Abstract
Epidemiological evidence has shown higher susceptibility of Children to the adverse effects of lead (Pb) exposure. However, experimental studies on Pb-induced neurotoxicity in prepubertal (PP) rats are limited. The present study aimed to examine the propensity of ferulic acid (FA), a commonly occurring phenolic acid in staple foods (fruits, vegetables, cereals, coffee etc.) to abrogate Pb-induced toxicity. Initially, we characterized Pb-induced adverse effects among PP rats exposed to Pb acetate (1,000–3,000 ppm in drinking water) for 5 weeks in terms of locomotor phenotype, activity of 5-aminolevulinic acid dehydratase (ALAD) in the blood, blood Pb levels and oxidative stress in brain regions. Further, the ameliorative effects of oral supplements of FA (25 mg/kg bw/day) were investigated in PP rats exposed to Pb (3,000 ppm). Pb intoxication increased the locomotor activity and FA supplements partially reversed the phenotype, while the reduced ALAD activity was also restored. FA significantly abrogated the enhanced oxidative stress in cerebellum (Cb) and hippocampus (Hc) as evidenced in terms of ROS generation, lipid peroxidation and protein carbonyls. Further, Pb-mediated perturbations in the glutathione levels and activity of enzymic antioxidants were also markedly restored. Furthermore, the protective effect of FA was discernible in striatum in terms of reduced oxidative stress, restored cholinergic activity and dopamine levels. Interestingly, reduced activity levels of mitochondrial complex I in Cb and enhanced levels in Hc among Pb-intoxicated rats were ameliorated by FA supplements. FA also decreased the number of damaged cells in cornu ammonis area CA1 and dentate gyrus as reflected by the histoarchitecture of Hc among Pb intoxicated rats. Collectively, our findings in the PP model allow us to hypothesize that ingestion of common phenolics such as FA may significantly alleviate the neurotoxic effects of Pb which may be largely attributed to its ability to abrogate oxidative stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pb is a ubiquitous and a non-essential toxic heavy metal widely distributed in the environment. Human exposure to Pb occurs via food, water, air and soil. It is also prevalent in occupational settings such as smelters and Pb manufacturing industries [1]. Despite improvements in public health policies and substantial reductions in blood Pb levels in adults, Pb exposure remains an important health problem worldwide. Pb is a systemic toxicant affecting virtually every organ system and notably, the central nervous system [2]. Nervous system is the primary target for the low levels of Pb-exposure, and the developing brain is highly vulnerable to Pb toxicity than the mature brain [3, 4]. Several mechanisms are implicated in the pathogenesis of Pb-induced neurotoxicity, including oxidative stress mitochondrial dysfunction and excitotoxicity [5]. Oxidative stress can induce brain injury via oxidative damage to critical biomolecules, such as lipids, proteins, and DNA [6].
Pb exposure is shown to cause generation of an excessive amount of ROS and alteration of defense system in animals and causes disruption of pro/antioxidant balance in the developing rat brain [7–9]. In addition, 5-aminolevulinic acid dehydratase (ALA-D), an enzyme of heme biosynthesis that is highly sensitive to lead [10] is inhibited by low blood lead levels. On the other hand, ALA-D inhibition contributes to ROS generation and is used as one of the most-reliable indicators of lead intoxication [11].
Phytochemicals are a heterogenous group of bioactive compounds that are extensively researched for their health-promoting potentials in humans [12]. The protective effect of several antioxidant nutrients against Pb-neurotoxicity has been investigated in experimental animals. Antioxidants such as ascorbic acid ameliorate oxidative damage induced by maternal low-level lead exposure in the hippocampus (Hc) of rat pups during gestation and lactation [13]. Recent studies have demonstrated the protective effect of quercetin, a major bioflavonoid abundant in fruits and vegetables against Pb-induced oxidative stress and impairment of synaptic plasticity [14]. Likewise previously, Gallic acid, a natural hydrolysis product of tannins (found abundantly in grapes, berries, and other fruits as well as in wine) was shown to attenuate Pb-induced oxidative and locomotor damage in rats [15]. Green tea extract and their components are partially efficacious in protection, and preventing disturbances of antioxidant defense system in the brain of Pb treated rats [16]. Other natural flavonoids such as puerarin, gossypin and curcumin, are also reported to protect against Pb-induced perturbations in hepatic antioxidant system, reduce brain oxidative stress and memory deficits in rats [2, 17, 18].
Ferulic acid (trans-4-hydroxy-3-methoxycinnamic acid; FA), one of the most-abundant phenolic compounds in the human diet, is synthesized in plants by the metabolism of phenylalanine and tyrosine. FA is present in seed plants (rice, wheat and oat), vegetables (tomato and carrot), and fruits (pineapple and orange) and possess excellent free radical scavenging and antioxidant properties [19–21]. FA possesses high antioxidant potential owing to its resonance-stabilized phenoxy radical structure. In the context of neurological disease, intravenous administration of FA has been shown to protect against neuronal cell death induced by cerebral ischemia [22, 23]. Interestingly, FA is also known to promote neural progenitor cell proliferation in vitro/in vivo, ameliorate stress-induced depression-like behavior in mice [24] and improves behavioral impairments and Alzheimer-like pathology in transgenic Mice [25].
The ubiquitous distribution of FA and its pleiotropic biological activity entails basic understanding on the potential neuroprotective effects under chronic Pb-intoxication in experimental animals. Accordingly, we examined the hypothesis that short-term oral supplements of FA may significantly alleviate Pb-induced phenotype, oxidative stress and neurotoxicity employing a prepubertal (PP) rat model. Initially, we recapitulated some of the adverse effects of Pb in PP rats employing 5-week regimen. Subsequently, the efficacy of oral FA supplements to ameliorate oxidative stress response in brain regions (Cb, Hc and St), cholinergic function and dopamine (DA) levels were investigated among PP rats subjected to Pb intoxication at the higher dosage. Since Pb exposure is known to cause specific effects on hippocampal function, we also examined the potential of FA to modulate histological lesions in Hc.
Materials and Methods
Chemicals and Reagents
Thiobarbituric acid (TBA), 1,1,3,3‐tetramethoxy propane, 2′,7′‐dichlorofluorescein (DCF), 2′,7′‐dichlorofluorescein diacetate (DCFH‐DA), 5-aminolevulinic acid hydrochloride and lead (II) acetate trihydrate, Ferulic acid (4-Hydroxy-3-methoxycinnamic acid) were purchased from M/s Sigma Chemical Co. St. Louis, USA. All other chemicals used were of analytical grade.
