Abstract
Purpose
Radiation necrosis (RN) represents a serious post-radiotherapy complication in patients with brain metastases. Bevacizumab and laser interstitial thermal therapy (LITT) are viable treatment options, but direct comparative data is scarce. We reviewed the literature to compare the two treatment strategies.
Methods
PubMed, EMBASE, Scopus, and Cochrane databases were searched. All studies of patients with RN from brain metastases treated with bevacizumab or LITT were included. Treatment outcomes were analyzed using indirect meta-analysis with random-effect modeling.
Results
Among the 18 studies included, 143 patients received bevacizumab and 148 underwent LITT. Both strategies were equally effective in providing post-treatment symptomatic improvement (P = 0.187, I2 = 54.8%), weaning off steroids (P = 0.614, I2 = 25.5%), and local lesion control (P = 0.5, I2 = 0%). Mean number of lesions per patient was not statistically significant among groups (P = 0.624). Similarly, mean T1-contrast-enhancing pre-treatment volumes were not statistically different (P = 0.582). Patterns of radiological responses differed at 6-month follow-ups, with rates of partial regression significantly higher in the bevacizumab group (P = 0.001, I2 = 88.9%), and stable disease significantly higher in the LITT group (P = 0.002, I2 = 81.9%). Survival rates were superior in the LITT cohort, and statistical significance was reached at 18 months (P = 0.038, I2 = 73.7%). Low rates of adverse events were reported in both groups (14.7% for bevacizumab and 12.2% for LITT).
Conclusion
Bevacizumab and LITT can be safe and effective treatments for RN from brain metastases. Clinical and radiological outcomes are mostly comparable, but LITT may relate with superior survival benefits in select patients. Further studies are required to identify the best patient candidates for each treatment group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain metastases (BM) are the most common intracranial neoplasms, with an estimated incidence of 9% in adults with systemic malignancies [1, 2]. Surgical resection and radiotherapy remain the mainstay of treatment, but expose patients to potential adverse events [3, 4]. Radiation necrosis (RN) is a known complication, occurring approximately 3–12 months after completion of radiotherapy. Incidence ranges between 5 and 25% based on modality of treatment, total dose, and fractionation [5,6,7]. Symptoms are non-specific and stem from necrotic foci mass effect, which may mimic tumor recurrence. The diagnosis is supported by characteristic “Swiss-cheese” or “soap-bubble” enhancement, and may be confirmed with biopsy [7, 8]. Steroids provide temporary symptomatic relief, but long-term use correlates with serious complications; similarly, surgical debulking is not risk-free [7, 9]. Bevacizumab has proven effective in treating RN by counteracting the upregulation of VEGF [10, 11]. Likewise, laser interstitial thermal therapy (LITT) can resolve necrotic foci by generating thermal thrombosis of abnormal surrounding vessels [12, 13].
Bevacizumab and LITT are both viable treatments for RN not amenable to surgical excision, but direct comparative data is scarce [14]. In this review, we assess the differences in clinical outcomes, radiological responses, and survival rates between bevacizumab and LITT in patients with RN from BM.
Methods
Literature search
A systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [15]. PubMed, EMBASE, Cochrane and Scopus databases were screened for eligible articles from inception to April 3, 2021 operating the Boolean full-text search [(radiation necrosis OR radionecrosis) AND (anti-VEGF OR bevacizumab OR laser interstitial thermal therapy OR LITT)]. Eligible studies were collected and exported to Mendeley; duplicates were removed.
Study selection
Inclusion and exclusion criteria were set a priori. Articles were included if they met the following criteria, in line with the PICOS format: (1) retrospective or prospective studies (Study design) including a minimum of 5 patients confirming the radiological or histological diagnosis of RN following radiotherapy for BM (Population); (2) treatment with bevacizumab or LITT (Intervention, Comparison); (3) available data on radiological response and clinical improvement (Outcome). We excluded: (1) systematic reviews, meta-analyses, case series with less than 5 patients, animal, cadaver, and laboratory studies; (2) studies lacking adequate reports on clinical/radiological outcomes; (3) studies with unclear radiological or histological distinction between patients with RN from BM, BM recurrences or other pathology.
Two authors (C.D.N. and P.P.) independently screened titles/abstracts of all identified articles and reviewed full-texts of studies that met the inclusion criteria. Disagreements were settled by a third author (A.S.H.). References of included articles were also searched to retrieve additional papers.
