Abstract
Purpose
Both laser interstitial thermal therapy (LITT) and bevacizumab have been used successfully to treat radiation necrosis (RN) after radiation for brain metastases. Our purpose is to compare pre-treatment patient characteristics and outcomes between the two treatment options.
Methods
Single-institution retrospective chart review identified brain metastasis patients who developed RN between 2011 and 2018. Pre-treatment factors and treatment responses were compared between those treated with LITT versus bevacizumab.
Results
Twenty-five patients underwent LITT and 13 patients were treated with bevacizumab. The LITT cohort had a longer overall survival (median 24.8 vs. 15.2 months for bevacizumab, p = 0.003) and trended to have a longer time to local recurrence (median 12.1 months vs. 2.0 for bevacizumab), although the latter failed to achieve statistical significance (p = 0.091). LITT resulted in an initial increase in lesional volume compared to bevacizumab (p < 0.001). However, this trend reversed in the long term follow-up, with LITT resulting in a median volume decrease at 1 year post-treatment of − 64.7% (range − 96.0% to + > 100%), while bevacizumab patients saw a median volume increase of + > 100% (range − 63.0% to + > 100%), p = 0.010.
Conclusions
Our study suggests that patients undergoing LITT for RN have longer overall survival and better long-term lesional volume reduction than those treated with bevacizumab. However, it remains unclear whether our findings are due only to a difference in efficacy of the treatments or the implications of selection bias.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radiation necrosis (RN) can occur in up to 46% of SRS-treated brain metastases patients radiographically and about 10–15% clinically [1, 2]. The incidence of RN can vary depending on multiple factors including radiation dose, fractionation, lesional volume, and systemic treatments [3, 4]. Initial treatment for RN is typically conservative. When symptoms develop, the treatment generally consists of corticosteroids. Although an effective tool, corticosteroids are associated with many long-term adverse effects including weight gain, hypertension, diabetes mellitus, impaired wound healing, infection, and GI intolerance. Other treatment modalities have emerged in the recent years as alternatives to steroids.
Laser interstitial thermal therapy (LITT) and bevacizumab have both been shown to be efficacious in the management of RN after radiation for brain metastases. LITT is a minimally invasive surgical treatment option for metastatic in-field recurrence and RN. Accessed via a single burr hole, LITT delivers thermal energy to the lesion and, when coupled with MRI-guidance, allows for greater accuracy of ablation. Although LITT was initially used for difficult-to-access lesions, its indications are rapidly expanding and now include the treatment of RN in cases where symptoms are difficult to control with steroids alone or in cases of progressive lesion growth in both deep and superficial locations. Bevacizumab, on the other hand, is an anti-vascular endothelial growth factor (VEGF) antibody which decreases vessel permeability and edema. It is the only agent that has been shown in a randomized controlled trial to be efficacious treatment for RN [5]. The indications for choosing LITT versus bevacizumab for management of RN however remain unclear. In this study, we aim to compare patient demographics, pretreatment factors, and outcomes for the patients undergoing LITT and bevacizumab for RN at our institution to better inform decision making.
Methods
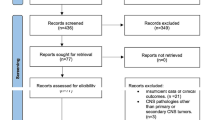
Single-institution retrospective chart review was performed of all patients who were treated with radiation for brain metastases and then developed RN between 2011 and 2018. Those who had subsequent treatment with either LITT or bevacizumab within 3 months after radiation were presumed to have acute or subacute radiation injury and were excluded from the study. Those without baseline imaging prior to treatment and those with follow-up of less than 6 weeks were also excluded from the study. For the LITT subgroup, RN was diagnosed by tissue biopsy performed at time of the procedure. For the bevacizumab subgroup, multidisciplinary review of serial MRI brain with and without contrast along with MRI perfusion and spectroscopy, when available, were used to make the diagnosis of RN. Associated patient demographics, cancer history, pre-treatment factors, steroid use, radiographic and clinical responses were collected.
Treatments
Indications for treatment included development of symptoms, concerns for imaging progression, and to facilitate steroid wean and/or commencement of immunotherapy. In the LITT cohort, indications also included need for diagnosis.