Animals and Care
Prepubertal (PP) Wistar rats (4 week‐old, 60 ± 5 g) drawn from the stock colony of our animal house facility were housed in rectangular polypropylene cages kept on racks built of slotted angles, in a controlled atmosphere with a 12 h light/dark cycle. They were acclimatized for 3 days prior to the start of the experiment. The animals were maintained on a commercial powdered diet and tap water ad libitum. Studies were conducted strictly in accordance with approved guidelines by the “Institute Animal Ethical Committee” regulated by the committee for the purpose of control and supervision of experiments on animals (CPCSEA), Ministry of Environment and Forests, Government of India, India. Handling, as well as care of animals during sacrifice, was strictly according to the standard guidelines of the Institutional Ethics Committee (registration No. 49/1,999/CPCSEA).
Experimental Design
Study 1:
For this study, male PP rats were randomly assigned to four groups (n = 6), while the first one as served as control, rats of other groups received lead acetate (PbA) daily in deionized drinking water at concentrations of 1,000, 2,000 and 3,000 ppm for 5-weeks. Terminally rats of all groups were sacrificed.
(1) Control group received Pb-free deionized water during the whole course of the experiment; (2) FA treated group, considered as positive control received daily oral supplements of FA at (25 mg/kg bw/day); (3) Pb group, animals received lead acetate (PbA) 3,000 ppm in deionized drinking water and (4) Pb + FA treated group, animals received PbA in drinking water and oral FA supplements. The dosage selection of Pb was based on the first study and published works. Further the criterion of selection of FA dosage was based on our preliminary dose finding studies in rats [26]. The duration of the experiment was 5 weeks and terminally rats were sacrificed, and blood samples and brain regions were processed as follows. Prior to sacrifice, rats of all groups were subjected to behavioral assessment.
In both studies, blood samples were drawn by cardiac puncture. Brain was excised and placed in 0.9 % ice-cold NaCl solution and cleaned with the physiological saline solution to remove blood cells, blotted on filter paper. The brain was immediately stored at −80 °C for later use.
Behavioral Assessment-Motor Activity
Spontaneous motor activity was assessed by placing rats in an open-field box (76 cm × 76 cm, divided by 8 lines into 25 squares). Each rat was individually placed in the centre of the open-field and allowed to explore it for 5 min. During this time, the motor activity was recorded in the open-field [27]. The number of squares crossed with all paws (crossing) and standing on legs (rearing) was evaluated during 5-min sessions. The crossing and rearing numbers were indicators of locomotor and exploratory activity, respectively. The arena was carefully cleaned with 70 % ethanol solution between animal tests to eliminate any olfactory cue derived from the previous rat located in the box.
Markers of Pb Intoxication
Activity of d-Aminolevulinic Acid Dehydratase in Blood
The activity of blood d-ALAD was assayed according to the procedure of [28]. The assay system consisted of 0.1 mL of heparinized blood and 0.75 mL of distilled water. After 10 min of incubation at 37 °C for complete hemolysis, 0.5 mL of standard d-aminolevulinic acid (ALA) was added to the tubes and incubated for 60 min at 37 °C. The reaction was stopped after 1 h by adding 0.5 mL of trichloroacetic acid. To the clear supernatant, an equal volume of Ehrlich’s reagent was added, and the absorbance was recorded at 555 nm after 5 min.
Blood Pb Levels
Blood samples were collected in heparinized tubes via cardiac puncture of anesthetized rats. Blood was digested with concentrated nitric acid and hydrogen peroxide. Digests were diluted to a constant volume with ultra-pure Nitric acid (2N). Blood lead levels were determined following the procedure [29] with some modifications using inductively coupled plasma optical emission spectrometer (ICPOES, Spectro Analytical Instruments, Model Ultima 2, Horiba Jobin–Yvon S.A.S, France) with axial view configuration and cross flow nebulizer coupled to a double pass-Scott type nebulization spray chamber. Lead was determined at 168.215 and 320.353 nm, which corresponds to the most-intense lines for the measurement of this element. Both wavelengths were monitored simultaneously to investigate possible spectral interferences in the quantification of the analyte. The operating conditions of the ICP OES equipment were radiofrequency power set at 1400 W, argon used for plasma generation, and plasma gas flow rates set at 14, 1.00, and 0.90 L/min for principal, auxiliary and nebulizer gas, respectively. The calibration standards were prepared from a Pb standard solution by sequential dilution in 5 % nitric acid in the range of 0.1–1 μg/L. The operating conditions used for ICP OES were selected according to the manufacturer’s recommendations. The samples were appropriately diluted in 5 % HNO3 (v/v) by the factor necessary for them to stay in the same concentration range of the calibration curve.
Histopathologic Evaluation
Brain tissue was placed in 10 % formaldehyde for 2 h. The brains were removed and placed in a new-formaldehyde solution for 24 h before being dehydrated using ethanol (70 % for 24 h, 90 % for 1 h and 100 % for 1 h) then cleaned in xylene and embedded in paraffin. Coronal Sections (5 μm thick) were cut with a microtome (Leica RM 2025, Germany), mounted on glass slides and stained with Hematoxylin and Eosin. The sections were analyzed by observing under light microscope (Leitz, Germany) at 20× magnification.
Biochemical Determinations
Preparation of Cytosol and Mitochondria:
Brain regions (Cb, Hc and St) were dissected over ice. A 10 % homogenate of was prepared in ice-cold Tris-sucrose buffer (0.25 M, pH 7.4) and centrifuged at 1,000g for 10 min at 4 °C to obtain the nuclear pellet. Differential centrifugation (700 and 4,500g, 10 min, 4 °C) was employed to isolate cytosol and mitochondria from different brain regions. The crude mitochondrial pellet was washed and resuspended using HEPES buffer (HEPES 10 mM, EDTA 0.1 mM, mannitol 200 mM, sucrose 70 mM, pH 7.4; [30]. All samples were stored in aliquots at −80 °C until next use.
Assessment of Lipid Peroxidation (LPO):
LPO was assessed by measuring the formation of thiobarbituric acid reactive substances (TBARS) following the method described previously [31]. Briefly, the reaction mixture contained 0.2 mL of cytosol, 1.5 mL of acetic acid (pH 3.5, 20 %), 1.5 mL of 0.8 % TBA (0.8 % w/v) and 0.2 mL SDS (8 % w/v). The mixture was heated to boiling for 45 min and TBARS adducts were extracted into 3 mL of 1-butanol and its absorbance was measured at 532 nm and quantified as malondialdehyde (MDA) equivalents using 1, 1, 3, 3-tetramethoxypropane as the standard.
Measurement of ROS Generation:
ROS generation in brain regions was assayed using dihydro dichlorofluorescein diacetate (H2 DCFH-DA) as described previously [32]. The reaction mixture (1 mL) containing Locke’s buffer (pH 7.4), 0.2 mL cytosol and 10 μl of DCFH-DA (5 μM) was incubated for 15 min at room temperature. After 30 min of further incubation, the formation of the fluorescent product DCF was measured in a spectrofluorimeter (excitation: 484 nm and emission: 530 nm). ROS formation was quantified from a DCF-standard curve and data are expressed as pmol DCF formed/min/mg protein.