Data extraction
Data were extracted by one reviewer (P.P.) and independently verified by two additional reviewers (A.S.H. and C.D.N.). Patient-level data were extracted directly or calculated from raw data. Data included: authors, year, study-design, sample-size, age, gender, primary tumor, radiation type, symptoms, imaging findings, steroids, bevacizumab dosage/cycles, hospital-stay, adverse-events, clinical outcome, radiological response, recurrence, progression-free survival (PFS), and overall survival (OS) [16, 17]. Clinical symptoms, steroid wean-off, and radiological responses were evaluated at 6-months after treatment or at the last available follow-up (at least > 1 month). Radiological response was assessed using the modified RANO criteria for BM: complete response (CR) = resolution, partial response (PR) = reduced volumes, stable disease (SD) = same volumes, progression (PD) = increased volumes [16, 17].
Data synthesis and quality assessment
The primary outcomes of interest were clinical and radiographic outcomes in patients with RN treated with bevacizumab or LITT. These included post-treatment symptomatic improvement, weaning off steroids, RN recurrence, radiological responses, and survival. Treatment-related adverse events were also evaluated. For each study, level of evidence was assessed using the 2011 Oxford Centre For Evidence-Based Medicine guidelines, and risk of bias evaluated with the Joanna Briggs Institute (JBI) checklists for case series and randomized controlled trials [18,19,20].
Statistical analysis
Continuous variables are presented as medians and ranges, and categorical variables as percentages. Two-sample weighted means t-test was performed to assess differences in the number of lesions per patient and volumes of treated lesions between bevacizumab and LITT cohorts. The time intervals between RN treatment and RN recurrence (PFS curve) or death (OS curve) were estimated with the Kaplan–Meier method. The survival analyses were conducted with the log-rank test. Indirect meta-analyses were performed for post-treatment symptomatic improvement, weaning off steroids, RN recurrence, radiological responses, and OS rates at 3–6-12–18 months. Outcomes were summarized with pooled proportions of events (effect size—ES), and confidence intervals (CI) were calculated with the Wilson score method, both graphically displayed with forest plots [21]. The Freeman-Tukey transformation was performed to include studies with 0 or 1 event rate and stabilize variance, and the DerSimonian-Laird approach for random effect models was used to account for high-variability between studies [22, 23]. Heterogeneity was assessed with the Higgins I-square (I2) and considered significant for I2 > 75% [24]. All analyses were bilateral and P-values < 0.05 were considered statistically significant. Statistical analyses were conducted using SPSS V.25 (IBM Corp, Armonk, NY) and STATA 16.1 (StataCorp LLC, College Station, TX).
Results
Study selection and quality assessment
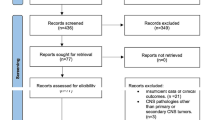
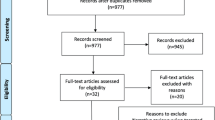
Figure 1 illustrates the flow diagram of the literature search and study selection. The search strategy yielded 477 citations (PubMed: 289, EMBASE: 117, Scopus: 40, Cochrane: 31), of which 18 were included in the qualitative and quantitative synthesis accordingly to the pre-specified criteria (Supplementary File 1). Nine studies reported the use of bevacizumab [25,26,27,28,29,30,31,32,33]. Eight studies described patients treated with LITT [13, 34,35,36,37,38,39,40]. One study compared bevacizumab and LITT [14]. Critical appraisal based on JBI criteria returned high quality (i.e., low risk of bias) for all included articles (Supplementary File 2).
Patient demographics, clinical and management characteristics
In total, 291 patients diagnosed with RN from BM were analyzed. Patients were divided in two treatment cohorts: 143 (49.1%) received bevacizumab and 148 (50.9%) underwent LITT (Table 1). Of note, 14 patients included in the LITT cohort received late bevacizumab courses for refractory lesions, but their outcome data referred to the period before receiving bevacizumab [13, 14, 38]. Median ages were 58 (range 27–79) and 60 (range 29–83) in the bevacizumab and LITT cohorts, respectively, with a male proportion of 53.1% and 33.3%. Lung cancers represented the prevalent primary tumors, followed by melanoma and breast cancer. BM were most treated with stereotactic radiotherapy (SRT) (95.1% in bevacizumab, 100% in LITT)—including intensity-modulated radiotherapy and stereotactic radiosurgery –, and less with whole brain radiotherapy (WBRT) (39.1% in bevacizumab, 14.6% in LITT), concomitant or without SRT.