All LITT patients underwent biopsy prior to the procedure and all cases of RN were confirmed by pathology. At our institution, the NeuroBlate system (Monteris Medical Inc., Minneapolis, MN) was used for LITT. Surgical procedures were performed using standard stereotactic guidance of a single laser fiber placed into the center of the lesion. Monitoring of lesion ablation was performed using continuous gradient echo sequence imaging. These procedures have been described elsewhere [6]. All patients were admitted for observation in the neuro intensive care unit (NICU) post-operatively and discharged home typically within 1–2 days after surgery.
Bevacizumab was administered as outpatient infusions, typically every 2 weeks at 7.5–15 mg/kg dose. Duration of bevacizumab dosing varied on a case-by-case basis but was typically administered as an abbreviated course unless bevacizumab was also being used for systemic disease treatment.
Five patients in the LITT group subsequently received bevacizumab treatment. Outcome for these patients were assigned to the cohort of their initial treatment. Adverse outcomes were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) [7]. Overall survival (OS) and progression free survival (PFS) were calculated from the date of the LITT treatment or the date of the first bevacizumab infusion.
Imaging analysis
When available, follow-up MRIs were assessed at 1 month, 3 months, 6 months, 12 months, and at the last follow-up. Both lesional volume change and a modified version of the Response Assessment in Neuro-Oncology Brain Metastases (RANO-BM) criteria were used to assess response. Lesional volumes were estimated from the T1-weighted post-contrast MRI using the formula volume = (length × width × height)/2. Response criteria were adapted from the original RANO-BM described by Lin et al. [8] by excluding an assessment of non-target lesions and new lesions which do not apply to assessment of surgical lesions. For simplicity, response in the index lesion in the bevacizumab group was compared to the surgically treated lesion in the LITT group. In bevacizumab patients with multiple lesions, the index lesion was defined as the lesion that expanded immediately prior to starting bevacizumab or the biggest lesion if multiple lesions expanded.
Statistical analysis
Data were analyzed using SPSS 26 (SPSS Inc.). Mann–Whitney U test was used to compare the medians of continuous variables. Fisher’s exact test was used to compare independent dichotomous variables. Simple logistic regression was used to assess a relationship between bevacizumab dosage and progression. All tests were two-sided. P-value of ≤ 0.05 was considered significant.
Results
Patient demographics and treatment data
One hundred patients were treated with LITT between 2011 and 2018 by a single neurosurgeon at a major academic institution. Sixty-eight patients underwent LITT for re-growing metastatic lesions and were excluded from the study. Four patients were also excluded for diagnoses other than brain metastasis. In the remaining 28 patients who had biopsy-proven RN, one patient died before 6 weeks, one was lost to follow-up, and one underwent LITT less than 3 months after radiation, making the remaining total number of patients 25 in the LITT cohort.
Two hundred and fifty patients were identified through a review of hospital pharmacy records to have received bevacizumab between 2011 and 2018 from neuro-oncologists and medical oncologists at our institution. Twenty-six patients remained after deduplications and after excluding those who had other pathologies, did not have previously radiated brain metastasis, or did not have pre-treatment brain imaging in the system. Five patients received bevacizumab at time of LITT failure and were analyzed with the LITT cohort. Of the remaining 21 patients, 2 died before 6 weeks, one was lost to follow-up, and 5 received bevacizumab within 3 months after radiation. The remaining total number of patients was 13 in the bevacizumab cohort.
Median age at time of treatment was 62 years for the LITT cohort (range 35–81 years) and 63 years for the bevacizumab cohort (range 33–72 years), p = 0.605. There were more females in the LITT cohort (64.0% vs. 23.1% in the bevacizumab cohort, p = 0.038). There was no statistically significant difference between the two groups in terms of pre-treatment KPS (median of 80 and range of 50–100 for bevacizumab vs. median of 90 and range of 60–100 for LITT, p = 0.057), as well as the presence of systemic metastases at time of treatment, defined as receiving systemic therapy or imaging demonstrating persistence of metastases outside the brain (69.2% for bevacizumab vs. 68.0% for LITT, p = 1.000). In both groups, the most common primary pathology was lung cancer (bevacizumab 46.2%, LITT 44.0%) followed by melanoma (bevacizumab 38.5%, LITT 32.0%). 46.2% of bevacizumab patients and 36.0% of LITT patients had prior craniotomy (p = 0.728). In the LITT group, 55.6% of the patients had prior craniotomy for the same problematic lesion, compared to 66.7% in the bevacizumab group. In both groups, the most common radiation modality used for treatment of the brain metastases of interest was SRS alone (84.6% for bevacizumab vs. 84.0% for LITT), with SRS dose ranging from 15 to 22 Gy single fraction SRS and 24 to 30 Gy hypofractionated SRS. Patients who received treatment less than 3 months after radiation were presumed to have acute or subacute radiation injury and were excluded. Median time from SRS to initiation of bevacizumab was 6 months (range 3–34 months), and median time from SRS to the LITT procedure was 13 months (range 6–48 months), p = 0.014.