Measurement of Hydroperoxide Levels:
An aliquot of cytosol (100 μg protein) was added to 1 mL FOX reagent (100 μM xylenol orange; 250 μM ammonium ferrous sulphate; 100 μM sorbitol; 25 mM H2SO4) and incubated for 30 min at room temperature [33]. The mixture was centrifuged at 600×g, and the supernatant was quantified at 560 nm in a spectrophotometer. Results were expressed as μmol hydroperoxide/mg protein.
Protein Carbonyls:
Protein carbonyl content in the brain regions were determined as described earlier [34]. An aliquot of cytosol was incubated with 2, 4-dinitrophenyl hydrazine (DNPH) for 60 min at room temperature. Following precipitation by adding 20 % trichloroacetic acid, the pellet was washed with acetone and dissolved in 1 mL of Tris buffer containing sodium dodecyl sulphate (8 % w/v, pH 7.4). The absorbance was measured at 360 nm and expressed as nmol carbonyls/mg protein (€-22,000 nmol/cm).
Determination of GSH:
GSH levels were quantified by a fluorimetric method [35]. An aliquot of cytosol was added to 1 mL formic acid (0.1 M) and centrifuged at 10,000×g for 10 min. An aliquot of the supernatant was used for the assay. Concentration of GSH was calculated from the standard curve and the expressed as μg GSH/mg protein.
Activities of Antioxidant Enzymes
Superoxide dismutase (SOD) activity was measured by monitoring the inhibition of quercetin autoxidation [36]. Glutathione reductase (GR) and Thioredoxin reductase (TRR) activity was measured as described previously [37, 38]. Glutathione-S-transferase (GST) activity was measured by monitoring the rate of enzyme–catalyzed conjugation of reduced glutathione with 1–chloro–2, 4–dinitrobenzene and expressed as µmol of conjugate formed per min per mg protein (€–9.6/mM/cm) [39]. The activity of glutathione peroxidase (GPx) was determined using t-butyl hydroperoxide as the substrate and activity expressed as nmol of NADPH oxidized per min per mg protein (€-6.22/mM/cm) [40].
Mitochondrial Functions
MTT Assay:
MTT reduction was determined as described previously [41]. Brain mitochondria were incubated in a Mannitol-Sucrose-HEPES buffer (pH 7.4) containing sodium succinate (20 mM) and 15 µL MTT (5 mg/mL) at 37 °C for 60 min. The formazan crystals formed were dissolved in aqueous 10 % SDS-45 % DMF buffer (v/v, pH 7.4). The absorbance of the clear solution was measured at 570 nm and expressed as OD/mg protein.
Activity of ETC Enzymes:
The activities of NADH-cytochrome C reductase (Complex I–III) and succinate-cytochrome C reductase (Complex II–III) were measured in brain mitochondria as described previously [42, 43]. Briefly mitochondrial protein (0.1 mg) was added to phosphate buffer (0.1 M, pH 7.4) containing NADH (0.2 mM) and KCN (1 mM). The reaction was initiated by the addition of 0.1 mM cytochrome C giving a final volume of 1 mL reaction mixture and decrease in absorbance was monitored for 3 min at 550 nm. The activity was expressed as nmol cytochrome C reduced/min/mg protein (€-19.6/mM/cm). To determine the activity of succinate-cytochrome C reductase, the substrate succinate (20 mM) was used.
Cholinergic Activity and Dopamine Levels
Acetylcholinesterase (AChE) activity and Butyrylcholinesterase (BChE): AChE and BChE activities were determined as described earlier [44]. To 1 mL reaction mixture containing phosphate buffer (0.1 M, pH 8.0), DTNB (10 mM), 50 μL cytosol and 20 μL acetylthiocholine iodide-/butyrylthiocholine iodide (78 mM) were added, and change in absorbance was monitored for 3 min at 412 nm. The enzyme activities were expressed as nmoles of substrate hydrolyzed/min/mg protein.
Dopamine (DA) Estimation by HPLC:
Dopamine (DA) levels in striatum were analyzed [45] using HPLC system (Model Shimadzu LC‐10 Avp, UV–Visible detector (SPD‐10 Avp), Pump (LC‐10 ATvp), Shimadzu, Japan) linked to the injection valve with 20 μL sample loop (Model 7125, Rheodyne, CA, USA). Sample was injected to a reversed‐phase column (Make: ‘Discovery’ Supelco Sigma‐Aldrich; C‐18, 25 cm, 4.6 mm, 5 μm) accessorized with an ultraviolet detector (280 nm). Analyses were all performed at room temperature. The sample was eluted using the mobile phase 0.2 % aqueous trifluoroacetic acid and methanol (70:30, v/v) at the flow rate of 1 mL/min. The peak corresponding to DA would appear between 3.4 and 3.7 min. DA levels were calculated from an external standard curve as μg DA/mg protein, and data is represented as percent change.
Determination of Protein
Protein concentration of cytosol and mitochondria was determined as described earlier [46] using bovine serum albumin as standard.
Data Presentation and Statistical Analysis
Data expressed as mean ± standard error (SE) for each experimental group were analyzed by one-way ANOVA followed by a post hoc ‘Turkey’ tests to compare the control and treatment groups; P-values <0.05 were considered as statistically significant. All statistical analysis was performed using SPSS statistical software package version 17.0.
Results
Effect of Pb Acetate
Effect on Locomotor Activity
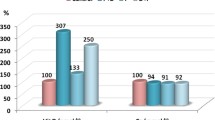
The effect of Pb exposure (low dose −1,000 ppm, mid dose −2,000 ppm and high dose −3,000 ppm) on motor activity in the open field test is shown in Fig. 1. While Pb exposure at low and mid dose had no significant effect on the crossing and rearing activity in open field test, a marked increase in crossing (62 %) and a decrease in rearing (40 %) activity was evident at the highest dose (Fig. 1a, b).
Effect of lead acetate (1,000–3,000 ppm in drinking water) in prepubertal rats evaluated as number of crossings (a) and rearing activity (b) in a open field box; Modulatory efficacy of Ferulic acid (FA) supplements on Pb-induced effect on crossing (c) and rearing (d) activity among rats exposed to Pb (3,000 ppm in drinking water). Values are mean ± SE (n = 6); Data analyzed by one way ANOVA (P ≤ 0.05) followed by Turkey’s test (* compared to control; # compared to Pb 3,000), CTR-Control; Pb-lead acetate-3,000 ppm, FA-ferulic acid 25 mg/kg bw/day oral for 5 weeks
Effect on Hb and ALAD Activity Levels
The Hb levels were not significantly affected at the low and mid dose of Pb, while only a marginal decrease was observed at the high dose (Fig. 2a). However, we could observe a significant diminution in the blood ALAD activity (65 %) at all the concentrations in relation to the control group (Fig. 2b).