Rates of symptomatic RN were 94.4% in bevacizumab cohort and 74.7% in LITT. Lesions were mostly diagnosed with imaging in the bevacizumab cohort (97.9%), and with biopsy prior to LITT (82.4%). The mean number of lesions per patient was 1.13 in the bevacizumab cohort and 1.05 in LITT, showing no significant difference (P = 0.624). Similarly, the mean contrast-enhancing pre-treatment volumes were not statistically different (P = 0.582), namely 30cm3 in bevacizumab cohort (n = 91) and 5cm3 in LITT (n = 44). Palliative steroids were administered in 86% and 46% patients before starting bevacizumab or LITT. In the bevacizumab cohort, patients completed a median of 4 treatment cycles (range 1–31) at dosages of 5 mg/kg q2w (38.5%), 7.5 mg/kg q3w (20%), 10 mg/kg q2w (21.5%) and 15 mg/kg q4-6w (3%). Zhuang et al.[31] also reported the use of low dose bevacizumab (1 mg/kg q3w) in 21 patients (16.1%). In LITT cohort, NeuroBlate (Monteris Medical Inc., Minneapolis, MN) and Visualase (Medtronic Inc., Dublin, Ireland) systems were used, and median post-treatment hospital-stay was 1.5 days (range 0.5–6).
Outcomes, adverse events, and survival analysis
Table 2 summarizes pooled treatment outcomes. Post-treatment symptomatic improvement—i.e., reduction or resolution of RN symptoms—occurred in 73.3% patients treated with bevacizumab and 60.8% with LITT, while 66.7% and 44.1% patients achieved post-treatment steroid wean-off. Follow-up radiological assessment returned higher rates of PR in bevacizumab cohort (79.6%) and SD in LITT (49.2%), with lower rates of CR (3.6% and 8.2%) and PD (10.2% and 13.1%) in both [16, 17].
Treatment-related adverse events, reported using the “Common Terminology Criteria for Adverse Events, v5.0”, showed rates of 14.7% and 12.2% respectively in bevacizumab and LITT cohorts [41]. In the bevacizumab cohort, the most common were grade 1 and 2 bleeding and proteinuria, and grade 3 hypertension. In LITT cohort, the most frequent were grade 1 headache, and grade 3 limb weakness and seizure. Sporadic cases of thromboembolic events (4.8%) and intracerebral hemorrhage (5.6%) have been associated with bevacizumab and LITT, respectively.
RN recurrence rates were 17.2% and 22.4% in the bevacizumab and LITT cohorts, and death occurred in 56.8% and 59.6% of patients, respectively. Median PFS and OS were 3.5 (range 0–22) and 6.5 (0–38.4) months in bevacizumab cohort, and 6 (0–64.6) and 11 (1–89) months in LITT. OS rates for bevacizumab and LITT cohorts were: 90.7% and 94.7% at 3 months, 80% and 91.6% at 6 months, 39.4% and 69.3% at 12 months, and 25% and 46.4% at 18 months. As shown in Fig. 2, PFS (P = 0.209) and OS (P = 0.484) were not significantly impacted by treatment strategy.
Kaplan–Meier survival curves of patients (no.) with available individual data: A PFS (n = 22) and B OS (n = 64) of the total pooled cohort; C PFS (n = 22) and D OS (n = 64) based on treatment strategies—bevacizumab versus LITT. PFS progression free survival, OS overall survival, LITT laser interstitial thermal therapy
Meta-analysis: comparison of clinical, radiological and survival outcomes rates
Table 2 summarizes the results of all indirect comparisons between bevacizumab and LITT, displayed as forest plots in Fig. 3. There were no significant differences in post-treatment symptomatic improvement (P = 0.187, I2 = 54.8%), steroid wean-off (P = 0.614, I2 = 25.5%), and RN recurrence (P = 0.5, I2 = 0%), between bevacizumab and LITT (Supplementary File 3).
Forest plots for indirect comparisons between bevacizumab and LITT treatments of radiation necrosis: post-treatment symptomatic improvement; post-treatment weaning off steroids; radiation necrosis recurrence; complete response, partial response, stable disease, and progression at 6-month radiological follow-up; overall survival rates at 3-month, 6-month, 12-month, 18-month. LITT laser interstitial thermal therapy, CI confidence interval, Effect effect size
Rates of CR (P = 0.29, I2 = 44.5%) and PD (P = 0.645, I2 = 61.6%) were comparable between the two cohorts. Rates of PR were significantly higher (P = 0.001, I2 = 88.9%) in bevacizumab (ES:0.77; 95% CI 0.51–0.96) compared to LITT (ES:0.28; 95% CI 0.17–0.41), while rates of SD were significantly higher (P = 0.002, I2 = 81.9%) in LITT (ES:0.42; 95% CI 0.17–0.69) compared to bevacizumab (ES:0.04; 95% CI 0–0.12); but both with significant heterogeneity (Supplementary File 4).
