Abstract
We investigated the effects of lead (Pb) and ascorbic acid co-administration on rat cerebellar development. Prior to mating, rats were randomly divided into control, Pb, and Pb plus ascorbic acid (PA) groups. Pregnant rats were administered Pb in drinking water (0.3% Pb acetate), and ascorbic acid (100 mg/kg) via oral intubation until the end of the experiment. Offspring were sacrificed at postnatal day 21, the age at which the morphology of the cerebellar cortex in developing pups is similar to that of the adult brain. In the cerebellum, Pb exposure significantly reduced Purkinje cells and ascorbic acid prevented their reduction. Along with the change of the Purkinje cells, long-term Pb exposure significantly reduced the expression of the synaptic marker (synaptophysin), γ-aminobutyric acid (GABA)–synthesizing enzyme (glutamic acid decarboxylase 67), and axonal myelin basic protein while ascorbic acid co-treatment attenuated Pb-mediated reduction of these proteins in the cerebellum of pups. However, glutamatergic N-methyl-d-aspartate receptor subtype 1 (NMDAR1), anchoring postsynaptic density protein 95 (PSD95), and antioxidant superoxide dismutases (SODs) were adversely changed; Pb exposure increased the expression of NMDAR1, PSD95, and SODs while ascorbic acid co-administration attenuated Pb-mediated induction. Although further studies are required about the neurotoxicity of the Pb exposure, the results presented here suggest that developmental Pb exposure disrupted normal development of Purkinje cells by increasing glutamatergic and oxidative stress in the cerebellum. Additionally, ascorbic acid co-treatment is beneficial in attenuating prenatal and postnatal Pb exposure–induced maldevelopment of Purkinje cells in the developing cerebellum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lead (Pb) is a nonessential heavy metal that is widely present in the environment. Recently, the burden of Pb has been greatly decreased by restriction of its use and omission from gasoline and paint in developed countries. However, environmental Pb contamination of air, soil, and water and past widespread use in batteries, water pipelines, Pb-based paints in old houses, cookware, toys, tobacco, and polyvinyl chloride products continue to threaten health in many countries [1]. Pb can be absorbed in various ways via inhalation of dust, skin contact, water ingestion, and placental/milk transmission from Pb-exposed mothers [1, 2]. Compared to the overt toxicity caused by high-dose Pb, the damage from exposure to low-dose Pb has been considered detrimental in neurodevelopment [3]. In addition to the brain, the liver, kidney, bones, and peripheral nerves are susceptible to Pb exposure [4,5,6]. Pb-induced toxicity is age-independent way in humans, but the highest risk occurs in children. During the developmental period, the brain is not protected from exogenous toxic agents including Pb, as the blood-brain barrier and glial cells are still immature [7]. Additionally, Pb has been reported to impair the blood-brain barrier and ingested Pb accumulates in the cerebellum, cortex, and hippocampus of the rat brain [8].
Pb-induced toxicity in the immature brain leads to maldevelopment, cognitive impairment, poor impulse control, attention deficit, and impaired language acquisition [9]. These prominent functional consequences of Pb exposure have attracted attention to the Pb-induced toxicity in the hippocampus and mesocorticolimbic systems [10,11,12,13,14]. Cory-Schlecta pointed out that neurotoxicity of Pb is mediated by altering the neurotransmission in the mesocorticolimbic system [10]. In addition, previous animal studies observed that Pb exposure during pregnancy is harmful to normal structural development of the cerebellar cortex [15, 16]. The age-dependent difference in Pb-induced toxicity of the cerebellum has been reported. Contrast to the developing cerebellum, mature cerebellum which has been reported as being resistant to the Pb-induced toxicity does not generate new neurons in the adult stage [17, 18]. Thus, the present study focused on the developmental period to examine the effect of early Pb exposure on the developing cerebellum at postnatal day (PND) 21, when the morphology is similar to that of the adult brain [19].
The primary goal of treatment for Pb exposure–induced toxicity is to induce its excretion. Calcium ethylenediaminetetraacetic acid is an effective chelating agent that removes Pb and prevents intoxication-induced damage and accumulation [20]. However, this chemical chelator has adverse effects including depression, vomiting, diarrhea, anorexia, and renal toxicity [21]. Ascorbic acid (vitamin C), an essential nutritional factor in humans and guinea pig, is useful against the Pb-induced toxicity by enhancing Pb excretion. Ascorbic acid exhibits scavenging ability for a wide range of reactive oxygen species, safety, and its low cost, making it useful for treatment of Pb poisoning in the present study. Additionally, ascorbic acid was effective in detoxifying the gestational Pb exposure–induced impairment in the cerebellum [16]. However, hitherto, the effect of Pb exposure during the prenatal and postnatal period on cerebellar development has not been clearly determined. Proper development of neurons, synapse formation, and subsequent signal transduction are required for synaptic plasticity and normal brain function, as well as control of balance and motor coordination, memory, and learning of motor skills [22, 23].
Therefore, the present study investigated the neurotoxicity of Pb exposure and the therapeutic effect of ascorbic acid co-administration on the developing cerebellum by investigating the neuronal, synaptic, and axonal development in the immature cerebellum of rat offspring.