Effect of lead acetate (1,000, 2,000 and 3,000 ppm in drinking water) on hemoglobin (a) and activity of aminolevulinic acid dehydratase-ALAD (b) in prepubertal rats; Modulatory effect of Ferulic acid (FA) supplements on hemoglobin level (c) and activity of ALAD (d) in prepubertal rats exposed to lead (Pb-3,000 ppm in drinking water). Values are mean ± SE (n = 6); Data analyzed by one way ANOVA (P ≤ 0.05) followed by Turkey’s test. (* compared to control; # compared to Pb 3,000), CTR-Control; Pb-lead acetate-3,000 ppm, FA-ferulic acid 25 mg/kg bw/day oral for 5 weeks
Effect of Pb per se on Oxidative Stress Markers
Pb intoxication resulted in consistent induction of oxidative stress in brain regions of PP rats as evidenced by enhanced generation of ROS levels. High dose induced a robust increase in reactive oxygen species formation in all regions (Cb-25 %; Hc-90 %; St-46 %). However, the increase in MDA levels were not consistent and showed no dose-dependency (Table 1). Further, high dose of Pb also caused only marginal depletion in reduced GSH levels in Hc and St, while significant increase in protein carbonyl levels was evident in all regions (Cb-26 %; Hc-19 % and St-30 %) excepting cortex (Table 2).
Ameliorative Effects of Ferulic Acid
-
Effect on Locomotor Activity
The locomotor activity measured as crossing activity among Pb exposed rats was only partially restored by FA supplements (Fig. 1c, d), while no effect was evident with respect to rearing activity.
-
Effect of FA on Hb and ALAD Activity and Blood Pb Levels
The modulatory effect of FA on Pb-induced alterations in blood Hb and ALAD activity are shown in Fig. 2. FA had no effect on the Pb-induced decrease in Hb levels (Fig. 2c). Pb exposure significantly decreased blood ALAD activity (65 %) in relation to the control group. FA alone had no effects on this variable, but the co-treatment (Pb + FA) partially reversed the effect (Fig. 2d). As anticipated rats exposed to Pb showed significantly higher blood levels of the metal than the controls. However, FA treatment had no significant effect on the blood Pb levels (Data not shown).
-
Ameliorative Effects of FA on Oxidative Markers
-
Modulatory Effect on Oxidative Markers in Cerebellum and Hippocampus
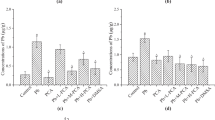
Pb caused differential degree of oxidative stress in brain regions. While the extent of reactive oxygen species generation was marginal in Cb, it was robust (93 %) in Hc clearly suggesting the higher susceptibility of this brain region. Interestingly, the ROS levels in both regions were restored to normal levels by FA supplements (Fig. 3a). Further Pb exposure decreased the hydroperoxide levels in Cb (18 %) and increased the levels in Hc (18 %), while FA supplements normalized the levels (Fig. 3b). Furthermore, Pb caused moderate elevation in MDA levels in both Cb (33 %) and Hc (30 %) while FA supplements completely normalized the MDA levels (Fig. 3c). Similarly, Pb caused a significant increase in protein carbonyl levels in Hc (16 %), while FA supplement marginally decreased the levels (Fig. 3d).
Modulatory effect of Ferulic acid (FA) supplements on ROS generation (a), Hydroperoxides (b), Malondialdehyde (c) and protein carbonyl content (d) in brain regions of prepubertal rats exposed to lead (Pb-3,000 ppm in drinking water). Values are mean ± SE (n = 6); Data analyzed by one way ANOVA (P ≤ 0.05) followed by Turkey’s test (* compared to control; # compared to Pb 3,000), CTR-Control; Pb-lead acetate-3,000 ppm, FA-ferulic acid 25 mg/kg bw/day oral for 5 weeks. Cb cerebellum, Hc hippocampus
-
Effect on Antioxidant Enzyme Activities in Cerebellum and Hippocampus
Pb exposure resulted in decreased SOD activity in Cb (27 %) and Hc (21 %), while FA supplement partially restored the levels (Fig. 4a). Further, Pb exposure resulted in increased GR activity in Cb (27 %) and Hc (29 %). However, FA supplements restored the elevated activity levels to normalcy in both the brain regions (Fig. 4b). Likewise, Pb exposure caused increased GPx activity in Cb (22 %) and decreased the activity levels in Hc (23 %). However, FA supplements enhanced the levels in Hc by 1.2 fold and restored the levels in Cb (Fig. 4c). Interestingly, Pb exposure caused enhanced TRR activity in both Cb and Hc. However, FA supplement had no effect on the elevated TRR activity levels (Fig. 4d). Among Pb exposed rats, GST activity was enhanced in Cb (45 %), and Hc (14 %) and FA supplements further enhanced the activity in Hc (Data not shown).
Modulatory effect of Ferulic acid (FA) on the activity levels of Superoxide dismutase (a), Glutathione reductase (b), Glutathione peroxidase (c) and thioredoxin reductase (d) in brain regions of prepubertal rats exposed to lead (Pb-3,000 ppm in drinking water). Values are mean ± SE (n = 6); Data analyzed by one way ANOVA (P ≤ 0.05) followed by Turkey’s test (* compared to control; # compared to Pb 3,000), CTR-Control; Pb-lead acetate-3,000 ppm, FA-ferulic acid 25 mg/kg bw/day oral for 5 weeks. Cb cerebellum, Hc hippocampus
-
Effect of FA on Mitochondria in Cerebellum and Hippocampus
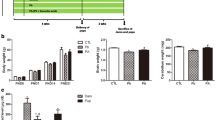
Pb exposure caused a significant decrease in the activity of complex I–III in Cb (31 %) and elevated the activity levels in Hc (64 %). However, FA supplements restored the activity levels to normalcy in both Cb and Hc (Fig. 5a). In contrast, Pb exposure caused a significant decrease in the activity of complex II–III in Cb (32 %) and Hc (15 %). However, FA supplements had no effect on diminished activity levels in Cb, but FA supplements further decreased the activity levels in Hc (Fig. 5b). Mitochondria of Pb exposed rats exhibited a significant reduction in the formation of formazan on exposure to MTT in Hc (23 %), and FA supplement offered significant protection in Hc (Fig. 5C).