OS rates at 3 months (P = 0.427, I2 = 61.9%), 6 months (P = 0.289, I2 = 75%), and 12 months (P = 0.183, I2 = 72.1%) were comparable between the two cohorts. OS rates at 18 months were significantly higher (P = 0.038, I2 = 73.7%) in LITT (ES:0.59; 95% CI 0.35–0.82) as compared to bevacizumab (ES:0.15; 95% CI 0–0.48) (Supplementary File 5).
Discussion
RN represents an inflammatory response of the brain parenchyma occurring months to years after radiotherapy. Our pooled patients most frequently received SRT for BM [11, 40, 42]. Stereotactic protocols pose higher risk of RN by delivering focused high-doses of radiation to specific targets, triggering endothelial injury, hypoxia, and local necrosis [5, 7]. Due to similarities between tumor recurrence and progression, the diagnosis of RN is challenging, mostly relying on the multidisciplinary review of clinical findings and advanced MRI scans [26, 43]. When feasible, a biopsy of the enhancing tissue may be pursued, but may render false negative or positive results due to intermingling necrosis and tumor cells [42]. We found that patients in the LITT group had higher rates of biopsy (82.4%) as compared to bevacizumab (5.6%). While pre-ablative biopsy and LITT ablation can be performed during the same session, patients receiving bevacizumab rarely undergo biopsy so as to avoid related surgical risks [13, 31, 33, 37]. Since tumor recurrence may be misinterpreted as RN on imaging but also on biopsy, the different diagnostic strategies remain a potential confounder in the analysis of treatment outcomes [14]. Based on our findings, LITT may be recommended in reasonable surgical candidates, while bevacizumab may be offered to patients with lower functional status.
Treatments may provide symptomatic relief and/or hamper lesion progression [5]. Early management with steroids suppresses inflammation and reduces brain edema, easing mass effect-related symptoms [9]. The majority of our pooled patients in both treatment groups were symptomatic upon clinical presentation (94.7% and 74.7%), mostly undergoing palliative therapy with steroids (86% and 46%). In the long-term, steroids may result in severe systemic toxicities and impaired quality of life; thus, adjunct treatments should be offered to prevent steroid dependance. Bevacizumab proved to be superior to steroids in treating RN from BM, nasopharyngeal carcinomas, and gliomas [10, 11, 44]. By neutralizing VEGF, bevacizumab counters vessel permeability and restores blood–brain-barrier function, improving short-interval clinical and radiological outcomes [5, 10]. In this review, treatment protocols were heterogeneous, ranging from 1 to 15 mg/kg cycles, corroborating the theory that the effectiveness of bevacizumab derives from its anti-angiogenic action rather than its dose [31]. The documented versatile dosage profile may increase worldwide accessibility to bevacizumab treatments by mitigating costs and dose-related adverse events [45].
Surgical excision of the necrotic foci may be pursued to resolve mass effect-related symptoms of aggressive RN lesions. However, the surgical risk and the poor baseline clinical status of patients with systemic malignancies make less invasive options more appealing [5, 9, 46]. LITT is a minimally invasive surgical ablative technique, which, by targeting peri-necrotic zones, induces the thermocoagulative necrosis of dysfunctional endothelial cells and removes the primary source of active VEGF [47]. LITT has been shown to improve functional and cognitive statuses, achieving prolonged lesion control and survival comparable to surgical resection [13, 37, 40]. The median hospital-stay of patients treated with LITT (1.5 days) was noted to be less than half that of patients undergoing craniotomy (3.9 days in previous cohorts) [13, 40]. Late bevacizumab courses were seen in 14 patients presenting with LITT-refractory RN lesions, but failed to improve clinical outcomes [13, 14, 38]. In these cases, therapeutic failures were likely related to the underlying poor clinical statuses of affected patients.