Materials and Methods
Experimental Animals
Female (n = 15) and male (n = 5) Sprague Dawley rats (8 weeks old) purchased from Narabiotec Co., Ltd. (Seoul, Republic of Korea) were housed under conditions of adequate temperature (23 °C) and humidity (60%), with a 12-h light/12-h dark cycle. After 1 week of acclimation to a conventional environment, the animals were used for experiments. Animals were allowed free access to food (Purina 5008, Purina Korea, Korea) and tap water. Pregnancy was confirmed when sperm were detected on vaginal smears, or when vaginal plugs were present. The day on which this confirmation took place was designated as day 0. Male and female rats were separated again and pregnant females were housed singly in each cage for safe delivery and caring of offspring until the end of the experiments. Animals were handled and cared for in accordance with the NIH Guide for the Care and Use of Laboratory Animals, which was issued by the Institute of Laboratory Animal Resources, USA, 1996. The protocol of the present study was approved by the Institutional Animal Care and Use Committee of the Konkuk University (approval number KU18133).
Pb and Ascorbic Acid Treatment
Female Sprague Dawley rats were randomly divided into three groups: a control group (n = 5), a Pb group (n = 5), and a Pb plus ascorbic acid (PA) group (n = 5). Chemically, both organic and inorganic forms of Pb have health-threatening effects. Organic Pb accumulates in greater amounts in body organs than inorganic Pb [24]. Among diverse compounds, we selected Pb acetate for its long history of use and toxicity, water solubility, and high bioavailability [25]. While previous studies reported the toxicity of low dose (0.1%, 0.2%) and high dose (0.54%, 1%) of Pb, few studies investigated the effect of a moderate dose (0.3%) of Pb [12, 14,15,16, 26]. The doses of ascorbic acid were adopted from previous study [16]. Pb acetate (0.3%; Sigma-Aldrich, USA) was dissolved in distilled water with glacial acetic acid (0.05%; Junsei Chemical Co., Tokyo, Japan) to prevent Pb precipitation. Ascorbic acid (100 mg/kg; Sigma-Aldrich, USA) for oral administration was freshly prepared in saline daily. To adjust for the effects of stress during oral intubation, rats in the control and Pb groups were orally administered the same volume of saline through intubation. Pb and ascorbic acid treatment was started at 1 week prior to mating day and continued during pregnancy and delivery of offspring until the end of the experiment at postnatal day (PND) 21. The body weight of pups was measured and averaged every Monday morning. At birth, to avoid the effect of litter size among experimental groups, 6 rat offspring per cage (5 cages per group, offspring n = 30 per group) were randomly selected and the remaining pups were discarded. Whenever possible, only male pups were kept within the litters and females were kept only if necessary to maintain equal litter sizes. Before sacrifice, the sex of every pup was recorded. The researchers in the present study conducted experimental procedures carefully to minimize suffering and the number of animals used.
Atomic Absorption Spectrometry for Blood Pb Level Analysis
At PND21, pups (n = 30 per group) and dams (n = 5 per group) were anesthetized using 1.5 g/kg urethane (Sigma-Aldrich; Merck KGaA). Blood samples were collected from the heart for analysis of Pb levels using an atomic absorption spectrophotometer (PerkinElmer Zeeman 5100; Norwalk, CT, USA) and an HGA-600 graphite furnace with Zeeman background correction. The absorption wavelength was 283.3 nm and the r2 of the calibration curve was above 0.995.
Nissl Staining and Immunohistochemistry
For histological studies, the pups (n = 15 per group) were anesthetized using 1.5 g/kg urethane (Sigma-Aldrich; Merck KGaA) at PND21 and perfused transcardially with heparinized phosphate-buffered saline (PBS; 0.1 M, pH 7.4), followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The cerebella were removed and post-fixed in the same fixative overnight at 4 °C. Cerebellar tissues (n = 15 per group) were embedded in paraffin blocks and midsagittal sections of the vermis were cut into 5-μm slices at multiple levels. Three slides per pup were selected for each stain, and a total of 45 paraffin sections per group were used in the histological analysis. Nissl staining was conducted using routine procedures. Briefly, immunohistochemistry for marker proteins was conducted as follows. Deparaffinized sections were subjected to antigen retrieval using citrate buffer (pH 6.0). The sections were then sequentially treated with 0.3% hydrogen peroxide (H2O2) to quench endogenous peroxidase activity, and with 10% normal horse serum for blocking. Next, the sections were incubated overnight at 4 °C in antibodies to synaptophysin (1:500; Abcam, Cambridge, UK), postsynaptic density protein 95 (PSD95, 1:500; Abcam), N-methyl-d-aspartate receptor (NMDAR1, 1:500; Millipore, Billerica, MA, USA), myelin basic protein (MBP, 1:500; Millipore), or GAD67 (1:500; Stressgen, USA). Subsequently, sections were exposed to biotinylated IgG (1:200; Vector, Burlingame, CA, USA) and streptavidin peroxidase complex (1:200; Vector). They were then visualized by reaction with 3,3′-diaminobenzidine tetrachloride (DAB; Sigma) in 0.1 M Tris-HCl buffer (pH 7.2) and mounted on gelatin-coated slides. The sections were finally dehydrated and mounted on a toluene-based mounting medium (Richard-Allan Scientific, Thermo Scientific).
