Abstract
Purpose
Postoperative stereotactic radiosurgery (SRS) has been shown to establish local control in patients with resected brain metastases, yet its efficacy may be limited, particularly for resected lesions with large post-operative resection cavities. We describe the efficacy of postoperative fractionated stereotactic radiotherapy (FSRT) for local control in patients who have undergone resection for brain metastases.
Methods
In this retrospective cohort study, we analyzed patients who received FSRT for resected brain metastases in 3 or 5 fractions. Time to local recurrence was the primary endpoint in this study.
Results
Sixty-seven patients (n = 29 female, n = 38 male) met study criteria for review. The median age of the cohort was 62 years (range 18–79 years). Median preoperative tumor volume was 11.1 cm3 (range 0.4–77.0 cm3). The rate of local control was 91.0% at 6 months, 85.1% at 12 months, and 85.1% at 18 months. Estimates of freedom from local recurrence at 6 and 12 months were 90.9% and 84.3%, respectively. Higher biologically equivalent doses (BED10) were found to be predictive of longer freedom from local recurrence on univariate and multivariable analysis. Larger cavity volumes were found to correspond to longer time to local recurrence on univariate and multivariable analysis.
Conclusion
Our results suggest that postoperative FSRT may be an effective method for providing local control to the surgical bed in patients with resected brain metastases, particularly for larger tumors not amenable to conventional, single-fraction SRS. Additional prospective studies are needed to confirm these findings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Brain metastases are the most common intracranial tumor in adults and confer a poor overall prognosis in the context of systemic malignancy [1]. Management of these patients depends on the size, location, number, and primary histology of the tumor. Stereotactic radiosurgery (SRS) has emerged as a primary treatment modality for the treatment of intracranial metastases. In the context of resected brain metastases, SRS improves local control at the resection cavity and is associated with better neurocognitive function particularly when compared to whole brain radiation therapy (WBRT) [2,3,4]. SRS has effectively become the standard-of-care for small tumors < 3 cm in diameter in locations that preclude a safe surgical resection [5]. Further, a number of studies, including two phase III trials, have reported good local control with postoperative SRS for patients with brain metastases, similar to WBRT [6, 7]. However, for metastases larger than 3 cm, SRS is associated with increased rates of cerebral radiation necrosis and diminished local control [8, 9]. Fractionated stereotactic radiotherapy (FSRT) has been utilized as one treatment alternative for these patients to provide local tumor control and decrease the risk of radiation necrosis. A number of studies have shown effective local control with FSRT for patients with brain metastases managed non-operatively [10,11,12,13,14,15,16]. However, evidence for the optimal fraction and dosing schedule for various tumor characteristics is only emerging [17,18,19,20,21,22,23,24,25,26]. In this retrospective study, we describe the efficacy of FSRT delivered in 3 or 5 fractions to maximize local control after surgical resection of brain metastasis(es).
Materials and methods
Study design, setting, and participants
This study was conducted under the auspices of an institutional review board (IRB)-approved protocol. Waivers of informed consent and authorization were granted. Patients who received postoperative FSRT for resected brain metastases between July 2013 and August 2018 were identified in the institutional database. A cohort of 67 patients were selected for inclusion in this study based on treatment with postoperative FSRT to an intracranial resection bed. No patients in the cohort were in any prior clinical trials or retrospective reviews. All demographic, clinical, radiographic, and pathologic data was attained with a retrospective review of the institutional electronic medical record.
Radiation treatment
Patients considered for radiation to the resection cavity are reviewed at a multidisciplinary conference. Decisions regarding the use of single session (typically SRS) or multi-session FSRT to the surgical cavity are primarily based on the size of the resection cavity, e.g. cavities ≥ 3 cm in diameter receive FSRT. FSRT for all patients was delivered with the Elekta Leksell Gamma Knife® Perfexion™ system (Elekta, Stockholm, Sweden) at our institution. Radiation plans were reviewed by the treating neurosurgeon, radiation oncologist, and medical physicist prior to radiation delivery to verify proper dosing and target volume. Following MRI-guided placement of the stereotactic head frame, a volumetric MRI is attained with 1 mm slice thickness on a 1.5 T magnet with a gap of 0 mm following administration of MultiHance® (Bracco, Milan, Italy) gadobenate dimeglumine contrast. The same day, the patient is brought to the Pinnacle AcQSim computed tomography (CT) simulator workstation (Phillips, London, UK) and properly positioned. Multiple axial CT images are obtained with a CT scanner through the volume of interest and isocenters for treatment planning are placed accordingly. The FSRT target volume was defined as the resection cavity as well as the resection tract on the pre-treatment MRI scan with an additional 1 mm margin. All patients received a cumulative FSRT dose of either 24 Gy, 25 Gy, 27 Gy, or 30 Gy in either 3 or 5 fractions. The selection of fraction scheme depends on the size of the lesion, prior radiation (dose and interval to current treatment), and nearby critical structures. We tend to use 5 fractions for patients with large lesions who received prior radiation at a short interval and/or is adjacent to critical neurovascular structures (e.g. optic chiasm).