Modulatory effect of Ferulic acid (FA) on the mitochondrial enzyme activity-Complex I–III (a) and Complex II–III (b), and MTT (c) in brain regions of prepubertal rats exposed to lead (Pb-3,000 ppm in drinking water). Values are mean ± SE (n = 6); Data analyzed by one way ANOVA (P ≤ 0.05) followed by Turkey’s test (* compared to control; # compared to Pb 3,000), CTR-Control; Pb-lead acetate-3,000 ppm, FA-ferulic acid 25 mg/kg bw/day oral for 5 weeks. Cb cerebellum, Hc hippocampus
-
Modulatory Effect of FA in Striatum
Data on the modulatory effect of FA against Pb (3,000 ppm)-induced perturbations in oxidative stress markers, protein carbonyls and activities of antioxidant enzymes in striatum of PP rats is presented in Table 3. FA supplements significantly diminished the ROS and HP levels, while there was no effect on MDA levels. The marginally depleted GSH levels among Pb rats were restored to normalcy by FA and a similar restorative effect was also observed in protein carbonyl levels. In contrast, Pb exposure resulted in marked diminution in the activity levels of SOD (43 %) and FA supplement further decreased the SOD levels (33 %). Similarly, Pb exposure resulted in increased activity levels of GR (89 %), GPx (47 %), TRR (27 %) and GST (20 %) levels. FA supplements restored the activity levels of GR and GPx, while no effect was evident in the activity levels of GST. On the other hand, the activity levels of TRR were elevated in Pb and FA supplemented rats. Interestingly, Pb caused a significant increase in the activity levels of complex I–III (45 %) and decreased the complex II–III (26 %) in striatum of PP rats. However, FA supplements restored the activity levels to normalcy suggesting the protective effect of FA in mitochondria. Further, FA supplements among Pb exposed rats, significantly offset the extent of MTT reduction as evident in the MTT reduction assay (Table 4).
-
Effect of FA on Cholinergic Function and Dopamine Levels in Striatum
Pb intoxication resulted in a marginal increase in the activity levels of AChE in striatum, while, FA supplements restored the activity levels (Fig. 6a). However, Pb exposure increased the BChE levels (by 16 %) and FA supplements further elevated the activity levels (Fig. 6b). Further, DA levels were significantly increased among Pb exposed rats, while the enhanced levels were completely restored with FA supplements (Fig. 6c).
Effect of Ferulic acid treatment on the activity levels of acetylcholinesterase (a), butyrylcholinesterase (b) and dopamine levels (c) in striatum among rats exposed to lead (Pb-3,000 ppm in drinking water). Values are mean ± SE (n = 6); Data analyzed by one way ANOVA (P ≤ 0.05) followed by Turkey’s test (* compared to control; # compared to Pb 3,000), CTR-Control; Pb-lead acetate-3,000 ppm, FA-ferulic acid 25 mg/kg bw/day oral for 5 weeks
-
Effect of FA on Pb-Induced Neuronal Damage in the Hippocampus
In order to determine implications of Pb-associated oxidative stress in the hippocampal region, we performed histopathologic analysis with H&E staining. Figure 7 represents the cornu ammonis area CA1 and the dentate gyrus (DG) regions of the Hc among control; Pb treated and Pb + FA treated groups. The hippocampi of the control group had a normal architecture and damaged cells were almost nonexistent in CA1and DG regions. The number of damaged neurons among the Pb-treated group was markedly increased in the CA1 and DG regions of the hippocampi compared to that of the control group. Interestingly, we found significantly reduced number of damaged neurons in CA1 and DG regions among the Pb + FA group compared to that of Pb-exposed group.
Hematoxylin and eosin stained sections of hippocampi of rats (5 week study). Control (CTR); Pb exposure (Pb 3,000 ppm); Pb + Ferulic acid (25 mg/kg bw). Degenerating neurons with shrunken and dark nuclei were markedly increased in cornu ammonis CA1 and DG hippocampal regions of Pb-exposed rats. The numbers of degenerating neurons were decreased among Pb + FA rats
Discussion
Pb toxicity is a persistent public health problem throughout the world and children are more susceptible than adults owing to their hand to mouth activity, increased respiratory rates and higher gastrointestinal absorption per unit body weight [5]. Epidemiological studies have shown that Pb exposure is associated with significant deficits in intelligence quotient of children and is associated with attention deficit hyperactivity disorder [47]. To alleviate Pb-associated toxic effects, several approaches such as chelation, antioxidant nutrients and their combination have been attempted [13–15]. It is in this context, that we examined the potential of FA supplements to ameliorate Pb-induced toxic effects employing a short-term dosing regimen. The criteria for using PP rats was based on the evidence that children exhibit enhanced susceptibility to Pb exposure and the brain is still in the process development of new inter neuronal connections which continue till the adult architecture is established by about 6–7 weeks. Hence, first we assessed the pattern of susceptibility of PP rats to varying dosages of Pb in terms of phenotype and induction of oxidative stress in brain regions. Further, with the objective of developing a pharmacological strategy, the propensity of FA, a ubiquitous naturally occurring antioxidant to ameliorate Pb-induced toxicity was investigated at the higher dosage.
In the present study, Pb exposure caused a significant reduction in the growth of rats, which is in agreement with previous reports [7]. Further, in the co-treatment paradigm, FA only marginally improved the reduction in the body weight. Pb exposure caused significant inhibition of ALAD activity, as shown previously [48]. ALAD is one of the most-reliable indicators of lead intoxication [11], whose inhibition contributes to the development of oxidative stress due to the accumulation of ALA, which is a substrate for ALAD. In fact, ALA may rapidly oxidize to generate ROS such as superoxide ion, hydroxyl radical, and hydrogen peroxides [49]. ALA is a potential endogenous source of free radicals and has been shown to cause oxidative damage to DNA in CHO cells in vitro through the formation of 8-OHdG adduct [50]. Further, ALAD inhibition decreases the biosynthesis of heme, which is the prosthetic group of many enzymes. We found that Pb-induced inhibition of ALAD activity was partially reversed by FA treatment. In contrast, FA supplements had no significant effect on the elevated blood Pb levels. Previously, several workers have adopted various strategies employing chelators to treat Pb poisoning and suggested that combinational therapy of antioxidants along with chelating agents may be a better treatment strategy than monotherapy to counter Pb-induced oxidative stress [51].
Evidence of oxidative damage associated with the presence of Pb in the brain clearly suggests a possible role of free radicals in the pathogenesis of Pb neurotoxicity [7, 52]. Oxidative stress can be induced as a result of a depressed antioxidant system, increased ROS production, or both. FA, a phenolic acid with pleiotropic biological activity and exhibits a wide range of pharmacological effects including anti-ageing, anti-inflammatory, anticancer, antidiabetic, antiapoptotic and neuroprotective [19]. It is an effective scavenger of free radicals and has been approved in certain countries as a food additive to prevent LPO [53]. It also acts as an antioxidant against peroxyl radical generator [54]. Hence, we reasoned that it may be relevant to understand the neuroprotective effects of FA in vivo under Pb intoxication. In the PP model, we evidenced significant elevation of ROS and MDA levels in Cb, Hc and St of Pb administered rats. Interestingly, FA supplements at the administered dose caused a significant decline of ROS and MDA levels in Cb and Hc. Accumulating evidence suggests that Pb causes oxidative stress and that MDA levels strongly correlate with Pb concentration in the brain of rats [55–57]. It is well established that Pb alters the lipid metabolism and enhances lipid peroxidation and increases brain TBARS through the inhibition of SOD in all brain regions [58]. Consistent with this the neuroprotective effect of epigallocatechin gallate (EGCG), the green tea polyphenol against Pb-induced neuronal damage is reported both in animal and cell model [59].