Bevacizumab and LITT had a positive impact on clinical and radiological outcomes of patients with RN. Both treatments showed favorable rates of symptomatic improvement and ability to wean off steroids, 73.3% and 66.7% in the bevacizumab group as compared to 60.8% and 44.1% in the LITT group (P-values 0.187 and 0.614, respectively) [14]. Bevacizumab may exhibit modest clinical advantages due to its direct effects on vessel and blood–brain-barrier permeability. However, our findings may be ascribed to the diversified clinical assessments amongst studies and to the complex multidisciplinary decision to modulate steroids therapy in patients with systemic malignancies. The introduction of standardized assessments and reporting of performance status scores may produce more accurate clinical data. Similarly, the pooled low rates of radiological RN recurrence were comparable between the two groups, but the absence of consistent histopathology reports may have failed to exclude cases of tumor progression [25, 30, 34, 35].
RN lesions treated with bevacizumab and LITT followed different patterns of radiological responses based on the modified RANO criteria for BM. At 6 months, while rates of complete response and disease progression were low and comparable between the two groups, rates of partial response were statistically higher in the bevacizumab cohort (P = 0.001, I2 = 88.9%) and rates of stable disease higher in LITT (P = 0.002, I2 = 81.9%); however, our findings are limited by their significant heterogeneity. This is likely related to the different mechanism-of-action of each treatment modality [14]. Bevacizumab may promptly restore blood–brain-barrier function, reducing brain edema and post-contrast lesion volumes upfront; but the lack of permanent anti-inflammatory effects may lead to increased volumes after prolonged cessation of treatment [14, 32, 33, 48]. In contrast, LITT ablation may directly and permanently inactivate inflammatory cells, resulting in apparent early disease progression due to transient increased lesion size; but, upon follow-up, the lesions decrease in volume and stabilize for a longer duration [14, 36, 37, 40]. Despite the rapid clinical effect of LITT, it is common knowledge that LITT-treated lesions expand radiologically during the first 4–6 months post-treatment due to the enlarging necrotic cores, but shrink and/or disappear upon late follow-up (12–15 months) [49].
In our pooled data, the median OS was longer in patients treated with LITT (11 months) as compared to bevacizumab (6.5 months), but the difference was not statistically significant. The low median OS in our pooled-data may reflect the limited patient-level survival data reported amongst studies, most of which restricted their follow-up times to 6 and 12 months [25, 27, 32,33,34, 37, 38, 40]. In regards to OS rates, we found longer survival rates in the LITT group, reaching statistical significance at 18 months (P = 0.038) [14, 26, 28, 31, 37, 38, 40]. The likely uneven distribution of patients between the two treatment groups may explain the observed differences. No significant difference was seen in the number-of-lesions per patient and pre-treatment volumes between the bevacizumab and LITT cohorts, although the mean volume of RN was much higher in the bevacizumab cohort (30 cm3) as compared to the LITT cohort (5 cm3). The low number of patients with data related to pre-treatment volumes did not allow statistical significance. However, it seems that patients who received bevacizumab had larger pre-treatment volumes to start with as compared to patients who received LITT, as most surgeons likely avoid treating larger lesions with LITT, which may provide a false impression that LITT is associated with better outcomes. This finding needs to be further studied in a controlled/randomized fashion, as a higher number of patients is probably needed to detect difference. Further, the intracranial extent of BM and RN lesions and their anatomical location in “eloquent” or “non-eloquent” areas were poorly defined in both groups. We speculate that patients with multiple lesions, or located in “eloquent” cortex, and with poor baseline functional status were probably not ideal candidates for LITT but were eligible for bevacizumab.
Both treatment modalities showed favorable toxicity profiles and proved to be safer than long-term steroids and, in some cases, surgical resection of RN [10, 40, 44]. The adverse events are listed in Table 2. In the bevacizumab cohort, bleeding and proteinuria were often self-limited after temporary treatment interruption [14, 25, 29, 31]. In the LITT cohort, headache and mild motor impairments were mostly transitory or managed with short-term steroids [36,37,38]. In rare cases, severe thromboembolic events and intracerebral hemorrhage were linked to bevacizumab and LITT, but no life-threatening complications were described [14, 27, 35]. Patient receiving bevacizumab may require up to 4-weeks of “wash-out” before qualifying for surgery or clinical trials [50]. These limitations may strongly influence the inclusion criteria among the two treatment strategies [11, 14].
Limitations
Except for three prospective studies, most included studies were retrospective with class IIIb-IV evidence, prone to selection and recall biases. Patient-level data was limited, with most data collected at study-level from heterogeneous populations and treatment centers. Possible clinical confounders could not be investigated due to the limited data on performance statuses in both cohorts and on pathology reports in the bevacizumab group. Data on lesion volume and per-patient number-of-lesions were also limited and not equally distributed between the two cohorts, thus likely responsible for the lack of statistical difference despite the numerical difference in averages. Finally, follow-up intervals varied amongst included studies and treatment groups, which made it hard to draw robust conclusions about survival outcomes.