Study variables
Study variables included age, gender, primary tumor histology, date of surgical resection, start and end date of FSRT treatment, tumor volume, cavity volume, cumulative radiation dose and number of fractions, pre- and postoperative Karnofsky performance scale (KPS) and graded prognostic assessment (GPA). Local tumor recurrence was the primary endpoint of this study. Time to local recurrence was defined as the time from the beginning of FSRT to the date of first radiographically-proven recurrence or date of last MRI if no recurrence was observed at last follow-up. Overall survival was defined as the time from the beginning of FSRT treatment to death, or the date of last follow-up if no death was observed. Melanoma, renal cell carcinoma, and sarcoma were considered radioresistant tumor histologies [27]. Functional location of the treated metastasis was classified as Grade I (non-eloquent), II (near-eloquent), and III (eloquent) per criteria described by Sawaya et al. [28]. Recurrence was defined using the Response Assessment in Neuro-Oncology Brain Metastases (RANO-BM) working group criteria [29]. Biological equivalent dose using a tumor α/β of 10 (BED10) and equivalent dose in 2 Gy (EQD2) was calculated from cumulative dose and number of fractions per patient. Radiation necrosis was defined by a diagnosis from the neuroradiologist in the electronic medical record or biopsy confirmation, if available.
Follow-up and volumetric analysis
All patients received MRI prior to surgical resection, prior to beginning adjuvant FSRT, and at three-months following completion of treatment. T1-weighted post-contrast (T1C+) MR images from these three time points were exported to Brainlab Elements™ software (Brainlab, Munich, Germany) from the electronic medical record. Using these T1C+ MRIs, manual tumor segmentation was completed by creating a tridimensional volumetric measure. The tumor margin was the enhancing lesion on preoperative imaging. The margin on all post-resection scans was the resection cavity. Single measurements of each lesion were calculated in Brainlab and volumes were verified by the senior author.
Statistical analysis
All statistical analyses were performed in R using the survival (https://CRAN.R-project.org/package=survival) and cmprisk (https://CRAN.R-project.org/package=cmprsk) packages. All plots were created in R using the survminer (https://CRAN.R-project.org/package=survminer) and default graphics packages. Categorical variables are reported with frequencies and percentages, while continuous variables are reported with medians and ranges. Categorical variables were compared with a Mantel-Cox (log-rank) test using a Kaplan–Meier method. Univariate and multivariable predictors of time to local recurrence and overall survival were separately assessed with a Cox proportional hazards model with confidence intervals (CI) set to 95%. Gender (male vs. female), number of lesions treated (1 vs. > 1), number of fractions (5 vs. 3), histology (radiosensitive vs. resistant histopathology as well as non-small cell lung cancer [NSCLC] vs. other), BED10, tumor volume, cavity volume, prior radiotherapy (yes vs. no), pre-FSRT KPS, pre-FSRT GPA, postoperative immune-modulating therapy (yes vs. no), extent of resection (gross total vs subtotal resection), and functional location were all variables studied. Overall survival and freedom from local recurrence were estimated with a competing risk analysis using the cumulative incidence function. Additionally, p-values < 0.05 were considered significant for all statistical analyses.