Earlier studies have shown that pre and neonatal Pb exposure altered the expression of antioxidant enzymes in the brain [9]. In the present model, Pb exposure caused significant perturbations in antioxidant enzyme activities such as SOD, catalase, and GPx, and changes in the concentrations of glutathione. SOD is considered as a first line of defense mechanism against ROS generation. Interestingly, FA supplements resulted in varying degree of restoration of SOD activity in Cb, which is in accordance with earlier results that FA effectively antagonizes the oxidative and nitrosative stress and inflammation as evidenced by down-regulated nitrite, LPO, IL-1β, TNF-α, and up-regulated GSH and SOD [60]. In the present study, Pb exposure resulted in altered GPx activity in Cb and Hc, which also corroborated with previous findings of decreased activity and protein expression of GPx in the frontal cortex, Cb and Hc. Furthermore, Pb exposure also resulted in altered activity levels of GR, TRR and GST, while FA supplements significantly restored the antioxidant enzyme activities clearly suggesting that its protective effects may be mediated through regulating oxidant/antioxidant defense, inflammatory and apoptotic signaling pathways. In the present study, Pb caused increased protein carbonyl levels in brain regions, viz., Cb, Hc, and St suggesting elevated protein oxidation as reported by previous workers [15]. FA supplements reduced the protein carbonyl levels only in the striatal region of the brain suggesting a specific effect of FA, which merits further study. In contrast, previously the antioxidant, gallic acid failed to reverse the Pb-induced protein carbonyl levels in rats [15].
Cerebral cortex and basal ganglia are brain areas involved in motor control and are intensely affected by Pb poisoning [3, 61, 62]. In view of this, in animal models, locomotor activity in open-field is often employed to assess the Pb-induced behavioral phenotype. PP rats exposed to Pb presented increased motor activity as observed by the increased number of crossings and decreased rearing movements, which are related to locomotor and exploratory activities, respectively. Interestingly, FA, supplements could partially reverse the locomotor and exploratory movements. The neuronal damage induced by Pb exposure is known to result in a reduction of the catecholaminergic transmission, either by inhibition of the DA synthesis and its release in the synaptic cleft or oxidative damages to the postsynaptic membranes [63, 64]. Since dopamine systems are important for central regulation of motor activity, we speculate that the observed behavioral phenotype reflects the effect of Pb on dopamine levels and its consequent oxidative stress.
Oral administration of FA has been demonstrated to increase the number of newly generated cells in the DG of the Hc of corticosterone treated mice, indicating that FA enhances the proliferation of neural stem/progenitor cells (NSC/NPCs) in vitro and in vivo [24]. Further FA was shown to increase cAMP response element binding protein (CREB) phosphorylation and brain-derived neurotrophic factor (BDNF) mRNA level in the Hc and ameliorative effect on the stress-induced depression-like behavior of mice. In the present model, evidence obtained data from histopathologic analysis indicated that supplementation of FA protected hippocampal neurons in the CA1 and DG regions against Pb-induced damage. Although speculative this specific effect of FA may be largely responsible for the ameliorative effect of FA against Pb-induced oxidative stress in the PP rat brain and may alleviate the associated cognitive deficits.
Despite intensive research efforts, the cellular mechanisms underlying the clinical manifestation of low-level Pb-induced neurotoxicity have remained elusive. Earlier studies have shown the role of neurotransmitter systems in Pb-induced behavioral perturbations and alterations in the properties of glutamatergic, cholinergic, and dopaminergic neurotransmitter function and signal transduction [64–66]. In the present study, Pb exposure resulted in increased cholinergic activity and enhanced DA levels in the striatal region, as reported previously [67]. Similarly, the locomotor activity and exploratory behavior were altered significantly among Pb-exposed animals corresponding to the alterations observed in cholinergic and aminergic systems [65]. Interestingly, FA supplements significantly altered both cholinergic and dopaminergic activity in PP rats. Further, recent studies have also shown that FA treatment reversed reserpine—induced behavioral abnormalities and decreased norepinephrine, serotonin and DA levels in the Hc and frontal cortex [60] and alleviated both learning/memory deficits in vascular dementia model of rats [68].
Collectively our findings clearly indicate that FA supplements during PP stages in rats could significantly ameliorate Pb-induced oxidative stress damage and reverse the altered antioxidant/mitochondrial enzyme activities. Although the precise mechanisms responsible for the neuroprotective efficacy of FA against Pb intoxication during PP stage warrants further investigation, our data for the first time, demonstrate its efficacy to attenuate Pb-mediated oxidative stress in brain regions and neurotoxicity. These findings emphasize the need for studies to understand the molecular events and the protective pathways which are responsible for the neuroprotective action of FA supplements under conditions of Pb-intoxication. Since oral FA supplementation is effective in reducing oxidative damage in brain regions and induces modifications in pathophysiological status under Pb-exposure, we propose its use as a potential complementary compound in the treatment of low-level Pb intoxication. Since FA is ubiquitously present in a variety of commonly consumed foods, increased consumption of FA-rich foods may reduce the neurotoxic implications of Pb among children.