All histopathological analyses described above were performed by an investigator blinded to the rat treatment. The quantification method of the present study was modified from the recent study [27]. The numbers of Purkinje cells in the cerebellum were counted in micrographs obtained at × 100 magnification of Purkinje cell layer using arbitrary line probe of the DP2-BSW software (Olympus, Tokyo, Japan). The observations were carried out in the second, fifth, and eighth lobules of the sagittal section of the cerebellar vermis.
Measurement of Cerebellar Weight and Immunoblotting
To detect synaptophysin, NMDAR1, PSD95, GAD67, and MBP protein expression in the cerebellum, the brains of PND21 pups (n = 15 per group) were immediately dissected. The cerebella were weighed and the samples were frozen until use. The cerebellar tissues were homogenized in lysis buffer, centrifuged at 15,000g, and the supernatant was separated. Proteins were quantified using the Thermo Pierce® BCA protein assay kit (Thermo Fisher Scientific, Rockford, IL, USA). Aliquots containing 40 μg of total protein were boiled in loading buffer containing 150 mM Tris (pH 6.8), 3 mM dithiothreitol, 6% SDS, 0.3% bromophenol blue, and 30% glycerol. The aliquots were then loaded onto a 10% sodium dodecyl sulfate-polyacrylamide gel and proteins were separated. After electrophoresis, the gels were transferred to polyvinylidene fluoride membranes (Roche, Penzberg, Germany). The membranes were blocked by incubation in 5% skimmed milk in Tris-buffered saline (TBS; pH 7.4) for 1 h. Next, they were incubated overnight at 4 °C with primary antibody against either synaptophysin (rabbit, 1:5000, Abcam), PSD95 (rabbit, 1:2000, Abcam), NMDAR1 (rabbit, 1:1000, Millipore), GAD67 (1:1000, Stressgen), MBP (rabbit, 1:3000, Millipore), Cu,Zn-SOD (rabbit, 1:1000, Santa Cruz, CA, USA), or Mn-SOD (goat, 1:1000, Santa Cruz). The blots were washed three times in TBS containing 0.1% Tween-20, and then incubated with a horseradish peroxidase–conjugated secondary antibody (1:2000). Bands were visualized using SuperSignal® West Pico Chemiluminescent Substrate (Thermo Fisher Scientific, Rockford, IL, USA). After several rounds of blotting, the relative optical density (ROD) of each band was measured using NIH ImageJ software.
Statistical Analysis
Data are expressed as means ± standard errors of the mean for each group, and the significance of the differences between these mean values was determined using one-way analysis of variance (ANOVA) followed by Tukey’s test for multiple comparisons. All analyses were carried out using GraphPad Prism 5.01 software (GraphPad Software, Inc., La Jolla, CA, USA). P values < 0.05 were considered significant.
Results
Body Weight, Brain Weight, Cerebellar Weight, and Blood Lead Levels
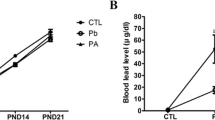
In the present study, there were no signs of Pb-induced toxicity on reproductivity of dams and external developmental features of pups during the experiment. The average number of pups (CTL 12.4, Pb 9.2, PA 10.2), sex ratio (♀:♂ = CTL 6:4, Pb 5:5, PA 5:5), and opening of both eyes in pups (PND13 and/or PND14) were not significantly affected by Pb exposure and ascorbic acid treatment. In addition, 0.3% Pb acetate (approximately 0.34 g/kg per body weight) exposure during pregnancy and lactation affected body weight gain, brain weight, and cerebellar weight in pups at PND21. A prominent decrease in brain and cerebellar weight were observed in the Pb-exposed pups while ascorbic acid treatment attenuated Pb-induced reduction. Atomic absorption spectrometry demonstrated that exposure increased the blood Pb level in dams and pups by the end of experiment, and ascorbic acid reduced the increase in blood levels with statistical significance only in dams (Fig. 1).
Experimental design of the study (a). Body weight of pups (n = 15 per group) during lactation and brain and cerebellar weight of pups at PND21 in the control (CTL), lead (Pb), and lead plus ascorbic acid (PA) groups (b). Blood lead level (c) of dams and pups at day 21 after delivery (*P < 0.05, compared with the CTL group; #P < 0.05, compared with the Pb group). The bars indicate means ± SE
Effects of Pb and Ascorbic Acid on Purkinje Cells in the Developing Cerebellum
To determine the effect of Pb and ascorbic acid on the cerebellum, we performed histopathologic analysis with Nissl staining. At PND21, Pb exposure significantly reduced the number of Purkinje cells in the cerebellum, while no changes were detected in the granule cell layer. Among the remaining Purkinje cells, degenerating pyknotic cells were detected in the Pb group. Ascorbic acid co-administration ameliorated the reduction of Purkinje cells in the cerebellum (Fig. 2).
