the proteasome. Amino Acids 42:23–38
Tramutola A, Di Domenico F, Barone E, Perluigi M, Butterfield DA (2016) It is all about (u)biquitin: role of altered ubiquitin-proteasome system and UCHL1 in Alzheimer disease. Oxid Med Cell Longev 2016:2756068. https://doi.org/10.1155/2016/2756068
Gentier RJ, van Leeuwen FW (2015) Misframed ubiquitin and impaired protein quality control: an early event in Alzheimer’s disease. Front Mol Neurosci. https://doi.org/10.3389/fnmol.2015.00047
Salon ML, Pasquini L, Moreno MB, Pasquini JM, Soto E (2003) Relationship between β-amyloid degradation and the 26S proteasome in neural cells. Exp Neurol 180:131–143
Hong L, Huang H-C, Jiang Z-F (2014) Relationship between amyloid-beta and the ubiquitin–proteasome system in Alzheimer’s disease. Neurol Res 36:276–282. https://doi.org/10.1179/1743132813Y.0000000288
Necchi D, Lomoio S, Scherini E (2011) Dysfunction of the ubiquitin–proteasome system in the cerebellum of aging Ts65Dn mice. Exp Neurol 232:114–118
Montero-Calle A, San Segundo-Acosta P, Garranzo-Asensio M, Rábano A, Barderas R (2020) The molecular misreading of APP and UBB induces a humoral immune response in Alzheimer’s disease patients with diagnostic ability. Mol Neurobiol 57:1009–1020. https://doi.org/10.1007/s12035-019-01809-0
Lam YA, Pickart CM, Alban A, Landon M, Jamieson C, Ramage R et al (2000) Inhibition of the ubiquitin-proteasome system in Alzheimer’s disease. Proc Natl Acad Sci 97:9902–9906
Lindsten K, de Vrij FMS, Verhoef LGGC, Fischer DF, van Leeuwen FW, Hol EM et al (2002) Mutant ubiquitin found in neurodegenerative disorders is a ubiquitin fusion degradation substrate that blocks proteasomal degradation. J Cell Biol 157:417–427
Ciechanover A, Kwon YT (2015) Degradation of misfolded proteins in neurodegenerative diseases: therapeutic targets and strategies. Exp Mol Med 47:e147–e147
Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R et al (2006) Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441:885–889. https://doi.org/10.1038/nature04724
Dikic I (2017) Proteasomal and autophagic degradation systems. Annu Rev 86:193–224. https://doi.org/10.1146/annurev-biochem-061516-044908
Johnson CW, Melia TJ, Yamamoto A (2012) Modulating macroautophagy: a neuronal perspective. Future Med Chem 4:1715–1731
Ichimura Y, Komatsu M (2010) Selective degradation of p62 by autophagy. Semin Immunopathol 32:431–436
Filimonenko M, Isakson P, Finley KD, Anderson M, Jeong H, Melia TJ et al (2010) The selective macroautophagic degradation of aggregated proteins requires the PI3P-binding protein Alfy. Mol Cell 38:265–279
Xie Z, Klionsky DJ (2007) Autophagosome formation: core machinery and adaptations. Nat Cell Biol 9:1102–1109
Nixon RA, Yang D-S (2011) Autophagy failure in Alzheimer’s disease—locating the primary defect. Neurobiol Dis 43:38–45
Di Meco A, Curtis ME, Lauretti E, Praticò D (2020) Autophagy dysfunction in Alzheimer’s disease: mechanistic insights and new therapeutic opportunities. Biol Psychiatry 87:797–807
Castellazzi M, Patergnani S, Donadio M, Giorgi C, Bonora M, Bosi C et al (2019) Autophagy and mitophagy biomarkers are reduced in sera of patients with Alzheimer’s disease and mild cognitive impairment. Sci Rep 9:1–7
Geng P, Zhang J, Dai W, Han X, Tan Q, Cheng D et al (2018) Autophagic degradation deficit involved in sevoflurane-induced amyloid pathology and spatial learning impairment in APP/PS1 transgenic mice. Front Cell Neurosci 12:185
Ling D, Magallanes M, Salvaterra PM (2014) Accumulation of amyloid-like Aβ1–42 in AEL (autophagy–endosomal–lysosomal) vesicles: potential implications for plaque biogenesis. ASN Neuro 6:AN20130044
Wen J, Fang F, Guo S-H, Zhang Y, Peng X-L, Sun W-M et al (2018) Amyloid β-derived diffusible ligands (ADDLs) induce abnormal autophagy associated with Aβ aggregation degree. J Mol Neurosci 64:162–174
