Abstract
Epilepsy is a common neurological disease. The dysregulated long noncoding RNAs (lncRNAs) are implicated in epileptogenesis. The aim of this research is to explore the role and mechanism of lncRNA zinc finger antisense 1 (ZFAS1) in status epilepticus (SE)-induced hippocampal neurons injury. SE mice model was established and primary hippocampal neurons were isolated. The expression levels of ZFAS1 and microRNA-421 (miR-421) were detected in hippocampus and hippocampal neurons via quantitative reverse transcription polymerase chain reaction. Hippocampal neurons viability, apoptosis and autophagy were analyzed via Cell Counting Kit-8, flow cytometry and western blot. The target relationship between ZFAS1 and miR-421 was analyzed via dual-luciferase reporter assay. The phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) pathway was blocked by LY294002 and related protein levels were detected via western blot. ZFAS1 expression was elevated in hippocampus and hippocampal neurons from SE mice. Knockdown of ZFAS1 increased SE hippocampal neurons viability and decreased apoptosis and autophagy. ZFAS1 could sponge miR-421. MiR-421 expression was declined in SE mice tissues and cells. Down-regulation of miR-421 abolished the suppressive effect of ZFAS1 knockdown on hippocampal neurons apoptosis and autophagy. Silencing of ZFAS1 induced activation of the PI3K/AKT pathway by up-regulating miR-421. Inhibition of the PI3K/AKT pathway reversed the effect of ZFAS1 knockdown on SE hippocampal neurons apoptosis and autophagy. Interference of ZFAS1 attenuated hippocampal neurons apoptosis and autophagy in SE by increasing miR-421 and activating the PI3K/AKT pathway, indicating a new mechanism for understanding the pathogenesis of SE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epilepsy is a common neurological disorder impairing life quality of patients with status epilepticus (SE) [1]. Great advances have been gained on the development, prediction and treatment of epilepsy [2]. The effective strategy for epilepsy remains a challenge. The hippocampal damage and dysfunction of hippocampal neurons are implicated in the development of epilepsy [3, 4]. Therefore, exploring the pathogenesis of hippocampal neurons dysfunction might be helpful to find out new target for the treatment of epilepsy.
The dysregulation of long noncoding RNAs (lncRNAs) is associated with the development of epilepsy [5]. Zinc finger antisense 1 (ZFAS1) is identified as a lncRNA having various roles in human diseases [6]. ZFAS1 could promote cancer malignancies by regulating cancer cell proliferation and apoptosis in different cancers, including glioma, colorectal cancer, nasopharyngeal carcinoma and non-small cell lung cancer [7,8,9,10]. By detecting 11 dysregulated lncRNAs, Han et al. find that ZFAS1 is up-regulated most except H19 in SE [11]. Hence, ZFAS1 might play important role in epilepsy development. However, the role and mechanism of ZFAS1 in epilepsy remain undetermined.
MiRNAs could mediate hippocampal neurogenesis via regulating gene expression, which might participate in the development of epilepsy [12]. MiR-421 is one of common miRNAs associated with cancer cell apoptosis [13]. Previous studies show that miR-421 could inhibit cell apoptosis in some cell lines [14, 15]. More importantly, emerging evidence suggests that miR-421 could repress hippocampal neurons apoptosis and autophagy by regulating toll-like receptor/myeloid differentiation factor-88 pathway in epilepsy [16]. Nevertheless, it is undetermined whether ZFAS1 could regulate miR-421.
Previous studies suggest that activation of phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) pathway protects against neuronal death, oxidative stress and occurrence of epilepsy [17,18,19]. However, whether the PI3K/AKT pathway is mediated by ZFAS1 in epilepsy remain unclear. It is documented that pilocarpine is widely used to induce SE of mice [20, 21]. In this research, we also established the mouse SE model via treatment of pilocarpine. Moreover, we measured the expression of ZFAS1 in hippocampus of mice with SE and investigated the effect of ZFAS1 on hippocampal neurons apoptosis and autophagy. Besides, we explored the interaction between ZFAS1 and miR-421/PI3K/AKT pathway.