Nissl stain (a–c) and immunohistochemistry for synaptophysin in the cerebellum (d–f) in pups at PND21 from the CTL, Pb, and PA groups. GCL, granule cell layer; ML, molecular layer; PL, Purkinje cell layer. Bar = 25 μm. g The number of Purkinje cells in the cerebellar cortex is expressed as a percentage of the value in the CTL group (n = 15 per group; *P < 0.05, compared with the CTL group; #P < 0.05, compared with the Pb group). The bars indicate the means ± SEM
Effect of Pb and Ascorbic Acid on Presynaptic Synaptophysin
Synaptophysin is expressed in presynaptic vesicular membranes. Synaptophysin was detected in the molecular layer and granule cell layer in the cerebellar cortex. The immunoblot results showed that the protein expression level of presynaptic synaptophysin was decreased by Pb exposure with statistical significance, while Pb and ascorbic co-administration increased its level compared to that in the Pb group. Additionally, synaptophysin immunoreactivity showed a change in the pattern of immunobloting in the cerebellum (Figs. 2 and 5).
Effect of Pb and Ascorbic Acid on NMDAR1 and PSD95
Postsynaptic NMDAR1 and PSD95 showed similar patterns of changes in the cerebellum. NMDAR1 translocation to the postsynaptic membrane is important for glutamatergic neuronal signal transduction. As immuonostaining results, NMDAR1 immunoreactivity was observed in Purkinje cells and some cells in the molecular layer. The reduction of NMDAR1-immunoreactive Purkinje cells did not parallel the change in the protein expressional level in the cerebellum. Its expression was increased by long-term Pb exposure and ascorbic acid co-administration ameliorated Pb-induced increases of the NMDAR1 in the cerebellum.
PSD95 was mainly detected in the Purkinje cell bodies and dendritic branches while it was also detected in cells and fibers in the molecular and granule cell layer of the cerebellum. In the cerebellum, the expression pattern was consistent to the NMDAR1 by showing a Pb-induced increase of PSD95 and ascorbic acid–mediated amelioration of induction of PSD95 by Pb. Abnormal increases of NMDAR1 and PSD95 in the cerebellum suggest that the long-term Pb exposure during gestation and lactation periods mediated excitotoxicity in the developing brain and ascorbic acid co-treatment reduced Pb-mediated toxicity in the brain (Figs. 3 and 5).
Immunohistochemistry for NMDAR1 (a–c) and PSD95 (d–f) in the cerebellum of pups at PND21 from the CTL, Pb, and PA groups. Note that numbers of NMDAR1-positive or PSD95-positive Purkinje cells are significantly reduced in the Pb group and ascorbic acid treatment ameliorated these reductions in the PA group. However, the protein expression level of NMDAR1 and PSD95 was increased by Pb treatment and ascorbic acid administration prominently ameliorated Pb-mediated induction in the cerebellum (Fig. 5). GCL, granule cell layer; ML, molecular layer; PL, Purkinje cell layer. Bar = 25 μm. e The number of NMDAR1-positive or PSD95-positive Purkinje cells is expressed as a percentage of the value in the CTL group in the cerebellar cortex (n = 15 per group; *P < 0.05, compared with the CTL group; #P < 0.05, compared with the Pb group). The bars indicate the means ± SEM
Effect of Pb and Ascorbic Acid on γ-Aminobutyric Acid–Synthesizing Enzyme (GAD67)
Cerebellar Purkinje cells are the only efferent neurons that mainly act as inhibitory regulators in the cerebellum [28]. γ-Aminobutyric Acid (GABA)–synthesizing GAD67 was detected in the somata, dendrites, and axonal projections of the Purkinje cells. GAD67 was also detected in the molecular layer of the cerebellum, where the GABAergic interneurons including the basket cells and stellate cells are located and dendrites of Purkinje cells are extended. After long-term exposure to Pb, the number of GAD67-immunoreactive Purkinje cells was prominently reduced and the dendritic branching of the Purkinje cells was poorly developed. However, ascorbic acid treatment restored the Pb-induced reduction and impairment of GAD67-immunoreactive Purkinje cells in the cerebellar cortex. The protein expression level of GAD67 in the whole cerebellum was also decreased by Pb exposure and ascorbic acid co-treatment upregulated GAD67 (Figs. 4 and 5).
Immunohistochemistry for GAD67 (a–c) and MBP (d–f) in the cerebellum of pups at PND21 from the CTL, Pb, and PA groups. Note that numbers of GAD67-positive Purkinje cells are significantly reduced in the Pb group and ascorbic acid treatment ameliorated these reductions in the PA group. MBP-positive myelinated fibers were detected in the granule cell layer in the gray matter (GM-GCL) and white matter (WM). GCL, granule cell layer; ML, molecular layer; PL, Purkinje cell layer. Bar = 25 μm. g The number of GAD67-positive Purkinje cells is expressed as a percentage of the value in the CTL group in the cerebellar cortex (n = 15 per group; *P < 0.05, compared with the CTL group; #P < 0.05, compared with the Pb group). The bars indicate the means ± SEM
Representative immunoblot for synaptophysin, NMDAR1, PSD95, GAD67, MBP, Cu,Zn-SOD, and Mn-SOD in the cerebellum (a) of pups at PND21 from the CTL, Pb, and PA groups. b Relative optical density (ROD) of immunoblot bands is demonstrated as a percentage of the value in the CTL group (n = 15 per group; *P < 0.05, compared with the CTL group; #P < 0.05, compared with the Pb group). The bars indicate the means ± SEM
Effect of Pb and Ascorbic Acid on Axonal Fibers and MBP
MBP is the main component protein of the myelin sheath of nerve fibers [29]. In the cerebellum, MBP was detected in the myelinated fibers of white matter tracts. After Pb exposure, the MBP immunoreactivity was decreased in the cerebellum. However, ascorbic acid co-administration ameliorated the reduction of MBP. Accordingly, the protein expression level of MBP was similar to that shown by staining by demonstrating Pb-induced reduction and ascorbic acid–induced amelioration (Figs. 4 and 5).