Ten out of 13 patients (76.9%) in the bevacizumab group had multiple lesions whereas 17 out of 25 patients (68.0%) treated with LITT had more than one lesion (p = 0.714). In the LITT group, 35.3% of the patients with multiple lesions had other lesions that showed an increase in size, but only one patient needed LITT treatment for more than one lesion. In the bevacizumab group, 40% of patients with multiple lesions had other lesions besides the index lesion that were also increasing in size. Median volume of the index lesion in the bevacizumab group was 3.1 cc (range 0.1–9.1 cc) compared to the median of 2.2 cc (range 0.3–12.6 cc) in the LITT group (p = 0.761).
In the bevacizumab group, the indications for using bevacizumab were symptomatic regrowth or inability to wean steroids in 11/13 (84.6%). Two patients (15.4%) did not have symptoms but were started on bevacizumab due to concerns for lesional progression on imaging. In the LITT group, the indications for treatment were symptomatic regrowth or inability to wean steroids in 13/25 (52.0%). Twelve patients (48.0%) did not have symptoms but underwent LITT due to lesional progression on imaging and need for diagnosis. One patient in this group also wished to start anti-PD1 clinical trial and was thus not a good candidate for steroids. Table 1 lists patient demographics and pre-treatment factors.
In the bevacizumab group, the median dose administered was 15 mg/kg (range 7.5–15 mg/kg), with a median total of 4 cycles (range 1–7 cycles), and a median total dose of 4150 mg (range 1200–12,900 mg). For patients who received more than one cycle (11/13), bevacizumab was administered over a median total of 16 weeks (range 3–54). For LITT patients, the median post-operative length of stay was 2 days (range 1–5 days). Median time in the OR was 6.2 h (range 5–10 h).
Survival outcomes
Median time to the last clinical follow-up was 110 weeks (range 23–370 weeks) in the LITT cohort compared to 52 weeks (range 6–110 weeks) in the bevacizumab cohort (p = 0.001). Median OS was 24.8 months (range 6.0–89.0 months) for the LITT group which was significantly longer than 15.2 months (range 1.6–25.4 months) for the bevacizumab group (p = 0.003). Median PFS was 12.1 months (range 0–64.6 months) in the LITT patients compared to 2.0 months (range 0–22.2 months) in the bevacizumab patients (p = 0.091). See Table 2, Figs. 1, and 2 for survival outcomes for both groups.
Lesional control
The median time to the last radiographic follow-up was 102 weeks (range 13–370 weeks) in the LITT cohort and 48 weeks (range 2–109 weeks) in the bevacizumab cohort (p = 0.002). Time to lesional progression was longer in the LITT patients: 12.1 months (range 0–64.6 months) for LITT vs. 2.0 months (range 0–22.2 months) for bevacizumab, however this result did not achieve statistical significance (p = 0.091). Ultimately, 44.0% of LITT patients and 38.5% of bevacizumab patients had lesional control until death or last radiographic follow up.
Radiographic outcome was assessed using both traditional volumetric estimation as well as a modified version of the RANO-BM criteria. The original criteria were adapted by excluding an assessment of non-target lesions and subsequent new lesions which do not apply in the surgical scenario.
Median volumetric changes at all follow-up time points were significantly different when comparing LITT lesions to bevacizumab-treated lesions. Median LITT lesion volumes initially increased more than 100% at one month follow-up but fell below starting volumes at 6 months and reached a 65% reduction in median volume at 12 months. In comparison, bevacizumab-treated lesions decreased 40% in median volume by 1 month, reached a maximal decrease of almost 85% at 6 months but then showed a significant re-increase in median volume to > 100% of starting volume by 12 months (Table 3 and Fig. 3).