Abbreviations
- ALAD:
-
Aminolevulinic acid dehydratase
- Pb:
-
Lead
- Cb:
-
Cerebellum
- St:
-
Straitum
- ROS:
-
Reactive oxygen species
- MDA:
-
Malondialdehyde
- HP:
-
Hydroperoxides
- AChE:
-
Acetylcholinesterase
- GSH:
-
Reduced Glutathione
- HPLC:
-
High performance Liquid chromatography
- DA:
-
Dopamine
References
Kim S, Hyun J, Kim H, Kim Y, Kim E, Jang J, Kim K (2011) Effects of lead exposure on nitric oxide-associated gene expression in the olfactory bulb of mice. Biol Trace Elem Res 142:683–692
Dairam A, Limson JL, Watkins GM, Antunes E, Daya S (2007) Curcuminoids, curcumin, and demethoxycurcumin reduce lead-induced memory deficits in male Wistar rats. J Agric Food Chem 55:1039–1044
Prasanthi RPJ, Devi CB, Basha DC, Reddy NS, Reddy GR (2010) Calcium and zinc supplementation protects lead (Pb)-induced perturbations in antioxidant enzymes and lipid peroxidation in developing mouse brain. Int J Dev Neurosci 28:161–167
Jin Y, Yu F, Liao Y, Liu S, Liu M, Xu J, Yang J (2011) Therapeutic efficiency of succimer used with calcium and ascorbic acid in the treatment of mild lead-poisoning. Environ Toxicol Pharmacol 31:137–142
Ahamed M, Siddiqui MKJ (2007) Low level lead exposure and oxidative stress: current opinions. Clin Chim Acta 383:57–64
Bokara KK, Brown E, McCormick R, Yallapragada PR, Rajanna S, Bettaiya R (2008) Lead-induced increase in antioxidant enzymes and lipid peroxidation products in developing rat brain. Biometals 21:9–16
Toscano CD, Guilarte TR (2005) Lead neurotoxicity: from exposure to molecular effects. Brain Res Review 49:529–554
Neal AP, Guilarte TR (2010) Molecular neurobiology of lead (Pb2+): effects on synaptic function. Mol Neurobiol 42:151–160
Baranowska-Bosiacka I, Gutowska I, Marchlewicz M, Marchetti C, Kurzawski M, Dziedziejko V, Kolasa A, Olszewska M, Rybicka M, Safranow K, Nowacki P, Wiszniewska B, Chlubek D (2012) Disrupted pro- and antioxidative balance as a mechanism of neurotoxicity induced by perinatal exposure to lead. Brain Res 1435:56–71
Papanikolaou NC, Hatzidaki EG, Belivanis S, Tzanakakis GN, Tsatsakis AM (2005) Lead toxicity update. A brief review. Med Sci Monit 11:RA329–RA336
Pappas JB, Nuttall KL, Ahlquist JT, Allen EM, Banner W Jr (1995) Oral dimercaptosuccinic acid and ongoing exposure to lead: effects on heme synthesis and lead distribution in a rat model. Toxicol Appl Pharmacol 133:121–129
van Duynhoven J, Vaughan EE, Jacobs DM, Kemperman RA, van Velzen EJ, Gross G, Roger LC, Possemiers S, Smilde AK, Dore J, Westerhuis JA, Van de Wiele T (2011) Metabolic fate of polyphenols in the human superorganism. Proc Natl Acad Sci USA 108:4531–4538
Chang B-J, Jang B-J, Son TG, Cho IH, Quan FS, Choe NH, Nahm SS, Lee JH (2012) Ascorbic acid ameliorates oxidative damage induced by maternal low-level lead exposure in the hippocampus of rat pups during gestation and lactation. Food Chem Toxicol 50:104–108
Liu C-M, Zheng G-H, Cheng C, Sun J-M (2013) Quercetin protects mouse brain against lead-induced neurotoxicity. J Agric Food Chem 61:7630–7635
Reckziegel P, Dias VT, Benvegnú D, Boufleur N, Silva Barcelos RC, Seqat HJ, Pase CS, Dos Santos CM, Flores EM, Burger ME (2011) Locomotor damage and brain oxidative stress induced by lead exposure are attenuated by gallic acid treatment. Toxicol Lett 203:74–81
Khalaf AA, Moselhy WA, Abdel-Hamed MI (2012) The protective effect of green tea extract on lead induced oxidative and DNA damage on rat brain. Neurotoxicology 33:280–289
Gautam P, Flora SJS (2010) Oral supplementation of gossypin during lead exposure protects alteration in heme synthesis pathway and brain oxidative stress in rats. Nutrition 26:563–570
Liu C-M, Ma J-Q, Sun Y-Z (2011) Protective role of puerarin on lead-induced alterations of the hepatic glutathione antioxidant system and hyperlipidemia in rats. Food Chem Toxicol 49:3119–3127
Srinivasan M, Sudheer AR, Menon VP (2007) Ferulic Acid: therapeutic potential through its antioxidant property. J Clin Biochem Nutr 40:92–100
Barone E, Calabrese V, Mancuso C (2009) Ferulic acid and its therapeutic potential as a hormetin for age-related diseases. Biogerontology 10:97–108
Sultana R (2012) Ferulic acid ethyl ester as a potential therapy in neurodegenerative disorders. Biochim Biophys Acta 1822:748–752
Cheng C-Y, Su S-Y, Tang N-Y, Ho TY, Chiang SY, Hsieh CL (2008) Ferulic acid provides neuroprotection against oxidative stress-related apoptosis after cerebral ischemia/reperfusion injury by inhibiting ICAM-1 mRNA expression in rats. Brain Res 1209:136–150
Koh P-O (2012) Ferulic acid prevents the cerebral ischemic injury-induced decrease of Akt and Bad phosphorylation. Neurosci Lett 507:156–160
Yabe T, Hirahara H, Harada N, Ito N, Nagai T, Sanagi T, Yamada H (2010) Ferulic acid induces neural progenitor cell proliferation in vitro and in vivo. Neuroscience 165:515–524
Mori T, Koyama N, Guillot-Sestier MV, Tan J, Town T (2013) Ferulic acid is a nutraceutical β-secretase modulator that improves behavioral impairment and alzheimer-like pathology in transgenic mice. PLoS One 8:e55774
Denny Joseph KM, Muralidhara (2014) Neuroprotective efficacy of a combination of fish oil and ferulic acid against 3-nitropropionic acid-induced oxidative stress and neurotoxicity in rats: behavioural and biochemical evidence. Appl Physiol Nutr Metab 39:487–496
Cauli O, Morelli M (2002) Subchronic caffeine administration sensitizes rats to the motor-activating effects of dopamine D(1) and D(2) receptor agonists. Psychopharmacology 162:246–254
Berlin A, Schaller KH (1974) European standardized method for the determination of delta-aminolevulinic acid dehydratase activity in blood. Z Klin Chem Klin Biochem 12:389–390
Peixoto NC, Rocha LC, Moraes DP, Bebianno MJ, Dressler VL, Flores EM, Pereira ME (2008) Changes in levels of essential elements in suckling rats exposed to zinc and mercury. Chemosphere 72:1327–1332
Moreadith RW, Fiskum G (1984) Isolation of mitochondria from ascites tumor cells permeabilized with digitonin. Anal Biochem 137:360–367
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Girish C, Muralidhara (2012) Propensity of Selaginella delicatula aqueous extract to offset rotenone-induced oxidative dysfunctions and neurotoxicity in Drosophila melanogaster: implications for Parkinson’s disease. NeuroToxicology 33:444–456
Farant JP, Wigfield DC (1982) Biomonitoring lead exposure with delta-aminolevulinate dehydratase (ALA-D) activity ratios. Int Arch Occup Environ Health 51:15–24
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478