Effect of Pb and Ascorbic Acid on Cu,Zn-SOD and Mn-SOD
The protein expression levels of antioxidant Cu,Zn-SOD and Mn-SOD were compared. Long-term Pb exposure significantly increased their levels in the whole cerebellum from the Pb group while ascorbic acid treatment reduced Pb-mediated induction of these endogenous antioxidants. Mitochondrial Mn-SOD was prominently reduced by ascorbic acid treatment; however, cytoplasmic Cu,Zn-SOD was still higher than that of the CTL group (Fig. 5).
Discussion
The developing cerebellum is susceptible to Pb intoxication during neurogensis, migration, and maturation, and the effect is persistent from the fetal to early postnatal period when these processes are vulnerable to the influence of external sources of stress [23, 30]. Unaltered generation and maturation of neurons and subsequent synapse formation are key steps in brain development. In our study, we investigated the effect of prenatal and postnatal Pb exposure and ascorbic acid co-administration on the developing cerebellum.
In advance, Pb exposure showed significant effects on several physiological parameters in pups; the weight of the body, brain, and cerebellum was reduced by Pb exposure while ascorbic acid treatment ameliorated the Pb-induced weight reduction. The blood Pb level was significantly increased by Pb exposure while ascorbic acid treatment reduced the blood Pb level in the dams and pups. Compared to the dams, blood Pb level in the pups did not show statistical significance. Ascorbic acid–mediated excretion of the maternal Pb can be transferred to offspring via placental and milk transmission [31]. This uncoupling effect of ascorbic acid between dams and pups is associated with the excretion of Pb along with ascorbic acid which increases their levels in the milk [32, 33]. Lihm et al. also reported that ascorbic acid treatment was effective in excreting Pb by urine and stool [34]. As previous studies reported [16, 35], gestational ascorbic acid co-administration is effective in lowering the blood Pb level; however, the present exposure to a higher Pb concentration (0.3% solution) for a longer period significantly increased the blood Pb level in both the dams and pups.
Nissl staining showed that Pb exposure during gestation and lactation significantly impaired the development of Purkinje cells by causing significant reduction of their number and degenerating changes including pyknosis and vacuolization. Similar to the finding of a protective effect of ascorbic acid against Pb exposure in the present study, others reported that Pb exposure is harmful to a normal cerebellar development and ascorbic acid treatment was effective in preventing the detrimental effects of Pb, mercury, and cadmium on brain development [16, 35,36,37,38,39,40]. Vitamin B, allicin, α-tocopherol, and garlic extract also exhibited protective effects against Pb-induced maldevelopment of Purkinje cells [37, 38].
To evaluate the effect of Pb and ascorbic acid on Purkinje cell–related structures in the cerebellum, we conducted immunostaining and immunoblotting. During brain development, newly generated neurons are connected to each other by synapse formation and these processes increase the volume of the brain. In the developing cerebellum of rats, neurogenesis takes place during the fetal and early postnatal periods. During the maturation process, synaptogenesis and axonal myelination continue as the subsequent event in the neuronal development. In the present study, presynaptic synaptophysin was significantly reduced in the cerebellum at PND21 by Pb exposure during gestation and lactation. Ascorbic acid increased the protein expression level of synaptophysin in the cerebellum in the PA group. In contrast to synaptophysin, postsynaptic NMDAR1 was significantly increased in the cerebellum with Pb exposure, while ascorbic acid treatment prevented Pb-induced changes in the cerebellum. Similarly, a previous study reported that developmental Pb exposure upregulated the mRNA level of NMDAR1 in the cerebellum and hippocampus [41]. Along with the NMDAR1, we focused on the expression change of PSD95 which serves as a scaffolding protein that facilitates the assembly and glutamate-mediated signal transduction of NMDAR in the neuron [42]. Long-term Pb treatment prominently increased PSD95 expression and ascorbic acid treatment ameliorated Pb-mediated induction. In the present study, compared to the reduction of the NMDAR1- and PSD95-immunoreactive Purkinje cells, protein expression levels of the NMDAR1 and PSD95 were increased. We suggest that one of the reasons for this inconsistency is that some cells in the molecular layer and granule cell layer which were immunoreactive against NMDAR1 and PSD95 increased in the cerebellum after long-term Pb exposure. The present study also indicates the need of further studies for a clearer understanding of the effect of Pb on NMDAR1 and PSD95 in the brain such as the difference in the protein expressional level of individual cells and the pattern of change in the synaptic and non-synaptic portions of NMDAR.