Materials and Methods
Mouse SE Model
The C57BL/6 mice (male, 20–25 g) were purchased from Vital River (Beijing, China) and used in this research. SE model was established via pilocarpine as previously reported with some modifications [22], and the mice at behavioral seizures stage 5 were regarded as SE. In brief, mice were given 2 mg/kg of atropine (Selleck, Shanghai, China) 30 min before the intraperitoneal injection of 280 mg/kg of pilocarpine (Selleck). The mice were monitored, and the behavioral seizures were investigated. The stages of behavioral seizures were determined based on the Racine scale [23]. The mice with stage 5 (rearing and falling) were selected for this study. After SE onset for 2 h, diazepam (10 mg/kg; Sigma, St. Louis, MO, USA) was intraperitoneally injected into mice to quell seizures. The mice in control group were injected with saline instead of pilocarpine. SE and control mice (n = 10/group) were used for the study. At 24 h after SE, the mice were killed and hippocampus were collected. The experiments were approved via the Institutional Animal Care and Use Committee of Jiangxi provincial People's Hospital Affiliated to Nanchang University.
Primary Hippocampal Neurons Isolation, Culture and Treatment
The primary hippocampal neurons were isolated from the hippocampus of SE mice or control group according to the procedures as previously reported [16]. Cells were grown in DMEM (Solarbio, Beijing, China) with 10% fetal bovine serum (Zhejiang Tianhang Biotechnology, Huzhou, China), and 1% penicillin/streptomycin (Thermo Fisher, Wilmington, DE, USA) at 37 °C in 5% CO2. For blocking the PI3K/AKT pathway, cells were incubated with 50 nM pathway inhibitor LY294002 for 24 h. The matched control group was incubated with dimethyl sulfoxide (DMSO; Solarbio).
Cell Transfection
The siRNA against ZFAS1 (si-ZFAS1, 5′-UUCUGUAUUCCAAAAGUACCU-3′), siRNA negative control (si-NC, 5′-CACUGACGGUGACCAGAACAAAGAU-3′), miR-421 mimic (5’-AUCAACAGACAUUAAUUGGGCGC-3′), mimic negative control (miR-NC, 5′-UUCUCCGAACGUGUCACGUTT-3′), miR-421 inhibitor (anti-miR-421, 5′-UAGUUGUCUGUAAUUAACCCGCG-3′), and corresponding negative controls (anti-NC, 5′-CAGUACUUUUGUGUAGUACAA-3′) were generated via Ribobio (Guangzhou, China). Hippocampal neurons with 60% confluence were transfected with these oligonucleotides (40 nM) via Lipofectamine 3000 reagent (Thermo Fisher) for 24 h.
Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
The RNA was extracted from hippocampus or hippocampal neurons using TRIzol reagent (Takara, Tokyo, Japan). The reverse transcription was carried out to synthesize cDNA using the specific reverse transcription kit (Thermo Fisher). The cDNA was used to perform qRT-PCR using SYBR Green (Takara) on an ABI 7900 system (ABI, Foster City, CA, USA). The primers (Sangon, Shanghai, China) were listed as: ZFAS1 (sense, 5′-AACCATTAGCTAGCTGGGGC-3′; antisense, 5′-CAAGTTAACCCCGGAGGGAC-3′), miR-421 (sense, 5′-TATGGTTGTTCTGCTCTCTGTGTC-3′; antisense, 5′-CTCACTCACATCAACAGACATTAATT-3′), U6 (sense, 5′-ACGCTTCACGAATTTGCGTGTC-3′; antisense, 5′-CTCGCTTCGGCAGCACA-3′), GAPDH (sense, 5′-ACGGGAAGCCCATCACGATT-3′; antisense, 5′-CAGAAGGGGCGGAGATGATG-3′). The relative expression was normalized to GAPDH (for ZFAS1) or U6 (for miR-421) according to the 2−ΔΔCt method [24].