Conclusion
This meta-analysis compared the role of bevacizumab and LITT in the treatment of RN in BM. Both strategies showed good safe toxicity-profiles and equal efficacy in relieving symptoms, weaning off steroids, and achieving local lesion control. Patterns of radiological responses were different and LITT resulted in longer overall survival likely related to its use in patients with smaller lesions and better baseline functional-status.
Data availability
All authors confirm the appropriateness of all dataset and software used for supporting the conclusion.
References
Nayak L, Lee EQ, Wen PY (2012) Epidemiology of brain metastases. Curr Oncol Rep 14:48–54. https://doi.org/10.1007/s11912-011-0203-y
Barnholtz-Sloan JS, Sloan AE, Davis FG et al (2004) Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the metropolitan detroit cancer surveillance system. J Clin Oncol 22:2865–2872. https://doi.org/10.1200/JCO.2004.12.149
Soussain C, Ricard D, Fike JR et al (2009) CNS complications of radiotherapy and chemotherapy. Lancet 374:1639–1651. https://doi.org/10.1016/S0140-6736(09)61299-X
Patchell RA (2003) The management of brain metastases. Cancer Treat Rev 29:533–540. https://doi.org/10.1016/S0305-7372(03)00105-1
Rahmathulla G, Marko NF, Weil RJ (2013) Cerebral radiation necrosis: a review of the pathobiology, diagnosis and management considerations. J Clin Neurosci 20:485–502. https://doi.org/10.1016/j.jocn.2012.09.011
Lawrence YR, Li XA, el Naqa I et al (2010) Radiation dose–volume effects in the brain. Int J Radiat Oncol 76:S20–S27. https://doi.org/10.1016/j.ijrobp.2009.02.091
Ali FS, Arevalo O, Zorofchian S et al (2019) Cerebral radiation necrosis: incidence, pathogenesis, diagnostic challenges, and future opportunities. Curr Oncol Rep 21:66. https://doi.org/10.1007/s11912-019-0818-y
Furuse M, Nonoguchi N, Yamada K et al (2019) Radiological diagnosis of brain radiation necrosis after cranial irradiation for brain tumor: a systematic review. Radiat Oncol 14:28. https://doi.org/10.1186/s13014-019-1228-x
Chung C, Bryant A, Brown PD (2018) Interventions for the treatment of brain radionecrosis after radiotherapy or radiosurgery. Cochrane Database Syst Rev 7:CD011492. https://doi.org/10.1002/14651858.CD011492.pub2
Levin VA, Bidaut L, Hou P et al (2011) Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol 79:1487–1495. https://doi.org/10.1016/j.ijrobp.2009.12.061
Khan M, Zhao Z, Arooj S, Liao G (2021) Bevacizumab for radiation necrosis following radiotherapy of brain metastatic disease: a systematic review & meta-analysis. BMC Cancer 21:167. https://doi.org/10.1186/s12885-021-07889-3
de Bastos DCA, Weinberg J, Kumar VA et al (2020) Laser interstitial thermal therapy in the treatment of brain metastases and radiation necrosis. Cancer Lett 489:9–18. https://doi.org/10.1016/j.canlet.2020.05.014
Hong CS, Deng D, Vera A, Chiang VL (2019) Laser-interstitial thermal therapy compared to craniotomy for treatment of radiation necrosis or recurrent tumor in brain metastases failing radiosurgery. J Neurooncol 142:309–317. https://doi.org/10.1007/s11060-019-03097-z
Sujijantarat N, Hong CS, Owusu KA et al (2020) Laser interstitial thermal therapy (LITT) vs. bevacizumab for radiation necrosis in previously irradiated brain metastases. J Neurooncol 148:641–649. https://doi.org/10.1007/s11060-020-03570-0
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. https://doi.org/10.1136/bmj.n71
Chukwueke UN, Wen PY (2019) Use of the response assessment in neuro-oncology (RANO) criteria in clinical trials and clinical practice. CNS Oncol 8:CNS28. https://doi.org/10.2217/cns-2018-0007
Lin NU, Lee EQ, Aoyama H et al (2015) Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol 16:e270–e278. https://doi.org/10.1016/S1470-2045(15)70057-4