Contrary to the Pb-induced upregulation of glutamatergic NMDAR1, the inhibitory GABA-synthesizing GAD67 was significantly decreased by Pb exposure and the Pb-induced reduction was attenuated by ascorbic acid co-administration in the cerebellum. The GAD67 enzyme was detected in the somata and dendrites of Purkinje cells and in the molecular layer of the cerebellum. The number of GAD67-immunoreactive Purkinje cells in the Pb group was lowest, while ascorbic acid co-treatment with Pb attenuated the reduction of GAD67-immunoreactive Purkinje cells in the cerebellum. Similarly, Bernocchi et al. also reported that platinum compounds negatively affect cerebellar development by reducing the GAD67 expression [43]. Along with the change in the GABA-synthesizing enzyme, Minnema and Michaelson previously reported that Pb can impair the release of GABA in the brain [44]. Based on these results, the disruption of the balance between excitation and inhibition might be associated with maldevelopment of the cerebellum.
MBP is required in the process of myelination of nerve fibers. MBP is an abundant myelin protein and it is a marker of oligodendrocytes, which form the myelin sheath around axons [29]. In this study, we confirmed that Pb exposure impaired MBP-immunoreactive fibers in the white matter in the cerebellum and reduced the expression of MBP while ascorbic acid attenuated Pb-induced loss. These are supported by an important study which demonstrated that Pb exposure causes hypomyelination in the rat brain [45]. Recent study also suggested that Pb induced disturbance of oligodendrocyte differentiation by inhibiting sodium/calcium exchanger [46]. Similar to the present protection effect of myelination in the cerebellum, ascorbic acid promoted myelination in in vitro cell cultures [47]. Additionally, we speculated that the reduction of efferent neuronal cells and axonal degeneration is linked with the reduction of MBP in the Pb-exposed cerebellum.
Oxidative stress is the primary mechanism of the Pb neurotoxicity. In response to the Pb-induced cellular oxidative stress, endogenous antioxidants including cytoplasmic Cu,Zn-SOD and mitochondrial Mn-SOD are increased in the cerebellum. This result suggests that long-term Pb-exposed cerebella are under the oxidative stress and Pb may continuously generate reactive oxygen species [48]. Cellular organelles in cytoplasm and mitochondria are the target of the Pb-induced toxicity and we previously observed that Pb-induced apoptotic cell death and mitochondrial pore-inducing Bax proteins were increased by Pb exposure [35, 49]. The subsequent reduction of Cu,Zn-SOD and Mn-SOD by ascorbic acid co-administration indicates that anti-oxidative property of ascorbic acid reduced the need of endogenous antioxidants induction.
Conclusion
Overall, this study demonstrated that Pb administration is toxic to the developing cerebellum by reducing Purkinje cells, synaptophysin, GABA-synthesizing GAD67, and MBP, while glutamatergic NMDAR1, scaffolding protein PSD95, and endogenous SODs were induced by Pb exposure. Along with the previously reported amelioration from Pb-induced apoptotic death [35], the present ascorbic acid treatment defended the cerebellum from excitotoxic glutamatergic activation and oxidative stresses. Although more future studies are required to clearly understand the mechanism of the Pb-induced neurotoxicity, the results presented here highlight Pb-induced developmental neurotoxicity and the potential protective effect of ascorbic acid in the cerebellum in the offspring of mothers who are continuously exposed to Pb during gestation and lactation.
References
Levin R, Brown MJ, Kashtock ME, Jacobs DE, Whelan EA, Rodman J, Schock MR, Padilla A, Sinks T (2008) Lead exposures in U.S. children, 2008: implications for prevention. Environ Health Perspect 116:1285–1293
Karita K, Shinozaki T, Tomita K, Yano E (1997) Possible oral lead intake via contaminated facial skin. Sci Total Environ 199:125–131
Neal AP, Guilarte TR (2010) Molecular neurobiology of lead (Pb2+): effects on synaptic function. Mol Neurobiol 42:151–160
Dai S, Yin Z, Yuan G, Lu H, Jia R, Xu J, Song X, Li L, Shu Y, Liang X, He C, Lv C, Zhang W (2013) Quantification of metallothionein on the liver and kidney of rats by subchronic lead and cadmium in combination. Environ Toxicol Pharmacol 36:1207–1216
Gangoso L, Alvarez-Lloret P, Rodríguez-Navarro AA, Mateo R, Hiraldo F, Donázar JA (2009) Long-term effects of lead poisoning on bone mineralization in vultures exposed to ammunition sources. Environ Pollut 157:569–574
Zahran S, Laidlaw MA, Rowe DB, Ball AS, Mielke HW (2017) Motor neuron disease mortality and lifetime petrol lead exposure: evidence from national age-specific and state-level age-standardized death rates in Australia. Environ Res 153:181–190