Cell Counting Kit-8 (CCK-8) Assay
The viability of hippocampal neurons was determined via CCK-8 assay. Hippocampal neurons were added into 96-well plates (1 × 104 cells/well) and then cultured for 48 h. Next, 10 μL CCK-8 reagent (Abbkine, Wuhan, China) was added. After culture for 3 h, the absorbance at 450 nm was detected through a microplate reader (Bio-Gene Technology, Guangzhou, China). The relative cell viability was normalized to control group.
Flow Cytometry
The apoptotic rate of hippocampal neurons was analyzed via Annexin V-FITC/PI detection kit (Vazyme, Nanjing, China) by flow cytometry. Hippocampal neurons were placed into 12-well plates (5 × 104 cells/well) and incubated for 48 h. Following the incubation of binding buffer, cells were stained with 5 μL Annexin V-FITC and PI solution. Next, cells were analyzed with a flow cytometer (Agilent, Hangzhou, China). The apoptotic rate of hippocampal neurons was presented as the percentage of cells at upper and lower right quadrants.
Western Blot
Hippocampal neurons were harvested and lysed using RIPA reagent (Beyotime, Shanghai, China) with 1 mM PMSF (Beyotime). The protein samples were quantified via BCA protein assay kit (Solarbio) and denatured at 100 °C for 5 min. Then, 30 μg samples were suffered from sodium dodecyl sulfate–polyacrylamide gel electrophoresis followed via transfer of nitrocellulose membranes (Millipore, Billerica, MA, USA). Subsequently, the membranes which were blocked in 5% non-fat milk were interacted with specific antibodies (Abcam, Cambridge, MA, USA), including anti-Cleaved caspase 3 (Cleaved-casp 3) (ab49822, 1:500 dilution), anti-B-cell lymphoma 2 (Bcl-2) (ab194583, 1:1000 dilution), anti-Bax (ab199677, 1:1000 dilution), anti-LC3-II/I (ab51520, 1:3000 dilution), anti-Beclin 1 (ab62557, 1:2000 dilution), anti-P62 (ab91526, 1:1000 dilution), anti-PI3K (ab227204, 1:500 dilution), anti-phosphorylated PI3K (p-PI3K) (ab182651, 1:500 dilution), anti-AKT (ab8805, 1:500 dilution), anti-p-AKT (ab38449, 1:500 dilution), and anti-GAPDH (ab37168, 1:1000 dilution), and horseradish peroxidase-conjugated IgG (ab6721, 1:8000 dilution). Next, the protein bands were exposed to ECL reagent (Amyjet Scientific, Wuhan, China). The relative protein level was normalized to GAPDH expression and control group.
Dual-Luciferase Reporter Assay
The complementary sites of ZFAS1 and miR-421 were predicted via starBase online (https://starbase.sysu.edu.cn/index.php). The sequence of ZFAS1 containing wild-type (WT) (CUGUUGA) or mutant (MUT) (AACCCAG) binding sites of miR-421 was inserted into the psiCHECK-2 vector (Promega, Madison, WI, USA), generating the corresponding luciferase reporter constructs ZFAS1-WT and ZFAS1-MUT. The hippocampal neurons were co-transfected with ZFAS1-WT or ZFAS1-MUT and miR-421 mimic or NC using Lipofectamine 3000 reagent for 24 h. A dual-luciferase assay system (Thermo Fisher) was exploited to analyze the luciferase activity.
Statistical Analysis
Statistical analysis was conducted via GraphPad Prism 6 (GraphPad, La Jolla, CA, USA). The experiments were conducted with 3 biological replicates and 3 technical replicates. The data were expressed as mean ± standard deviation. The linear correlation in Fig. 3f was analyzed via Pearson correlation analysis. Student’s t-test was performed in Figs. 1 and 3; ANOVA followed via Tukey test was conducted in Figs. 2, 4, 5 and 6. The difference was significant when P < 0.05.