Alternatively, lesional control assessment by RANO-BM showed that at one month, for both LITT and bevacizumab, the majority of lesions remained in the stable disease (SD) category although 30% of bevacizumab-treated lesions had already progressed (PD). By 3 months, 15% of the bevacizumab-treated lesions had attained complete response (CR) and 40% had now progressed compared to 12% of the LITT-treated lesions in the PD category. By 6 months, 15% of the bevacizumab-treated lesions remained at CR but over 50% of the lesions were now PD whereas in the LITT-treated group 20% had changed to PR and only 4% of lesions remained in the PD category. By the last follow-up only 8% of lesions were CR in both cohorts but 77% of bevacizumab-treated lesions were PD compared to 36% in the LITT group (Table 4). No statistically significant difference in post-treatment modified RANO-BM was found at any time point between the LITT and bevacizumab cohorts. There was no statistically significant correlation between the total dose of bevacizumab received with progression at any time point (p = 0.881 at one month, 0.797 at 3 months, and 0.927 at the last follow-up).
Five patients from the LITT cohort later received bevacizumab. Bevacizumab was started at a median of 6 months post-LITT as a salvage therapy (range 4–18 months). Lesions were enlarging in 4 patients prior to bevacizumab treatment. In one patient, bevacizumab was started despite stable imaging for inability to taper steroids.
Neurological outcome and steroid cessation
For the LITT group, post-treatment KPS was 100 (range 80–100) at one month, 80 (range 60–100) at 3 months, 80 (range 40–100) at 6 months, and 80 (range 40–100) at the last clinical follow-up. For the bevacizumab group, post-treatment KPS was 80 (range 30–100) at one month, 80 (range 30–100) at 3 months, 80 (range 40–100) at 6 months, and 60 (range 30–100) at the last clinical follow-up.
Pre-treatment symptoms were documented in 13/25 (52.0%) LITT patients. Symptoms consisted of headache in 4 patients, weakness in 4 patients, seizures in 3 patients, speech changes in 3 patients, visual changes in 4 patients, and imbalance in 3 patients. Eight patients had more than one symptom. Nine of the 13 symptomatic patients had symptom relief after LITT. Headache eventually improved in all patients (2 at one-month follow-up, and 2 at 6-month follow-up). At 1-month follow-up, weakness improved in 2 patients (50.0%), seizures in 1 patient (33.3%), speech changes in 1 patient (33.3%), visual changes in 1 patient (25.0%), and imbalance in 2 patients (66.7%).
In the bevacizumab cohort, pre-treatment symptoms were documented in 11/13 patients (84.6%). Symptoms consisted of headache and pain in 3 patients, nausea in 2 patients, confusion in 2 patients, weakness in 2 patients, seizure in 2 patients, and imbalance in 2 patients. Two patients had more than one symptom. Four out of 11 symptomatic patients had symptom relief after starting bevacizumab. At 1 month, headache improved in 2 patients (66.7%), confusion in 1 patient (50.0%), weakness in 1 patient (50.0%), and imbalance in 1 patient (50.0%). Nausea and seizures did not improve at any point during follow-up.
53.8% of the patients were on steroids prior to treatment with bevacizumab, and 56.0% of the patients were on steroids prior to LITT (p = 1.000). In the LITT cohort, 37.5% of the patients were on steroids at 1 month, 34.8% at 3 months, 29.2% at 6 months, and 32.0% at the last follow-up. Of the 14 LITT patients with pre-operative steroids use, 5 were able to wean off steroids at 1 month, while 5 out of 9 remaining patients were able to decrease steroid dosage at 1 month. Of the remaining 3 patients who were unable to taper steroids at 1 month, one required steroids for chronic adrenal insufficiency, one for COPD, and one declined our recommendation to taper due to increased weakness with taper. The latter patient was started on bevacizumab 6 months post-op and was able to slowly taper steroids off 3 months later. The patient with COPD was able to taper down to half the baseline dosage. One LITT patient who was not on steroids pre-op was started on steroids at 1-month follow-up due fatigue and lethargy. This patient was able to taper steroids to off at 3 months and remained off steroids at the last follow-up. At the last follow-up, 88% patients had stable to decreased steroid dosage in the LITT group.