Jeremy Howick, Iain Chalmers, Paul Glasziou, Trish Greenhalgh, Carl Heneghan, Alessandro Liberati, Ivan Moschetti, Bob Phillips and HT (2011) Explanation of the 2011 Oxford Centre for Evidence-Based Medicine (OCEBM) Levels of Evidence (Background Document). In: Oxford Cent. Evidence-Based Med. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence. Accessed 19 Apr 2021
Joanna Briggs Institute (2020) Checklist for case series. https://jbi.global/critical-appraisal-tools. Accessed 19 Apr 2021
Joanna Briggs Institute (2020) Checklist for randomized controlled trials. https://jbi.global/critical-appraisal-tools. Accessed 19 Apr 2021
Wilson EB (1927) Probable inference, the law of succession, and statistical inference. J Am Stat Assoc 22:209–212. https://doi.org/10.1080/01621459.1927.10502953
Freeman MF, Tukey JW (1950) Transformations related to the angular and the square root. Ann Math Stat 21:607–611. https://doi.org/10.1214/aoms/1177729756
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188. https://doi.org/10.1016/0197-2456(86)90046-2
Higgins JPT (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560. https://doi.org/10.1136/bmj.327.7414.557
Wang Y, Pan L, Sheng X et al (2012) Reversal of cerebral radiation necrosis with bevacizumab treatment in 17 Chinese patients. Eur J Med Res 17:25. https://doi.org/10.1186/2047-783X-17-25
Boothe D, Young R, Yamada Y et al (2013) Bevacizumab as a treatment for radiation necrosis of brain metastases post stereotactic radiosurgery. Neuro Oncol 15:1257–1263. https://doi.org/10.1093/neuonc/not085
Sadraei NH, Dahiya S, Chao ST et al (2015) Treatment of cerebral radiation necrosis with bevacizumab. Am J Clin Oncol 38:304–310. https://doi.org/10.1097/COC.0b013e31829c3139
Yomo S, Hayashi M (2016) Salvage stereotactic radiosurgery with adjuvant use of bevacizumab for heavily treated recurrent brain metastases: a preliminary report. J Neurooncol 127:119–126. https://doi.org/10.1007/s11060-015-2019-3
Zhuang H, Yuan X, Zheng Y et al (2016) A study on the evaluation method and recent clinical efficacy of bevacizumab on the treatment of radiation cerebral necrosis. Sci Rep 6:24364. https://doi.org/10.1038/srep24364
Glitza IC, Guha-Thakurta N, D’Souza NM et al (2017) Bevacizumab as an effective treatment for radiation necrosis after radiotherapy for melanoma brain metastases. Melanoma Res 27:580–584. https://doi.org/10.1097/CMR.0000000000000389
Zhuang H, Zhuang H, Shi S, Wang Y (2019) Ultra-low-dose bevacizumab for cerebral radiation necrosis: a prospective phase ii clinical study. Onco Targets Ther 12:8447–8453. https://doi.org/10.2147/OTT.S223258
Park M, Gwak H-S, Lee SH et al (2020) Clinical experience of bevacizumab for radiation necrosis in patients with brain metastasis. Brain Tumor Res Treat 8:93. https://doi.org/10.14791/btrt.2020.8.e11
Li J, He J, Cai L et al (2021) Bevacizumab as a treatment for radiation necrosis following stereotactic radiosurgery for brain metastases: clinical and radiation dosimetric impacts. Ann Palliat Med 10:2018–2026. https://doi.org/10.21037/apm-20-2417
Torres-Reveron J, Tomasiewicz HC, Shetty A et al (2013) Stereotactic laser induced thermotherapy (LITT): a novel treatment for brain lesions regrowing after radiosurgery. J Neurooncol 113:495–503. https://doi.org/10.1007/s11060-013-1142-2
Rao MS, Hargreaves EL, Khan AJ et al (2014) Magnetic resonance-guided laser ablation improves local control for postradiosurgery recurrence and/or radiation necrosis. Neurosurgery 74:658–667. https://doi.org/10.1227/NEU.0000000000000332
Smith CJ, Myers CS, Chapple KM, Smith KA (2016) Long-term follow-up of 25 cases of biopsy-proven radiation necrosis or post-radiation treatment effect treated with magnetic resonance-guided laser interstitial thermal therapy. Neurosurgery 79:S59–S72. https://doi.org/10.1227/NEU.0000000000001438
Ahluwalia M, Barnett GH, Deng D et al (2019) Laser ablation after stereotactic radiosurgery: a multicenter prospective study in patients with metastatic brain tumors and radiation necrosis. J Neurosurg 130:804–811. https://doi.org/10.3171/2017.11.JNS171273