Results
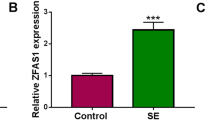
The Expression of ZFAS1 is Up-regulated in Epilepsy
To measure the expression of ZFAS1 in epilepsy, the SE mice model was established. By detecting the level of ZFAS1 in hippocampus, results showed that ZFAS1 expression was higher in SE group than that in control group (Fig. 1a). Furthermore, the hippocampal neurons were isolated from SE mice or control group. The expression of ZFAS1 was evidently elevated in hippocampal neurons from SE mice compared with that in control group (Fig. 1b). These data indicated that high expression of ZFAS1 might play important role in epilepsy.
Knockdown of ZFAS1 Represses SE-Induced Hippocampal Neurons Apoptosis and Autophagy
To explore the effect of ZFAS1 on SE-induced hippocampal injury, the hippocampal neurons from SE mice were transfected with si-NC or si-ZFAS1. The transfection of si-ZFAS1 effectively decreased the level of ZFAS1 in hippocampal neurons from SE mice (Fig. 2a). The functional assay showed that hippocampal neurons viability was obviously reduced in SE group compared with control group, while it was restored by knockdown of ZFAS1 (Fig. 2b). Moreover, silence of ZFAS1 protected against SE-induced hippocampal neurons apoptosis (Fig. 2c). In addition, the apoptotic proteins (Bcl-2, Bax and Cleaved-casp 3) and autophagy-related proteins (LC3-II/I and Beclin 1) were measured. As shown in Fig. 2d–f, reduction of Bcl-2 and P62, and elevation of Bax, Cleaved-casp 3, LC3-II/I and Beclin 1 were displayed in SE hippocampal neurons, while these protein levels were reversed via down-regulation of ZFAS1. These results suggested that inhibition of ZFAS1 repressed SE-induced hippocampal neurons apoptosis and autophagy.
The effect of ZFAS1 on SE-induced hippocampal neurons apoptosis and autophagy. a The level of ZFAS1 was examined in hippocampal neurons from SE mice transfected with si-ZFAS1 or si-NC via qRT-PCR. b The viability of hippocampal neurons from SE mice transfected with si-ZFAS1 or si-NC was measured via CCK-8 assay. c The apoptotic rate of hippocampal neurons from SE mice transfected with si-ZFAS1 or si-NC was detected via flow cytometry. d–f The apoptotic and autophagy-related protein levels were measured in hippocampal neurons from SE mice transfected with si-ZFAS1 or si-NC by western blot. *P < 0.05
ZFAS1 is a Sponge of miR-421
To explore the mechanism of ZFAS1 in hippocampal injury, the targets of ZFAS1 were analyzed via starBase, which indicated miR-421 was a candidate target of ZFAS1. As predicted miR-421 expression was remarkably reduced in hippocampus and hippocampal neurons of SE group in comparison to control group (Fig. 3a and b). For validation of this target association, the wild-type and mutant luciferase reporter vectors were constructed (Fig. 3c), and the luciferase activity was analyzed. As shown in Fig. 3d, overexpression of miR-421 led to a 48% loss of luciferase activity in ZFAS1-WT group, whereas the mutant of binding sites in ZFAS1-MUT group abolished this effect. Moreover, miR-421 expression was significantly increased via knockdown of ZFAS1 in hippocampal neurons (Fig. 3e). In addition, miR-421 level was inversely correlated with ZFAS1 expression in hippocampus from SE group (Fig. 3f). These findings uncovered that ZFAS1 could sponge miR-421.