Saunders NR, Liddelow SA, Dziegielewska KM (2012) Barrier mechanisms in the developing brain. Front Pharmacol 3:46
Wang Q, Luo W, Zheng W, Liu Y, Xu H, Zheng G, Dai Z, Zhang W, Chen Y, Chen J (2007) Iron supplement prevents lead-induced disruption of the blood-brain barrier during rat development. Toxicol Appl Pharmacol 219:33–41
Mason LH, Harp JP, Han DY (2014) Pb neurotoxicity: neuropsychological effects of lead toxicity. Biomed Res Int 2014:840547
Cory-Schlecta DA (1995) Relationships between lead-induced learning impairments and changes in dopaminergic, cholinergic, and glutamatergic neurotransmitter system functions. Annu Rev Pharmacol Toxicol 35:391–415
Gilbert ME, Mack CM, Lasley SM (1999) The influence of developmental period of lead exposure on long-term potentiation in the adult rat dentate gyrus in vivo. Neurotoxicology 20:57–69
Huang J, Huang K, Shang L, Wang H, Zhang M, Fan CL, Chen D, Yan X, Xiong K (2012) Chronic lead exposure reduces doublecortin-expressing immature neurons in young adult guinea pig cerebral cortex. BMC Neurosci 13:82
Jaako-Movits K, Zharkovsky T, Romantchik O, Jurgenson M, Merisalu E, Heidmets LT, Zharkovsky A (2005) Developmental lead exposure impairs contextual fear conditioning and reduces adult hippocampal neurogenesis in the rat brain. Int J Dev Neurosci 23:627–635
Struzyñska L, Bubko I, Walski M, Rafałowska U (2001) Astroglial reaction during the early phase of acute lead toxicity in the adult rat brain. Toxicology 165:121–131
Mousa AM, Al-Fahdi AS, Rao MS, Kilarkaje N (2015) Gestational lead exposure induces developmental abnormalities and upregulates apoptosis of fetal cerebellar cells in rats. Drud Chem Toxicol 38:73–83
Nam SM, Ahn SC, Go TH, Seo JS, Nahm SS, Chang BJ, Lee JH (2018) Ascorbic acid ameliorates gestational lead exposure-induced developmental alteration in GAD67 and c-Kit expression in the rat cerebellar cortex. Biol Trace Elem Res 182:278–286
Holtzman D, De Vries C, Nguyen H, Jameson N, Olson J, Carrithers M, Bensch K (1982) Development of resistance to lead encephalopathy during maturation in the rat pup. J Neuropathol Exp Neurol 41:652–663
Su X, Guan W, Yu YC, Fu Y (2014) Cerebellar stem cells do not produce neurons and astrocytes in adult mouse. Biochem Biophys Res Commun 450:378–383
Altman J, Bayer SA (1990) Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J Comp Neurol 301:365–381
Mikirova M, Casciari JJ, Hunninghake R, Riordan N (2011) EDTA chelation therapy in treatment of toxic metal exposure. Journal of Complementary medicine and drug discovery 1:81–89
Oehme FW, Rumbeiha WK (1999) Veterinary toxicology. In: Ballantyne B, Marrs T, Syversen T (eds) General and applied toxicology, 2nd edn. Macmillan Reference Ltd., Basingstoke, pp 1509–1526
Martinez S, Andreu A, Mecklenburg N, Echevarria D (2013) Cellular and molecular basis of cerebellar development. Front Neuroanat 7:18
Rice D, Barone S Jr (2000) Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect 108:511–533
Timbrell JA (2008) Biochemical mechanisms of toxicity: specific examples. In: Timbrell JA (ed) Principles of biochemical toxicology, 4th edn. Informa Health Care, New York, pp 293–403
Dieter MP, Matthews HB, Jeffcoat RA, Moseman RF (1993) Comparison of lead bioavailability in F344 rats fed lead acetate, lead oxide, lead sulfide, or lead ore concentrate from Skagway, Alaska. J Toxicol Environ Health 39:79–93
Lalith Kumar V, Muralidhara (2014) Ameliorative effects of ferulic acid against lead acetate-induced oxidative stress, mitochondrial dysfunctions and toxicity in prepubertal rat brain. Neurochem Res 39:2501–2515
Kandeel S, Elhosary NM, El-Noor MMA, Balaha M (2017) Electric injury-induced Purkinje cell apoptosis in rat cerebellum: histological and immunohistochemical study. J Chem Neuroanat 81:87–96
Plioplys AV, Thibault J, Hawkes R (1985) Selective staining of a subset of Purkinje cells in the human cerebellum with monoclonal antibody mabQ113. J Neurol Sci 70:245–256
Weil MT, Möbius W, Winkler A, Ruhwedel T, Wrzos C, Romanelli E, Bennett JL, Enz L, Goebels N, Nave KA, Kerschensteiner M, Schaeren-Wiemers N, Stadelmann C, Simons M (2016) Loss of myelin basic protein function triggers myelin breakdown in models of demyelinating diseases. Cell Rep 16:314–322
Faustino LC, Ortiga-Carvalho TM (2014) Thyroid hormone role on cerebellar development and maintenance: a perspective based on transgenic mouse models. Front Endocrinol 5:75
Kim HC, Jang TW, Chae HJ, Choi WJ, Ha MN, Ye BJ, Kim BG, Jeon MJ, Kim SY, Hong YS (2015) Evaluation and management of lead exposure. Ann Occup Environ Med 27:30
Daneel-Otterbech S, Davidsson L, Hurrell R (2005) Ascorbic acid supplementation and regular consumption of fresh orange juice increase the ascorbic acid content of human milk: studies in European and African lactating women. Am J Clin Nutr 81:1088–1093