Jiang S, Zhao Y, Zhang T, Lan J, Yang J, Yuan L et al (2018) Galantamine inhibits β-amyloid-induced cytostatic autophagy in PC 12 cells through decreasing ROS production. Cell Prolif 51:e12427
Sakamoto KM, Kim KB, Kumagai A, Mercurio F, Crews CM, Deshaies RJ (2001) Protacs: chimeric molecules that target proteins to the Skp1–Cullin–F box complex for ubiquitination and degradation. Proc Natl Acad Sci 98:8554–8559
Sakamoto KM, Kim KB, Verma R, Ransick A, Stein B, Crews CM et al (2003) Development of Protacs to target cancer-promoting proteins for ubiquitination and degradation. Mol Cell Proteomics 2:1350–1358
Sakamoto KM (2005) Chimeric molecules to target proteins for ubiquitination and degradation. Ubiquitin and protein degradation, part B. Elsevier, Amsterdam, pp 833–847
An S, Fu L (2018) Small-molecule PROTACs: an emerging and promising approach for the development of targeted therapy drugs. EBioMedicine 36:553–562
Arora P, Singh M, Singh V, Bhatia S, Arora S (2021) PROTACs in treatment of cancer: a review. Mini Rev Med Chem. https://doi.org/10.2174/1389557521666210226150740
Bondeson DP, Mares A, Smith IED, Ko E, Campos S, Miah AH et al (2015) Catalytic in vivo protein knockdown by small-molecule PROTACs. Nat Chem Biol 11:611–617. https://doi.org/10.1038/nchembio.1858
Bondeson DP, Crews CM (2017) Targeted protein degradation by small molecules. Annu Rev Pharmacol Toxicol 57:107–123. https://doi.org/10.1146/annurev-pharmtox-010715-103507
Burslem GM, Crews CM (2017) Small-molecule modulation of protein homeostasis. Chem Rev 117:11269–11301. https://doi.org/10.1021/acs.chemrev.7b00077
Burslem GM, Crews CM (2020) Proteolysis-targeting chimeras as therapeutics and tools for biological discovery. Cell 181:102–114
Salami J, Crews CM (2017) Waste disposal—an attractive strategy for cancer therapy. Science 355:1163–1167
Nalawansha DA, Crews CM (2020) PROTACs: an emerging therapeutic modality in precision medicine. Cell Chem Biol 27:998–1014
Konstantinidou M, Li J, Zhang B, Wang Z, Shaabani S, Ter Brake F et al (2019) PROTACs—a game-changing technology. Expert Opin Drug Discov 14:1255–1268. https://doi.org/10.1080/17460441.2019.1659242
Neklesa TK, Winkler JD, Crews CM (2017) Targeted protein degradation by PROTACs. Pharmacol Ther 174:138–144
Bondeson DP, Smith BE, Burslem GM, Buhimschi AD, Hines J, Jaime-Figueroa S et al (2018) Lessons in PROTAC design from selective degradation with a promiscuous warhead. Cell Chem Biol 25:78-87.e5
Buhimschi AD, Armstrong HA, Toure M, Jaime-Figueroa S, Chen TL, Lehman AM et al (2018) Targeting the C481S ibrutinib-resistance mutation in Bruton’s tyrosine kinase using PROTAC-mediated degradation. Biochemistry 57:3564–3575. https://doi.org/10.1021/acs.biochem.8b00391
Gadd MS, Testa A, Lucas X, Chan K-H, Chen W, Lamont DJ et al (2017) Structural basis of PROTAC cooperative recognition for selective protein degradation. Nat Chem Biol 13:514–521. https://doi.org/10.1038/nchembio.2329
Iqbal K, Liu F, Gong C-X, Grundke-Iqbal I (2010) Tau in Alzheimer disease and related tauopathies. Curr Alzheimer Res 7:656–664
James OG, Doraiswamy PM, Borges-Neto S (2015) PET imaging of Tau pathology in Alzheimer’s disease and tauopathies. Front Neurol. https://doi.org/10.3389/fneur.2015.00038
Ruan Z (2022) Extracellular vesicles drive tau spreading in Alzheimer’s disease. Neural Regen Res 17:328–329
Frisoni GB, Altomare D, Thal DR, Ribaldi F, van der Kant R, Ossenkoppele R et al (2022) The probabilistic model of Alzheimer disease: the amyloid hypothesis revised. Nat Rev Neurosci 23:53–66. https://doi.org/10.1038/s41583-021-00533-w
Lu M, Liu T, Jiao Q, Ji J, Tao M, Liu Y et al (2018) Discovery of a Keap1-dependent peptide PROTAC to knockdown Tau by ubiquitination-proteasome degradation pathway. Eur J Med Chem 146:251–259
Wilson AJ, Kerns JK, Callahan JF, Moody CJ (2013) Keap calm, and carry on covalently. J Med Chem 56:7463–7476. https://doi.org/10.1021/jm400224q