The target association between ZFAS1 and miR-421. a and b miR-421 expression was detected by qRT-PCR in hippocampus and hippocampal neurons of SE mice or control group. c The complementary sites between ZFAS1 and miR-421 were predicted via starBase. d Luciferase activity was performed in hippocampal neurons transfected with ZFAS1-WT or ZFAS1-MUT and miR-421 mimic or miR-NC. e The level of miR-421 was examined in hippocampal neurons from SE mice transfected with si-ZFAS1 or si-NC by qRT-PCR. f The linear relationship between the levels of ZFAS1 and miR-421 was analyzed in SE hippocampus. *P < 0.05
Knockdown of miR-421 Abates the Suppressive Effect of ZFAS1 Silence on SE-Induced Hippocampal Neurons Apoptosis and Autophagy
To explore whether miR-421 was required for ZFAS1 in regulating hippocampal injury, the hippocampal neurons from SE mice were transfected with si-NC, si-ZFAS1, si-ZFAS1 + anti-NC or anti-miR-421. The transfection of anti-miR-421 effectively declined the abundance of miR-421 in the presence of si-ZFAS1 in hippocampal neurons from SE mice (Fig. 4a). Moreover, down-regulation of miR-421 weakened silence of ZFAS1-induced viability restoration of SE hippocampal neurons (Fig. 4b). In addition, inhibition of miR-421 reversed the inhibitive role of ZFAS1 knockdown in hippocampal neurons apoptosis (Fig. 4c–e). Besides, knockdown of miR-421 alleviated silence of ZFAS1-mediated regulation on autophagy-related protein expression (Fig. 4d and f). These data suggested that ZFAS1 could regulate SE-induced hippocampal injury via mediating miR-421.
The rescue effect of miR-421 on ZFAS1-mediated SE-induced hippocampal neurons apoptosis and autophagy. a The abundance of miR-421 was examined in SE hippocampal neurons transfected with si-NC, si-ZFAS1, si-ZFAS1 + anti-NC or anti-miR-421 via qRT-PCR. Cell viability (b), apoptotic rate (c), apoptotic and autophagy-related protein levels (d–f) were measured in hippocampal neurons from SE mice transfected with si-NC, si-ZFAS1, si-ZFAS1 + anti-NC or anti-miR-421 by CCK-8, flow cytometry and western blot. *P < 0.05
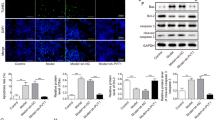
Knockdown of ZFAS1 Activates the PI3K/AKT Pathway by Regulating miR-421 in Hippocampal Neurons
To explore whether the PI3K/AKT pathway was mediated by ZFAS1, the related protein levels were measured in the hippocampal neurons from SE mice. As shown in Fig. 5a and b, the phosphorylation levels of PI3K and AKT were significantly inhibited in SE group, while they were promoted via knockdown of ZFAS1 in hippocampal neurons from SE mice. And the effect of ZFAS1 knockdown was abated via inhibition of miR-421 or use of LY294002 (an inhibitor of the PI3K/AKT pathway). These results showed that ZFAS1/miR-421 axis could regulate the activation of the PI3K/AKT pathway in hippocampal neurons.
The effect of ZFAS1 and miR-421 on the PI3K/AKT pathway in SE hippocampal neurons. a and b The expression levels of p-PI3K, PI3K, p-AKT and AKT were detected in hippocampal neurons from SE mice transfected with si-NC, si-ZFAS1, si-ZFAS1 + anti-NC or anti-miR-421, or treated by LY294002 or DMSO via western blot. *P < 0.05
Knockdown of ZFAS1 Inhibits SE-Induced Hippocampal Neurons Apoptosis and Autophagy by Activating the PI3K/AKT Pathway
To explore whether the PI3K/AKT pathway was involved in ZFAS1-mediated regulation of hippocampal injury, the hippocampal neurons from SE mice transfected with si-NC or si-ZFAS1 were treated by the pathway inhibitor LY294002. As shown in Fig. 6a–e, inhibition of the PI3K/AKT pathway using LY294002 abrogated knockdown of ZFAS1-induced increase of viability and inhibition of apoptosis and autophagy in hippocampal neurons from SE mice. These findings implied that silence of ZFAS1 inhibited SE-induced hippocampal injury via activating the PI3K/AKT signaling.