Rammo R, Asmaro K, Schultz L et al (2018) The safety of magnetic resonance imaging-guided laser interstitial thermal therapy for cerebral radiation necrosis. J Neurooncol 138:609–617. https://doi.org/10.1007/s11060-018-2828-2
Shah AH, Semonche A, Eichberg DG et al (2020) The role of laser interstitial thermal therapy in surgical neuro-oncology: series of 100 consecutive patients. Neurosurgery 87:266–275. https://doi.org/10.1093/neuros/nyz424
Kim AH, Tatter S, Rao G et al (2020) Laser Ablation of abnormal neurological tissue using robotic neuroblate system (LAANTERN): 12-month outcomes and quality of life after brain tumor ablation. Neurosurgery 87:E338–E346. https://doi.org/10.1093/neuros/nyaa071
National Cancer Institute (2017) Common Terminology Criteria for Adverse Events (CTCAE) v5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_50. Accessed 19 Apr 2021
Telera S, Fabi A, Pace A et al (2013) Radionecrosis induced by stereotactic radiosurgery of brain metastases: results of surgery and outcome of disease. J Neurooncol 113:313–325. https://doi.org/10.1007/s11060-013-1120-8
Dequesada IM, Quisling RG, Yachnis A, Friedman WA (2008) Can standard magnetic resonance imaging reliably distinguish recurrent tumor from radiation necrosis after radiosurgery for brain metastases? A radiographic-pathological study. Neurosurgery 63:898–904. https://doi.org/10.1227/01.NEU.0000333263.31870.31
Xu Y, Rong X, Hu W et al (2018) Bevacizumab monotherapy reduces radiation-induced brain necrosis in nasopharyngeal carcinoma patients: a randomized controlled trial. Int J Radiat Oncol 101:1087–1095. https://doi.org/10.1016/j.ijrobp.2018.04.068
Lubelski D, Abdullah KG, Weil RJ, Marko NF (2013) Bevacizumab for radiation necrosis following treatment of high grade glioma: a systematic review of the literature. J Neurooncol 115:317–322. https://doi.org/10.1007/s11060-013-1233-0
McPherson CM, Warnick RE (2004) Results of contemporary surgical management of radiation necrosis using frameless stereotaxis and intraoperative magnetic resonance imaging. J Neurooncol 68:41–47. https://doi.org/10.1023/B:NEON.0000024744.16031.e9
Rahmathulla G, Recinos PF, Valerio JE et al (2012) Laser interstitial thermal therapy for focal cerebral radiation necrosis: a case report and literature review. Stereotact Funct Neurosurg 90:192–200. https://doi.org/10.1159/000338251
Furuse M, Nonoguchi N, Kuroiwa T et al (2016) A prospective, multicentre, single-arm clinical trial of bevacizumab for patients with surgically untreatable, symptomatic brain radiation necrosis†. Neuro-Oncology Pract 3:272–280. https://doi.org/10.1093/nop/npv064
Traylor JI, Bastos DCA, Fuentes D et al (2019) Dynamic contrast-enhanced MRI in patients with brain metastases undergoing laser interstitial thermal therapy: a pilot study. Am J Neuroradiol. https://doi.org/10.3174/ajnr.A6144
Abrams DA, Hanson JA, Brown JM et al (2015) Timing of surgery and bevacizumab therapy in neurosurgical patients with recurrent high grade glioma. J Clin Neurosci 22:35–39. https://doi.org/10.1016/j.jocn.2014.05.054
Funding
No funds, grants, or other support was received.
Author information
Authors and Affiliations
Contributions
PP—Conceptualization, methodology, data analysis, writing (original draft). ASH—Conceptualization, writing (review & editing). CDN—Literature search, writing (review & editing). WW—Data analysis, writing (review & editing). SGA—Methodology, writing (review & editing). KGA—Methodology, writing (review & editing). TYE—Conceptualization, methodology, writing (review & editing).
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Palmisciano, P., Haider, A.S., Nwagwu, C.D. et al. Bevacizumab vs laser interstitial thermal therapy in cerebral radiation necrosis from brain metastases: a systematic review and meta-analysis. J Neurooncol 154, 13–23 (2021). https://doi.org/10.1007/s11060-021-03802-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-021-03802-x