Lu M-C, Ji J-A, Jiang Z-Y, You Q-D (2016) The Keap1–Nrf2–ARE pathway as a potential preventive and therapeutic target: an update. Med Res Rev 36:924–963. https://doi.org/10.1002/med.21396
Rivas CI, Vera JC, Maccioni RB (1988) Anti-idiotypic antibodies that react with microtubule-associated proteins are present in the sera of rabbits immunized with synthetic peptides from tubulin’s regulatory domain. Proc Natl Acad Sci 85:6092–6096. https://doi.org/10.1073/pnas.85.16.6092
Maccioni RB, Rivas CI, Vera JC (1988) Differential interaction of synthetic peptides from the carboxyl-terminal regulatory domain of tubulin with microtubule-associated proteins. EMBO J 7:1957–1963. https://doi.org/10.1002/j.1460-2075.1988.tb03033.x
Yaron A, Hatzubai A, Davis M, Lavon I, Amit S, Manning AM et al (1998) Identification of the receptor component of the IκBα–ubiquitin ligase. Nature 396:590–594
Yaron A, Gonen H, Alkalay I, Hatzubai A, Jung S, Beyth S et al (1997) Inhibition of NF-kappa-B cellular function via specific targeting of the I-kappa-B-ubiquitin ligase. EMBO J 16:6486–6494
Schneekloth JS, Fonseca FN, Koldobskiy M, Mandal A, Deshaies R, Sakamoto K et al (2004) Chemical genetic control of protein levels: selective in vivo targeted degradation. J Am Chem Soc 126:3748–3754. https://doi.org/10.1021/ja039025z
Chu T-T, Gao N, Li Q-Q, Chen P-G, Yang X-F, Chen Y-X et al (2016) Specific knockdown of endogenous Tau protein by peptide-directed ubiquitin-proteasome degradation. Cell Chem Biol 23:453–461
Wang W, Zhou Q, Jiang T, Li S, Ye J, Zheng J et al (2021) A novel small-molecule PROTAC selectively promotes tau clearance to improve cognitive functions in Alzheimer-like models. Theranostics 11:5279–5295
Berson A, Nativio R, Berger SL, Bonini NM (2018) Epigenetic regulation in neurodegenerative diseases. Trends Neurosci 41:587–598
Sen P, Shah PP, Nativio R, Berger SL (2016) Epigenetic mechanisms of longevity and aging. Cell 166:822–839
Roth SY, Denu JM, Allis CD (2001) Histone acetyltransferases. Annu Rev Biochem 70:81–120. https://doi.org/10.1146/annurev.biochem.70.1.81
Gräff J, Rei D, Guan J-S, Wang W-Y, Seo J, Hennig KM et al (2012) An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature 483:222–226. https://doi.org/10.1038/nature10849
Janczura KJ, Volmar C-H, Sartor GC, Rao SJ, Ricciardi NR, Lambert G et al (2018) Inhibition of HDAC3 reverses Alzheimer’s disease-related pathologies in vitro and in the 3xTg-AD mouse model. Proc Natl Acad Sci 115:E11148–E11157
Volmar C-H, Salah-Uddin H, Janczura KJ, Halley P, Lambert G, Wodrich A et al (2017) M344 promotes nonamyloidogenic amyloid precursor protein processing while normalizing Alzheimer’s disease genes and improving memory. Proc Natl Acad Sci 114:E9135–E9144
Smalley JP, Cowley SM, Hodgkinson JT (2020) Bifunctional HDAC therapeutics: one drug to rule them all? Molecules 25:4394
Schiedel M, Herp D, Hammelmann S, Swyter S, Lehotzky A, Robaa D et al (2018) Chemically induced degradation of sirtuin 2 (Sirt2) by a proteolysis targeting chimera (PROTAC) based on sirtuin rearranging ligands (SirReals). J Med Chem 61:482–491. https://doi.org/10.1021/acs.jmedchem.6b01872
Singh MB, Sartor GC (2020) BET bromodomains as novel epigenetic targets for brain health and disease. Neuropharmacology 181:108306
Ma K, Han X-X, Yang X-M, Zhou S-L (2021) Proteolysis targeting chimera technology: a novel strategy for treating diseases of the central nervous system. Neural Regen Res 16:1944
Korb E, Herre M, Zucker-Scharff I, Darnell RB, Allis CD (2015) BET protein Brd4 activates transcription in neurons and BET inhibitor Jq1 blocks memory in mice. Nat Neurosci 18:1464–1473. https://doi.org/10.1038/nn.4095
Magistri M, Velmeshev D, Makhmutova M, Patel P, Sartor GC, Volmar C-H et al (2016) The BET-bromodomain inhibitor JQ1 reduces inflammation and Tau phosphorylation at Ser396 in the brain of the 3xTg model of Alzheimer’s disease. Curr Alzheimer Res 13:985–995