Results
Patient demographic and clinical characteristics
Patient demographic and clinical information is summarized in Table 1. A median age of 62 years (18–79 years) was calculated for a cohort of 67 patients (n = 29 female, n = 38 male) at the time of FSRT. Median preoperative tumor volume was 11.1 cm3 (range 0.4–77.0 cm3). Median cavity volume was 6.4 cm3 (range 0.2–61.4 cm3) from the immediate postoperative period and 2.7 cm3 (range 0–39.6 cm3) at 3 months follow-up. Median KPS at the time of FSRT and at 12 months follow-up was 80 (range 70–90) and 80 (range 50–100), respectively. Median GPA score preceding FSRT and at 12 months follow-up was 2.5 (range 1–4) and 2.25 (range 0–4), respectively. A subset of the cohort (n = 15, 22.4%) received cranial radiotherapy prior to FSRT. The majority of these patients (n = 12) received stereotactic radiotherapy, either single or multi-fraction, while three patients received prior WBRT. Of these 15 patients, 13 received radiotherapy (n = 11 SRS, n = 2 WBRT) to the same lesion treated by FSRT prior to resection. Median length of follow-up from the time of FSRT to last imaging follow-up for non-deceased patients was 12.9 months (range 0–35.8 months). Of the cohort of 67 patients, 16.4% (n = 11) were observed to have local recurrence before last follow-up. A minority of these patients (n = 5) had dural-based loci of recurrence. FSRT was delivered in four regimens for the entire cohort; BED10 = 51.3 Gy (EQD2 = 42.75 Gy) (n = 32), BED10 = 48 Gy (EQD2 = 40 Gy) (n = 2), BED10 = 43.2 Gy (EQD2 = 36) (n = 26), BED10 = 37.5 Gy (EQD2 = 31.25) (n = 7). Radiation necrosis was observed in 13.4% (n = 9) of patients before last follow-up. Of these, six patients (66.7%) experienced symptoms associated with this diagnosis. Three patients required operative management of associated radiation necrosis (n = 2 surgical resection, n = 1 laser interstitial thermal therapy), while the remaining six patients were medically managed with steroids or bevacizumab. Median time to diagnosis of radiation necrosis from last FSRT session was 9 months (range 1–11 months). Of these seven patients, one received SRS for local recurrence following FSRT but preceding diagnosis of radiation necrosis on follow-up imaging.
Predictors of local recurrence and overall survival
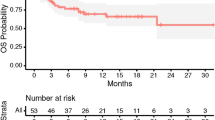
The rate of local control was 91.0% at 6 months, 85.1% at 12 months, and 85.1% at 18 months. Estimates of overall survival at 6 (76.8%), 12 (63.3%) and 18 months (51.5%) were calculated using a competing risk approach as well as estimates of freedom from local recurrence at 6 (90.9%), 12 (84.3%), and 18 months (84.3%). Both overall survival and time to local recurrence are depicted by a competing risk plot in Fig. 1. When treated as continuous variables, neither pre-treatment KPS (p = 0.44, HR 0.98 [CI 0.92–1.03]) nor GPA score (p = 0.27, HR 1.81 [CI 0.19–1.61]) were predictive of local recurrence on Cox univariate analysis. Prior radiotherapy (either WBRT, SRS, or FSRT) was not found to be predictive of local recurrence on log-rank (p = 0.66) or Cox univariate analysis (p = 0.66, HR 0.71 [CI 0.15–3.30]). Multiple lesions (one vs. more than one) did not affect freedom from local recurrence on Kaplan–Meier (p = 0.91) or Cox univariate analysis (p = 0.91, HR 0.91 [CI 0.20–4.26]). When treated as a continuous variable, BED10 (and corresponding EQD2) was found to be predictive of time to local recurrence (p = 0.01). When compared as a categorical variable ( ≥ 48 Gy vs < 48 Gy), BED10 was significant on log-rank (p = 0.04) and near-significant on Cox univariate analysis (p = 0.06) (Fig. 2a). Radioresistant histology was not predictive of time to local recurrence on Cox univariate analysis (p = 0.99). Similarly, non-NSCLC histology was not predictive of time to local recurrence (p = 0.82). When treated as a continuous variable, postoperative cavity volume was found to be predictive of time to local recurrence (p = 0.05, HR 0.82 [CI 0.67–1.00]). For resection cavities above and below the median (6 cc), a relationship can be observed on a Kaplan–Meier curve that is near significant on log-rank (p = 0.06) (Fig. 2b). Similarly, preoperative tumor volume is a near-significant predictor of freedom from local recurrence on Cox univariate analysis (p = 0.09). Treatment with immune-modulating drugs did not significantly prolong time to local recurrence on Cox univariate analysis (p = 0.21). Neither extent of resection (p = 0.50) nor functional location (p = 0.45) of the tumor contributed to freedom from local recurrence. Cox multivariable analysis demonstrated a significant relationship between time to local recurrence and BED10 as well as cavity volume. All multivariable predictors of freedom from local recurrence are summarized in Table 2.