The effect of inhibition of PI3K/AKT signaling on ZFAS1-mediated SE-induced hippocampal neurons apoptosis and autophagy. Cell viability (a), apoptotic rate (b), apoptotic and autophagy-related protein levels (c–e) were examined in hippocampal neurons from SE mice transfected with si-NC or si-ZFAS1, or treated by LY294002 or DMSO by CCK-8, flow cytometry and western blot. *P < 0.05
Discussion
Epilepsy is a serious brain problem with more than 70 million cases in the world [25]. 30% of persons with epilepsy have refractory disease despite the therapeutic improvement [26]. Hence, it is urgent to explore new strategy for the treatment of epilepsy. LncRNAs could take part in epilepsy development by regulating neuron dysfunction [27]. Hippocampal neurons apoptosis and autophagy contribute to epilepsy progression [28, 29]. This study was the first to demonstrate the protective effect of ZFAS1 knockdown on hippocampal neurons via decreasing neurons apoptosis and autophagy. Moreover, the target association between ZFAS1 and miR-421 was first confirmed in this research.
In this research, increased expression of ZFAS1 was measured in hippocampus of SE mice, which was in agreement with previous study [11]. This result indicated that ZFAS1 might play important role in epilepsy development. In Bcl-2 family, anti-apoptotic Bcl-2 protein and pro-apoptotic Bax regulate the apoptotic process [30]. Moreover, activation of caspase 3 contributes to cell apoptosis [31]. Here we found that knockdown of ZFAS1 weakened hippocampal neurons apoptosis in SE mice. Furthermore, LC3 and P62 are important proteins involved in autophagosome development, and Beclin 1 controls autophagocytosis, which are associated with alteration of autophagy in epilepsy [32,33,34]. By detecting these autophagy-related biomarkers, we found that knockdown of ZFAS1 mitigated SE-induced hippocampal neurons autophagy. Thus, ZFAS1 might serve as an important target for treatment of epilepsy.
Previous studies suggested that ZFAS1 could regulate cancer progression via regulating multiple miRNAs, such as miR-432-5p, miR-7-5p, miR-135a and miR-193a-3p [7,8,9,10]. To explore an additional target of ZFAS1, here we validated that ZFAS1 could target miR-421. Previous study reported that miR-421 expression was decreased in hippocampus of SE mice, and this miRNA played a protective function via decreasing hippocampal neurons apoptosis and autophagy [16]. Similarly, we also found the reduced expression of miR-421 in hippocampus of SE mice. Furthermore, down-regulation of miR-421 abolished silence of ZFAS1-mediated inhibitive effect on hippocampal neurons apoptosis and autophagy, uncovering that ZFAS1 controlled SE-induced hippocampal injury by regulating miR-421.
The PI3K/AKT pathway is associated with autophagy process in neurodegenerative diseases [35]. Our data showed that the PI3K/AKT pathway was inactivated in epilepsy, and ZFAS1 knockdown activated this pathway via increasing miR-421. This result was opposite with that in acute lymphoblastic leukemia and nasopharyngeal carcinoma [36, 37]. We hypothesized it might be due to the different microenvironments or different targets in the upstream of the PI3K/AKT pathway. Moreover, activation of the PI3K/AKT pathway inhibited hippocampal neurons apoptosis and autophagy [19, 29]. In this work, we also found that inhibition of the PI3K/AKT pathway attenuated the effect of ZFAS1 knockdown on hippocampal neurons, indicating that ZFAS1 regulated hippocampal neurons apoptosis and autophagy via inhibiting the PI3K/AKT pathway.
In conclusion, knockdown of ZFAS1 alleviated hippocampal neurons apoptosis and autophagy, possibly via up-regulating miR-421 and activating the PI3K/AKT pathway. This study indicates a novel target for the treatment of epilepsy.