In the bevacizumab cohort, 38.5% of the patients were on steroids at 1 month, 27.3% at 3 months, 25.0% at 6 months, and 53.8% at the last follow-up. Of the 7 patients with pre-treatment steroids use, 4 were able to taper off steroids at 1 month. Of the 3 remaining patients, 2 were able to decrease the dosage, while one had to increase dosage at 1 month due to headache and nausea. Of the 6 patients who were not on steroids pre-treatment, 2 were started on steroids at some point after treatment due to nausea and seizure, and one was started on steroids for colitis related to immunotherapy. Two patients who initially were able to be weaned off steroids at 1 month had to restart steroids by the last follow-up due to fatigue and systemic symptoms. At the last follow-up, 61.6% patients had stable to decreased steroids dosage in the bevacizumab group. See Fig. 4 for steroids usage in both cohorts.
Adverse outcomes
Three patients experienced complications in the LITT group. This included one case of confusion and headache, one case of worsening left sided weakness, and one case of seizure and bilateral deep vein thrombosis. The last case eventually also developed cystic regrowth requiring a salvage craniotomy. In the bevacizumab group, one patient developed grade 1 bleeding during dental procedure, one patient developed grade 2 epistaxis requiring discontinuation of bevacizumab, one patient developed grade 3 hypertension requiring long-term antihypertensive pharmacotherapy, and one patient developed grade 1 flushing, myalgia, and epistaxis which eventually led to discontinuation of bevacizumab after 4 cycles.
Discussion
Radiation necrosis (RN) is thought to be an inflammatory response to radiation injury that occurs typically months to years following radiosurgery and is found clinically in up to 10–15% of brain metastases patients surviving beyond 1 year [4]. The pathophysiology of RN is unclear, with multiple hypotheses ranging from glial cell damage to endothelial cell dysfunction causing local injury [3, 9]. When follow-up imaging shows persistent regrowth of a previously radiated lesion or when symptoms develop, additional treatment is often required. Laser interstitial thermal therapy (LITT) and bevacizumab have both been utilized in this setting. In patients eligible to have surgery with LITT-amenable lesions however, it remains unclear how to decide which option to choose.
LITT is a novel ablative technology that is gaining acceptance by neurosurgeons. Due to its minimally invasive surgical access to lesions, its use has also become attractive to patients. Initially, LITT was predominantly indicated for tumors < 30 mm that had exhausted all other treatment options, or were difficult to access via open craniotomy [10, 11]. These indications have rapidly expanded and many authors have now described the use of LITT in patients with metastatic in-field recurrent lesions regardless of their locations and whether the pathology is consistent with RN or tumor regrowth [12,13,14]. Most recently, the results from the Laser Ablation After Stereotactic Radiosurgery (LAASR) study have shown that LITT treatment of RN is associated with good outcomes in terms of survival and local control [12] and for lesions less than 3 cm in diameter, the results of LITT are comparable to craniotomy [15]. LITT therefore is rapidly becoming a first line surgical treatment for RN.
Bevacizumab is an anti-VEGF antibody that inhibits angiogenesis and decreases vessel permeability. VEGF has been shown to be overexpressed in RN lesions and the degree of expression has been shown to correlate with the degree of radiation injury [16, 17]. Bevacizumab is the only agent that has ever been shown in a randomized study to be efficacious for RN treatment [5]. In contrast to LITT, however, bevacizumab alone is insufficient for treatment of re-growing brain metastases. Due to the risk of intracerebral hemorrhage and out of concern for issues with wound healing, biopsy is also often not performed immediately prior to initiation of bevacizumab as a standard surgical practice [18]. Thus, the diagnosis of RN prior to treatment is often made radiographically.
To our knowledge, this is the first study to compare these two treatment options for the management of RN. Overall, LITT patients had significantly longer overall survival compared to bevacizumab patients. LITT patients also trended towards longer progression-free survival although the difference did not reach statistical significance. A likely explanation for this difference is that patients and lesions treated within the two groups may have been unevenly distributed. Patients in the bevacizumab cohort had a lower median KPS of 80 compared to the median KPS of 90 in the LITT group suggesting they may be sicker systemically making them less ideal surgical candidates. In addition, 80% of the lesions treated with bevacizumab were symptomatic compared to only 50% of those treated with LITT. This might also suggest that treatment might be more successful before these lesions become symptomatic.