Benito E, Ramachandran B, Schroeder H, Schmidt G, Urbanke H, Burkhardt S et al (2017) The BET/BRD inhibitor JQ1 improves brain plasticity in WT and APP mice. Transl Psychiatry 7:e1239–e1239. https://doi.org/10.1038/tp.2017.202
Rippin I, Eldar-Finkelman H (2021) Mechanisms and therapeutic implications of GSK-3 in treating neurodegeneration. Cells 10:262
Llorens-Marítin M, Jurado J, Hernández F, Ávila J (2014) GSK-3β, a pivotal kinase in Alzheimer disease. Front Mol Neurosci. https://doi.org/10.3389/fnmol.2014.00046
Culbreth M, Aschner M (2018) GSK-3β, a double-edged sword in Nrf2 regulation: Implications for neurological dysfunction and disease. F1000Research 7:1043
Sayas CL, Ávila J (2021) GSK-3 and Tau: a key duet in Alzheimer’s disease. Cells 10:721
Sirerol-Piquer M, Gomez-Ramos P, Hernández F, Perez M, Morán MA, Fuster-Matanzo A et al (2011) GSK3β overexpression induces neuronal death and a depletion of the neurogenic niches in the dentate gyrus. Hippocampus 21:910–922. https://doi.org/10.1002/hipo.20805
Domínguez JM, Fuertes A, Orozco L, del Monte-Millán M, Delgado E, Medina M (2012) Evidence for irreversible inhibition of glycogen synthase kinase-3β by Tideglusib*. J Biol Chem 287:893–904
Jiang X, Zhou J, Wang Y, Liu X, Xu K, Xu J et al (2021) PROTACs suppression of GSK-3β, a crucial kinase in neurodegenerative diseases. Eur J Med Chem 210:112949
Gray NS, Haggarty SJ, Cai QT, Baptista Lima Da Silva MC, Zhang T, Ferguson FM (2017) Compounds for Tau protein degradation
Kargbo RB (2019) Treatment of Alzheimer’s by PROTAC-Tau protein degradation. ACS Med Chem Lett 1:699–700
Kargbo RB (2020) PROTAC compounds targeting α-synuclein protein for treating neurogenerative disorders: Alzheimer’s and Parkinson’s diseases. ACS Med Chem Lett 11:1086–1087. https://doi.org/10.1021/acsmedchemlett.0c00192
Crew AP, Dong H, Berlin M, Sparks SM (2020) Proteolysis targeting chimeric (PROTAC) compound with E3 ubiquitin ligase binding activity and targeting alpha-synuclein protein for treating neurodegenerative diseases
Crew AP, Berlin M, Flanagan JJ, Dong H, Ishchenko A (2018) Tau-protein targeting protacs and associated methods of use. Google Patents
Crew AP, Berlin M, Flanagan JJ, Dong H, Ishchenko A (2018) Tau-protein targeting PROTACS and associated methods of use
Kargbo RB (2019) Treatment of cancer and Alzheimer’s disease by PROTAC degradation of EGFR. ACS Med Chem Lett. https://doi.org/10.1021/acsmedchemlett.9b00283
Tomoshige S, Ishikawa M (2020) PROTACs and other chemical protein degradation technologies for the treatment of neurodegenerative disorders. Angew Chemie Int Ed. https://doi.org/10.1002/anie.202004746
Pettersson M, Crews CM (2019) PROteolysis TArgeting Chimeras (PROTACs)—past, present and future. Drug Discov Today Technol 31:15–27
Schapira M, Calabrese MF, Bullock AN, Crews CM (2019) Targeted protein degradation: expanding the toolbox. Nat Rev Drug Discov 18:949–963. https://doi.org/10.1038/s41573-019-0047-y
Inuzuka H, Liu J, Wei W, Rezaeian A-H (2022) PROTAC technology for the treatment of Alzheimer’s disease: advances and perspectives. Acta Mater Med. https://doi.org/10.15212/AMM-2021-0001
Pradeepkiran JA, Reddy PH (2021) Phosphorylated tau targeted small-molecule PROTACs for the treatment of Alzheimer’s disease and tauopathies. Biochim Biophys Acta 1867:166162
Acknowledgements
I am thankful to all my colleagues of Chitkara College of Pharmacy, Chitkara University, Punjab, India, who have provided constant moral support and gave inspiration during my review.
Funding
This review received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bhatia, S., Singh, M., Singh, T. et al. Scrutinizing the Therapeutic Potential of PROTACs in the Management of Alzheimer’s Disease. Neurochem Res 48, 13–25 (2023). https://doi.org/10.1007/s11064-022-03722-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03722-w