Hossain S, Bhowmick S, Jahan S, Rozario L, Sarkar M, Islam S, Basunia MA, Rahman A, Choudhury BK, Shahjalal H (2016) Maternal lead exposure decreases the levels of brain development and cognition-related proteins with concomitant upsurges of oxidative stress, inflammatory response and apoptosis in the offspring rats. Neurotoxicology 56:150–158
Lihm H, Kim H, Chang H, Yoon M, Lee K, Choi J (2013) Vitamin C modulates lead excretion in rats. Anat Cell Biol 46:239–245
Nam SM, Chang BJ, Kim JH, Nahm SS, Lee JH (2018) Ascorbic acid ameliorates lead-induced apoptosis in the cerebellar cortex of developing rats. Brain Res 1686:10–18
Eltony SA, Othman MA, Mohamed AA (2010) Histological study on the effect of low level perinatal lead exposure on the cerebellar cortex of adult male albino rat. Egypt J Histol 33:781–797
Mustafa HN, Hussein AM (2016) Does allicin combined with vitamin B-complex have superior potentials than alpha-tocopherol alone in ameliorating lead acetate-induced Purkinje cell alterations in rats? An immunohistochemical and ultrastructural study. Folia Morphol (Warsz) 75:76–86
Saleh HA, Abdel El-Aziz GS, Mustafa HN, Saleh AHA, Mal AO, Deifalla AHS, Aburas M (2018) Protective effect of garlic extract against maternal and fetal cerebellar damage induced by lead administration during pregnancy in rats. Folia Morphol (Warsz) 77:1–15
El-Sokkary GH, Awadalla EA (2011) The protective role of vitamin C against cerebral and pulmonary damage induced by cadmium chloride in male adult albino rat. Open Neuroendocrinol J 4:1–8
Ibegbu AO, Animoku Abdulrazaq A, Ayuba M, Brosu D, Adamu Sadeeq A, Akpulu P, Hamman WO, Umana UE, Musa SA (2014) Histomorphological effect of ascorbic acid on mercury chloride-induced changes on the cerebellum of adult Wistar rats. J Morphol Sci 31:219–224
Altmann L, Gutowski M, Wiegand H (1994) Effects of maternal lead exposure on functional plasticity in the visual cortex and hippocampus of immature rats. Dev Brain Res 81:50–56
Cousins SL, Stephenson FA (2012) Identification of N-methyl-D-aspartic acid (NMDA) receptor subtype-specific binding sites that mediate direct interactions with scaffold protein PSD-95. J Biol Chem 287:13465–13476
Bernocchi G, Bottone MG, Piccolini VM, Dal Bo V, Santin G, De Pascali SA, Migoni D, Fanizzi FP (2011) Developing central nervous system and vulnerability to platinum compounds. Chemother Res Pract 2011:315418
Minnema DJ, Michaelson IA (1986) Differential effects of inorganic lead and δ-aminolevulinic acid in vitro on synaptosomal γ-aminobutyric acid release. Toxicol Appl Pharmacol 86:437–447
Coria F, Berciano MT, Berciano J, Lafarga M (1984) Axon membrane remodeling in the lead-induced demyelinating neuropathy of the rat. Brain Res 291:369–372
Ma T, Wu X, Cai Q, Wang Y, Xiao L, Tian Y, Li H (2015) Lead poisoning disturbs oligodendrocytes differentiation involved in decreased expression of NCX3 inducing intracellular calcium overload. Int J Mol Sci 16:19096–19110
Zhang L, Ma Z, Smith GM, Wen X, Pressman Y, Wood PM, Xu XM (2009) GDNF-enhanced axonal regeneration and myelination following spinal cord injury is mediated by primary effects on neurons. Glia 57:1178–1191
Hsu PC, Liu MY, Hsu CC, Chen LY, Guo YL (1997) Lead exposure causes generation of reactive oxygen species and functional impairment in rat sperm. Toxicology 122:133–143
Ma SB, Nguyen TN, Tan I, Ninnis R, Iyer S, Stroud DA, Menard M, Kluck RM, Ryan MT, Dewson G (2014) Bax targets mitochondria by distinct mechanisms before or during apoptotic cell death: a requirement for VDAC2 or Bak for efficient Bax apoptotic function. Cell Death Differ 21:1925–1935
Contributions
SMN, JSS, THG, SSN, and BJC conceived the study and conducted the animal modeling, histological staining, and immunoblotting. SMN analyzed the data and wrote the manuscript. SSN and BJC participated in the writing of manuscript, design of the study, and subsequent discussion. All authors read and approved the final version of the manuscript.
Funding
This research was supported by the faculty research fund of Konkuk University and the Veterinary Science Research Institute of the Konkuk University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The protocol of the present study was approved by the Institutional Animal Care and Use Committee of the Konkuk University (approval number KU18133). All procedures were in accordance with the ethical standards of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Nam, S.M., Seo, J.S., Go, TH. et al. Ascorbic Acid Supplementation Prevents the Detrimental Effects of Prenatal and Postnatal Lead Exposure on the Purkinje Cell and Related Proteins in the Cerebellum of Developing Rats. Biol Trace Elem Res 190, 446–456 (2019). https://doi.org/10.1007/s12011-018-1572-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1572-y