This study also found that not only were the changes in lesional volumes post-treatment significantly different between the LITT and bevacizumab groups, but their patterns of change were also different. Using 3-dimensional volumetric assessments, LITT resulted in an initial increase in volume due to expected post-surgical changes, the lesional volume trended down over subsequent follow-ups such that there was an overall reduction in volume by 1-year post-treatment. In contrast, bevacizumab resulted in a post-contrast volume reduction up front, but this response was not sustained after cessation of treatment. In fact, there was a trend towards increased lesional volume compared to pre-treatment baseline at 1 year. These results are in line with previously published results for both LITT and bevacizumab [19,20,21]. A likely explanation for the discrepancy in radiographic responses may be the difference in the mechanisms of action of the two treatments. In the LITT patient, thermocoagulation causes cell death and therefore likely inactivation of the inflammatory cells. Bevacizumab, on the other hand, works by decreasing vessel permeability and may not in fact have a permanent effect on the inflammatory process but may have a role in diminishing symptomatology from mass effect early on in the course of RN management. Interestingly, based on the modified version of the RANO-BM criteria, the response of lesions to bevacizumab also follows a very different pattern than that following LITT. In the bevacizumab group, treatment can result in complete lesion resolution in a small percentage of cases but mostly results in stabilization of the lesion until time of further progression. On the other hand, LITT falsely appears to result in early disease progression—a phenomenon that is more appropriately explained by the expected transient increase in lesion size after effective LITT treatment. Ultimately, the majority of lesions will show partial response although very few show a complete response even in the long term.
In this study, no statistical difference was found between the two groups as far as the ability to wean off steroids. We found the ability to wean off steroids in our bevacizumab group to be lower than what was reported in other studies [5, 19, 21]. For example, in Boothe et al. cohort, 77.8% of the patients were able to be weaned off steroids by the first follow-up following bevacizumab treatment [19]. However, their population only included one patient with multiple lesions, compared to 76.9% of patients with multiple lesions from our cohort. Another study by Levin et al. reported that 4 out of 5 patients who were on steroids pre-treatment were able to reduce their steroid dose by 12 weeks after starting bevacizumab, but the information on whether these patients were able to wean off steroids completely was not available [5]. The ability to wean off steroids in our LITT cohort was also lower than that reported by Chaunzwa et al., who found that 73.3% of the patients were off steroids at 4.5 weeks [13]. This may also reflect the differences in patient selection, as their population included patients with re-growing tumor in addition to RN, as well as those who underwent LITT as early as 1 month after stereotactic radiosurgery [13]. The decision making involved in use or maintenance of steroids in this population treated with multimodality therapy, in our experience, is complex, and may be influenced by many factors unrelated to the intracranial lesion including the need to wean off steroids to facilitate commencement or continuation of immunotherapy or treatment of ongoing systemic conditions such as anorexia, fatigue, or COPD.
The limitations of this study obviously include the small sample size, its retrospective nature, and possible heterogeneity between the two cohorts. For example, although pre-treatment KPS were similar between the two groups, it is possible that patients in the bevacizumab cohort may have more extensive comorbidities compared to the LITT patients. Since LITT is a surgical procedure with potential complications not dissimilar to craniotomy, patients who were deemed to be poor surgical candidates may not be eligible for this procedure. Bevacizumab, on the other hand, is an IV infusion typically given in an outpatient setting. It can therefore be administered to a wide range of patients, including those with poor functional status who may not be good candidates for surgery. Additionally, we found that median time between radiation and initiation of RN treatment was significantly different between the two groups. While this difference may be attributable to the small size of the cohorts, it is possible that it may reflect also differing diagnoses. All LITT patients underwent a biopsy and had tissue proven RN. Most of the bevacizumab patients however did not have tissue diagnosis, as discussed previously, and the diagnosis instead was dependent upon imaging only. With multiple lesions progressing simultaneously, it is possible that some of them in fact represented tumor relapse. For both RN and tumor regrowth, bevacizumab may have resulted in an initial improvement in radiographic findings since it can cause dramatic changes in the degree of edema and lesional enhancement regardless of its etiology. With tumor progression, edema and enhancement would be expected to increase again over time.
Based on our findings, we would recommend considering LITT over bevacizumab for a patient suspected of having RN if the patient is a reasonable surgical candidate. Because the patterns of referrals vary widely, however, it has been difficult to standardize patient selection for each treatment in this setting. A larger study, preferably prospective and randomized, is needed to verify these findings.