References
Devinsky O, Vezzani A, O'Brien TJ, Jette N, Scheffer IE, de Curtis M, Perucca P (2018) Epilepsy. Nat Rev Dis Primers 4:18024
Pitkanen A, Loscher W, Vezzani A, Becker AJ, Simonato M, Lukasiuk K, Grohn O, Bankstahl JP, Friedman A, Aronica E, Gorter JA, Ravizza T, Sisodiya SM, Kokaia M, Beck H (2016) Advances in the development of biomarkers for epilepsy. Lancet Neurol 15:843–856
Huberfeld G, Blauwblomme T, Miles R (2015) Hippocampus and epilepsy: findings from human tissues. Rev Neurol (Paris) 171:236–251
Liu YQ, Yu F, Liu WH, He XH, Peng BW (2014) Dysfunction of hippocampal interneurons in epilepsy. Neurosci Bull 30:985–998
Hashemian F, Ghafouri-Fard S, Arsang-Jang S, Mirzajani S, Fallah H, Mehvari HJ, Sayad A, Taheri M (2019) Epilepsy is associated with dysregulation of long non-coding RNAs in the peripheral blood. Front Mol Biosci 6:113
Jiang X, Yang Z, Li Z (2019) Zinc finger antisense 1: a long noncoding RNA with complex roles in human cancers. Gene 688:26–33
Yang G, Han B, Feng T (2019) ZFAS1 knockdown inhibits viability and enhances cisplatin cytotoxicity by up-regulating miR-432-5p in glioma cells. Basic Clin Pharmacol Toxicol 125:518–526
Mo D, Liu W, Li Y, Cui W (2019) Long non-coding RNA zinc finger antisense 1 (ZFAS1) regulates proliferation, migration, invasion, and apoptosis by targeting MiR-7-5p in colorectal cancer. Med Sci Monit 25:5150–5158
Wang M, Ji YQ, Song ZB, Ma XX, Zou YY, Li XS (2019) Knockdown of lncRNA ZFAS1 inhibits progression of nasopharyngeal carcinoma by sponging miR-135a. Neoplasma 66:939–945
Ge HB, Chen S, Huang SR, Zhu J (2019) Long noncoding RNA ZFAS1 acts as an oncogene by targeting miR-193a-3p in human non-small cell lung cancer. Eur Rev Med Pharmacol Sci 23:6516–6523
Han CL, Ge M, Liu YP, Zhao XM, Wang KL, Chen N, Hu W, Zhang JG, Li L, Meng FG (2018) Long non-coding RNA H19 contributes to apoptosis of hippocampal neurons by inhibiting let-7b in a rat model of temporal lobe epilepsy. Cell Death Dis 9:617
Bielefeld P, Mooney C, Henshall DC, Fitzsimons CP (2017) miRNA-mediated regulation of adult hippocampal neurogenesis; implications for epilepsy. Brain Plast 3:43–59
Farooqi AA, Qureshi MZ, Coskunpinar E, Naqvi SK, Yaylim I, Ismail M (2014) MiR-421, miR-155 and miR-650: emerging trends of regulation of cancer and apoptosis. Asian Pac J Cancer Prev 15:1909–1912
Hu TB, Chen HS, Cao MQ, Guo FD, Cheng XY, Han ZB, Li MQ (2018) MicroRNA-421 inhibits caspase-10 expression and promotes breast cancer progression. Neoplasma 65:49–54
Li X, Chen SH, Zeng JW (2019) MiR-421 is overexpressed and promotes cell proliferation in non-small cell lung cancer. Med Princ Pract 29:80–89
Wen X, Han XR, Wang YJ, Wang S, Shen M, Zhang ZF, Fan SH, Shan Q, Wang L, Li MQ, Hu B, Sun CH, Wu DM, Lu J, Zheng YL (2018) MicroRNA-421 suppresses the apoptosis and autophagy of hippocampal neurons in epilepsy mice model by inhibition of the TLR/MYD88 pathway. J Cell Physiol 233:7022–7034
Lee SH, Chun W, Kong PJ, Han JA, Cho BP, Kwon OY, Lee HJ, Kim SS (2006) Sustained activation of Akt by melatonin contributes to the protection against kainic acid-induced neuronal death in hippocampus. J Pineal Res 40:79–85
Duan W, Chen Y, Wang XR (2018) MicroRNA155 contributes to the occurrence of epilepsy through the PI3K/Akt/mTOR signaling pathway. Int J Mol Med 42:1577–1584
Liu AH, Chu M, Wang YP (2019) Up-regulation of Trem2 inhibits hippocampal neuronal apoptosis and alleviates oxidative stress in epilepsy via the PI3K/Akt pathway in mice. Neurosci Bull 35:471–485