Mokrasch LC, Teschke EJ (1984) Glutathione content of cultured cells and rodent brain regions: a specific fluorometric assay. Anal Biochem 140:506–509
Kostyuk VA, Potapovich AI (1989) Superoxide–driven oxidation of quercetin and a simple sensitive assay for determination of superoxide dismutase. Biochem Int 19:1117–1124
Luthman M, Holmgren A (1982) Rat liver thioredoxin and thioredoxin reductase: purification and characterization. Biochemistry (Mosc) 21:6628–6633
Carlberg I, Mannervik B (1985) Glutathione reductase. Methods Enzymol 113:484–490
Guthenberg C, Alin P, Mannervik B (1985) Glutathione transferase from rat testis. Methods Enzymol 113:507–510
Flohé L, Günzler WA (1984) Assays of glutathione peroxidase. Methods Enzymol 105:114–121
Berridge MV, Tan AS (1993) Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch Biochem Biophys 303:474–482
Navarro A, Sánchez Del Pino MJ, Gómez C, Peralta JL, Boveris A (2002) Behavioral dysfunction, brain oxidative stress, and impaired mitochondrial electron transfer in aging mice. Am J Physiol Regul Integr Comp Physiol 282:R985–R992
Navarro A, Gomez C, López-Cepero JM, Boveris A (2004) Beneficial effects of moderate exercise on mice aging: survival, behavior, oxidative stress, and mitochondrial electron transfer. Am J Physiol Regul Integr Comp Physiol 286:R505–R511
Ellman GL, Courtney KD, Andres V, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Dalpiaz A, Filosa R, de Caprariis P, Conte G, Bortolotti F, Biondi C, Scatturin A, Prasad PD, Pavan B (2007) Molecular mechanism involved in the transport of a prodrug dopamine glycosyl conjugate. Int J Pharm 336:133–139
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, Canfield RL, Dietrich KN, Bornschein R, Greene T, Rothenberg SJ, Needleman HL, Schnaas L, Wasserman G, Graziano J, Roberts R (2005) Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect 113:894–899
Pande M, Flora SJS (2002) Lead induced oxidative damage and its response to combined administration of alpha-lipoic acid and succimers in rats. Toxicology 177:187–196
Hermes-Lima M, Pereira B, Bechara EJ (1991) Are free radicals involved in lead poisoning? Xenobiotica 21:1085–1090
Yusof M, Yildiz D, Ercal N (1999) N-acetyl-l-cysteine protects against delta-aminolevulinic acid-induced 8-hydroxydeoxyguanosine formation. Toxicol Lett 106:41–47
Velaga MK, Basuri CK, Robinson Taylor KS, Yallapragada PR, Rajanna S, Rajanna B (2014) Ameliorative effects of Bacopa monniera on lead-induced oxidative stress in different regions of rat brain. Drug Chem Toxicol 37:357–364
Vaziri ND, Lin C-Y, Farmand F, Sindhu RK (2003) Superoxide dismutase, catalase, glutathione peroxidase and NADPH oxidase in lead-induced hypertension. Kidney Int 63:186–194
Adam A, Crespy V, Levrat-Verny MA, Leenhardt F, Leuillet M, Demigne C, Remesy C (2002) The bioavailability of ferulic acid is governed primarily by the food matrix rather than its metabolism in intestine and liver in rats. J Nutr 132:1962–1968
Kanski J, Aksenova M, Stoyanova A, Butterfield DA (2002) Ferulic acid antioxidant protection against hydroxyl and peroxyl radical oxidation in synaptosomal and neuronal cell culture systems in vitro: structure-activity studies. J Nutr Biochem 13:273–281
Nehru B, Kanwar SS (2004) N-acetylcysteine exposure on lead-induced lipid peroxidative damage and oxidative defense system in brain regions of rats. Biol Trace Elem Res 101:257–264
Zhang J, Wang X-F, Lu ZB, Liu NQ, Zhao BL (2004) The effects of meso-2,3-dimercaptosuccinic acid and oligomeric procyanidins on acute lead neurotoxicity in rat hippocampus. Free Radic Biol Med 37:1037–1050
Zhang L, Corona-Morales AA, Vega-González A, Garcia-Estrada J, Escobar A (2009) Dietary tryptophan restriction in rats triggers astrocyte cytoskeletal hypertrophy in hippocampus and amygdala. Neurosci Lett 450:242–245
Wang J, Wu J, Zhang Z (2006) Oxidative stress in mouse brain exposed to lead. Ann Occup Hyg 50:405–409
Yin S-T, Tang M-L, Su L, Chen L, Hu P, Wang HL, Wang M, Ruan DY (2008) Effects of Epigallocatechin-3-gallate on lead-induced oxidative damage. Toxicology 249:45–54
Xu Y, Zhang L, Shao T, Ruan L, Wang L, Sun J, Li J, Zhu X, O’Donnell JM, Pan J (2013) Ferulic acid increases pain threshold and ameliorates depression-like behaviors in reserpine-treated mice: behavioral and neurobiological analyses. Metab Brain Dis 28:571–583
Moreira EG, Vassilieff I, Vassilieff VS (2001) Developmental lead exposure: behavioral alterations in the short and long term. Neurotoxicol Teratol 23:489–495
Ramesh GT, Manna SK, Aggarwal BB, Jadhav AL (2001) Lead exposure activates nuclear factor kappa B, activator protein-1, c-Jun N-terminal kinase and caspases in the rat brain. Toxicol Lett 123:195–207
NourEddine D, Miloud S, Abdelkader A (2005) Effect of lead exposure on dopaminergic transmission in the rat brain. Toxicology 207:363–368
Cory-Slechta DA, Stern S, Weston D, Allen JL, Liu S (2010) Enhanced learning deficits in female rats following lifetime pb exposure combined with prenatal stress. Toxicol Sci 117:427–438
Basha DC, Rani MU, Devi CB, Kumar MR, Reddy GR (2012) Perinatal lead exposure alters postnatal cholinergic and aminergic system in rat brain: reversal effect of calcium co-administration. Int J Dev Neurosci 30:343–350
Lasley SM, Gilbert ME (2000) Glutamatergic components underlying lead-induced impairments in hippocampal synaptic plasticity. NeuroToxicology 21:1057–1068
Nowak P, Szczerbak G, Nitka D, Kostrzewa RM, Sitkiewicz T, Brus R (2008) Effect of prenatal lead exposure on nigrostriatal neurotransmission and hydroxyl radical formation in rat neostriatum: dopaminergic-nitrergic interaction. Toxicology 246:83–89
Luo Y, Zhao HP, Zhang J, Wang J, Yang WL, Yang M, Liao ZG (2012) Effect of ferulic acid on learning and memory impairments of vascular dementia rats and its mechanism of action. Yao Xue Xue Bao 47:256–260
Acknowledgments
We wish to thank the Director, CFTRI for his keen interest in this study. The first author thank the Indian Council Medical Research (ICMR), New Delhi, for the award of a Senior Research Fellowship.
Conflict of interest
The authors declare that there are no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lalith Kumar, V., Muralidhara Ameliorative Effects of Ferulic Acid Against Lead Acetate-Induced Oxidative Stress, Mitochondrial Dysfunctions and Toxicity in Prepubertal Rat Brain. Neurochem Res 39, 2501–2515 (2014). https://doi.org/10.1007/s11064-014-1451-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-014-1451-7