Conclusion
In this study, LITT treatment of radiation necrosis lesions resulted in a long-term reduction of lesional volume and a longer overall survival. There also appears to be a trend towards a sustained reduction in steroids and a longer time to progression in the LITT group although this failed to achieve statistical significance in this small sample size. Comparison of the two cohorts however showed significant heterogeneity. Time from radiation to RN treatment was significantly shorter in the bevacizumab cohort and RN diagnosis was made radiographically in the bevacizumab group versus by tissue biopsy in the LITT group. Given the significant differences between the cohorts, these findings need to be confirmed in a larger and perhaps randomized study.
Abbreviations
- KPS:
-
Karnofsky performance score
- LITT:
-
Laser interstitial thermal therapy
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- RN:
-
Radiation necrosis
- SRS:
-
Stereotactic radiosurgery
- WBRT:
-
Whole brain radiation therapy
References
Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, Werner-Wasik M, Demas W, Ryu J, Bahary JP, Souhami L, Rotman M, Mehta MP, Curran WJ Jr (2004) Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet (London, England) 363(9422):1665–1672. https://doi.org/10.1016/s0140-6736(04)16250-8
Glantz MJ, Burger PC, Friedman AH, Radtke RA, Massey EW, Schold SC Jr (1994) Treatment of radiation-induced nervous system injury with heparin and warfarin. Neurology 44(11):2020–2027. https://doi.org/10.1212/wnl.44.11.2020
Miyatake S, Nonoguchi N, Furuse M, Yoritsune E, Miyata T, Kawabata S, Kuroiwa T (2015) Pathophysiology, diagnosis, and treatment of radiation necrosis in the brain. Neurol Med Chir (Tokyo) 55(1):50–59. https://doi.org/10.2176/nmc.ra.2014-0188
Sneed PK, Mendez J, Vemer-van den Hoek JG, Seymour ZA, Ma L, Molinaro AM, Fogh SE, Nakamura JL, McDermott MW (2015) Adverse radiation effect after stereotactic radiosurgery for brain metastases: incidence, time course, and risk factors. J Neurosurg 123(2):373–386. https://doi.org/10.3171/2014.10.Jns141610
Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel JS, Bekele BN, Grewal J, Prabhu S, Loghin M, Gilbert MR, Jackson EF (2011) Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys 79(5):1487–1495. https://doi.org/10.1016/j.ijrobp.2009.12.061
Sloan AE, Ahluwalia MS, Valerio-Pascua J, Manjila S, Torchia MG, Jones SE, Sunshine JL, Phillips M, Griswold MA, Clampitt M, Brewer C, Jochum J, McGraw MV, Diorio D, Ditz G, Barnett GH (2013) Results of the NeuroBlate System first-in-humans Phase I clinical trial for recurrent glioblastoma: clinical article. J Neurosurg 118(6):1202–1219. https://doi.org/10.3171/2013.1.Jns1291
Common Terminology Criteria for Adverse Events (CTCAE) v5.0. (2017) National Cancer Institute. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf. 2020
Lin NU, Lee EQ, Aoyama H, Barani IJ, Barboriak DP, Baumert BG, Bendszus M, Brown PD, Camidge DR, Chang SM, Dancey J, de Vries EG, Gaspar LE, Harris GJ, Hodi FS, Kalkanis SN, Linskey ME, Macdonald DR, Margolin K, Mehta MP, Schiff D, Soffietti R, Suh JH, van den Bent MJ, Vogelbaum MA, Wen PY (2015) Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol 16(6):e270–278. https://doi.org/10.1016/s1470-2045(15)70057-4
Furuse M, Nonoguchi N, Kawabata S, Miyatake S, Kuroiwa T (2015) Delayed brain radiation necrosis: pathological review and new molecular targets for treatment. Med Mol Morphol 48(4):183–190. https://doi.org/10.1007/s00795-015-0123-2
Carpentier A, McNichols RJ, Stafford RJ, Itzcovitz J, Guichard JP, Reizine D, Delaloge S, Vicaut E, Payen D, Gowda A, George B (2008) Real-time magnetic resonance-guided laser thermal therapy for focal metastatic brain tumors. J Neurosurg: https://doi.org/10.1227/01.neu.0000335007.07381