Leite JP, Garcia-Cairasco N, Cavalheiro EA (2002) New insights from the use of pilocarpine and kainate models. Epilepsy Res 50:93–103
Peng J, Wang K, Xiang W, Li Y, Hao Y, Guan Y (2019) Rosiglitazone polarizes microglia and protects against pilocarpine-induced status epilepticus. CNS Neurosci Ther 25:1363–1372
Jang HJ, Kim JE, Jeong KH, Lim SC, Kim SY, Cho KO (2019) The neuroprotective effect of Hericium erinaceus extracts in mouse hippocampus after pilocarpine-induced status epilepticus. Int J Mol Sci 20:859
Racine RJ (1972) Modification of seizure activity by electrical stimulation II Motor seizure. Electroencephalogr Clin Neurophysiol 32:281–294
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Thijs RD, Surges R, O'Brien TJ, Sander JW (2019) Epilepsy in adults. Lancet 393:689–701
Billakota S, Devinsky O, Kim KW (2019) Why we urgently need improved epilepsy therapies for adult patients. Neuropharmacology 170:107855
Villa C, Lavitrano M, Combi R (2019) Long non-coding RNAs and related molecular pathways in the pathogenesis of epilepsy. Int J Mol Sci 20:4898
Wang L, Song LF, Chen XY, Ma YL, Suo JF, Shi JH, Chen GH (2019) MiR-181b inhibits P38/JNK signaling pathway to attenuate autophagy and apoptosis in juvenile rats with kainic acid-induced epilepsy via targeting TLR4. CNS Neurosci Ther 25:112–122
Wu Q, Yi X (2018) Down-regulation of long noncoding RNA MALAT1 protects hippocampal neurons against excessive autophagy and apoptosis via the PI3K/Akt signaling pathway in rats with epilepsy. J Mol Neurosci 65:234–245
Strasser A, Vaux DL (2018) Viewing BCL2 and cell death control from an evolutionary perspective. Cell Death Differ 25:13–20
Nagata S (2018) Apoptosis and clearance of apoptotic cells. Annu Rev Immunol 36:489–517
Schaaf MB, Keulers TG, Vooijs MA, Rouschop KM (2016) LC3/GABARAP family proteins: autophagy-(un)related functions. FASEB J 30:3961–3978
Salminen A, Kaarniranta K, Kauppinen A (2013) Beclin 1 interactome controls the crosstalk between apoptosis, autophagy and inflammasome activation: impact on the aging process. Ageing Res Rev 12:520–534
Li Q, Han Y, Du J, Jin H, Zhang J, Niu M, Qin J (2018) Alterations of apoptosis and autophagy in developing brain of rats with epilepsy: changes in LC3, P62, Beclin-1 and Bcl-2 levels. Neurosci Res 130:47–55
Heras-Sandoval D, Perez-Rojas JM, Pedraza-Chaverri J (2020) Novel compounds for the modulation of mTOR and autophagy to treat neurodegenerative diseases. Cell Signal 65:109442
Liu Q, Ma H, Sun X, Liu B, Xiao Y, Pan S, Zhou H, Dong W, Jia L (2019) The regulatory ZFAS1/miR-150/ST6GAL1 crosstalk modulates sialylation of EGFR via PI3K/Akt pathway in T-cell acute lymphoblastic leukemia. J Exp Clin Cancer Res 38:199
Wang X, Jin Q, Wang X, Chen W, Cai Z (2019) LncRNA ZFAS1 promotes proliferation and migration and inhibits apoptosis in nasopharyngeal carcinoma via the PI3K/AKT pathway in vitro. Cancer Biomark 26:171–182
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no financial conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hu, F., Shao, L., Zhang, J. et al. Knockdown of ZFAS1 Inhibits Hippocampal Neurons Apoptosis and Autophagy by Activating the PI3K/AKT Pathway via Up-regulating miR-421 in Epilepsy. Neurochem Res 45, 2433–2441 (2020). https://doi.org/10.1007/s11064-020-03103-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-020-03103-1