Torres-Reveron J, Tomasiewicz HC, Shetty A, Amankulor NM, Chiang VL (2013) Stereotactic laser induced thermotherapy (LITT): a novel treatment for brain lesions regrowing after radiosurgery. J Neurooncol 113(3):495–503. https://doi.org/10.1007/s11060-013-1142-2
Ahluwalia M, Barnett GH, Deng D, Tatter SB, Laxton AW, Mohammadi AM, Leuthardt E, Chamoun R, Judy K, Asher A, Essig M, Dietrich J, Chiang VL (2018) Laser ablation after stereotactic radiosurgery: a multicenter prospective study in patients with metastatic brain tumors and radiation necrosis. J Neurosurg 130(3):804–811. https://doi.org/10.3171/2017.11.Jns171273
Chaunzwa TL, Deng D, Leuthardt EC, Tatter SB, Mohammadi AM, Barnett GH, Chiang VL (2018) Laser thermal ablation for metastases failing radiosurgery: a multicentered retrospective study. Neurosurgery 82(1):56–63. https://doi.org/10.1093/neuros/nyx142
Rao MS, Hargreaves EL, Khan AJ, Haffty BG, Danish SF (2014) Magnetic resonance-guided laser ablation improves local control for postradiosurgery recurrence and/or radiation necrosis. J Neurosurg: https://doi.org/10.1227/neu.0000000000000332
Hong CS, Deng D, Vera A, Chiang VL (2019) Laser-interstitial thermal therapy compared to craniotomy for treatment of radiation necrosis or recurrent tumor in brain metastases failing radiosurgery. J Neurooncol 142(2):309–317. https://doi.org/10.1007/s11060-019-03097-z
Kim JH, Chung YG, Kim CY, Kim HK, Lee HK (2004) Upregulation of VEGF and FGF2 in normal rat brain after experimental intraoperative radiation therapy. J Korean Med Sci 19(6):879–886. https://doi.org/10.3346/jkms.2004.19.6.879
Nonoguchi N, Miyatake S, Fukumoto M, Furuse M, Hiramatsu R, Kawabata S, Kuroiwa T, Tsuji M, Fukumoto M, Ono K (2011) The distribution of vascular endothelial growth factor-producing cells in clinical radiation necrosis of the brain: pathological consideration of their potential roles. J Neurooncol 105(2):423–431. https://doi.org/10.1007/s11060-011-0610-9
Francisco S, Genentech CA (2018) US Food and Drug Administration website. J Neurooncol. https://doi.org/10.1001/jamanetworkopen.2019.11111
Boothe D, Young R, Yamada Y, Prager A, Chan T, Beal K (2013) Bevacizumab as a treatment for radiation necrosis of brain metastases post stereotactic radiosurgery. Neuro-oncology 15(9):1257–1263. https://doi.org/10.1093/neuonc/not085
Carpentier A, McNichols RJ, Stafford RJ, Guichard JP, Reizine D, Delaloge S, Vicaut E, Payen D, Gowda A, George B (2011) Laser thermal therapy: real-time MRI-guided and computer-controlled procedures for metastatic brain tumors. Lasers Surg Med 43(10):943–950. https://doi.org/10.1002/lsm.21138
Torcuator R, Zuniga R, Mohan YS, Rock J, Doyle T, Anderson J, Gutierrez J, Ryu S, Jain R, Rosenblum M, Mikkelsen T (2009) Initial experience with bevacizumab treatment for biopsy confirmed cerebral radiation necrosis. J Neurooncol 94(1):63–68. https://doi.org/10.1007/s11060-009-9801-z
Funding
None.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Nanthiya Sujijantarat, MD, Christopher S. Hong, MD, Kent A. Owusu, PharmD, BCCCP, BCPS, Aladine A. Elsamadicy, MD, Joseph P. Antonios, MD, PhD, and Andrew B. Koo, MD. The first draft of the manuscript was written by Nanthiya Sujijantarat, MD, and edited by Veronica L. Chiang, MD and Joachim Baehring, MD. All authors commented on subsequent versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The senior author of this paper (VC) is a consultant for Monteris Medical Inc. (Minnesota, USA) and Clearpoint Neuro (California, USA). No other authors have any conflicts of interest to disclose.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sujijantarat, N., Hong, C.S., Owusu, K.A. et al. Laser interstitial thermal therapy (LITT) vs. bevacizumab for radiation necrosis in previously irradiated brain metastases. J Neurooncol 148, 641–649 (2020). https://doi.org/10.1007/s11060-020-03570-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-020-03570-0