Discussion
SRS and FSRT both work by intersecting multiple low-dose radiation beams over a target with stereotactic precision. SRS is conventionally delivered in a single, high dose, while FSRT divides the prescribed dose into multiple sessions, which is thought to increase the likelihood of targeting tumor cells in a radiation-sensitive phase of the cell cycle [30]. Although the efficacy of SRS for the management of brain metastases has been established, the exponentially increased radiation required to meet the BED10 for larger tumors delivered in a single dose results in a significantly increased risk of radiation necrosis [8]. For larger tumors, there is emerging evidence to support a few alternative therapies including intraoperative radiotherapy (IORT) and brachytherapy as well as FSRT to reduce the risk of radiation necrosis [31, 32]. However, much of this evidence is still nascent and larger studies are needed to confirm the safety and efficacy of these adjunctive therapies. Although there are few studies comparing SRS and FSRT directly, a retrospective study by Minniti et al. found lower rates of radiation necrosis in patients receiving FSRT compared to SRS (9% vs. 18%, respectively) [11]. Despite the smaller sample size in our study, the observed rates of radiation necrosis are similar (13.4%) and give additional evidence for the reduced risk of radiotoxic complications. Moreover, the high, single dose of SRS often precludes its applicability to tumors adjacent to critical white matter structures [33, 34]. FSRT, on the other hand, can mitigate the risk of radiation necrosis and collateral radiotoxicity while providing adequate dosage to the tumor region of interest. Another disadvantage of SRS is the associated ‘pseudoprogression’ observed on follow-up imaging resulting from reactionary peritumoral edema, inflammation, and transient tumor growth secondary to high dose radiation that can confound the detection of local recurrence [35]. Conversely, FSRT is associated with a less robust tissue response to treatment with similar rates of tumor control [36].
Although FSRT was previously described as one method for providing stereotactic radiotherapy to the resection bed of patients with brain metastases, Steinmann et al. were the first to investigate its efficacy in a dedicated cohort of patients and described a 12-month local control rate of 71% [17, 37]. In 2015, Eaton et al. described lower rates of radiation necrosis in patients receiving FSRT compared to single-fraction SRS and concluded FSRT to be a favorable technique for providing local control in larger cavities [23]. Two years later, three retrospective cohort studies, described 12-month local control rates between 84 and 89%, respectively for patients receiving FSRT to the resection bed in 3, 5, or 10 fractions [18, 19, 24, 25]. A recent multicenter study published by Combs et al. reported a 12-month local control rate of 75% for patients receiving FSRT in 6 or 7 fractions to the surgical bed [26]. Kumar et al. further investigated the role of FSRT for establishing local control in patients with resected brain metastases with higher local control observed at higher BED10 [20].
The findings in our study suggest that FSRT delivered in 3 or 5 fractions is a safe and effective adjunctive therapy for improving local control in patients with resected intracranial metastases. Notably, rates of local recurrence at 6 and 12 months were similar to previous reports in the literature for both FSRT and single fraction SRS indicating that fractionated dosing may not sacrifice local control, particularly for larger tumors [7, 20]. Further, higher BED10 (and associated EQD2) doses would appear to be associated with better local control, a relationship established by previous studies [38]. Specifically, the longer freedom from local recurrence associated with BED10 doses ≥ 48 Gy in our cohort echoes the findings of Kumar et al. who observed a 100% 12-month freedom from local recurrence at the same dose threshold [20]. Interestingly, a longer freedom from local recurrence was observed, paradoxically, in patients with larger tumor resection cavities in our cohort. This parallel relationship between tumor volume and local failure is not reflected by previous studies investigating postoperative SRS or FSRT [7, 19, 20, 22]. Reoxygenation of hypoxic tumor cells with FSRT has been shown to increase tumor sensitivity to subsequent irradiation in murine models, a phenomenon that may be exaggerated with larger metastatic lesions [39]. There is controversy in the literature on the applicability of BED10 derived from the linear-quadratic model to FSRT and the reliability of this approach for different fractionation schedules should be evaluated [21].
Our results are limited by the retrospective design of the study. Additionally, the low rates of overall survival in our cohort can confound estimates of local recurrence, which we attempted to mitigate with competing risk analysis. Although the relationship between FSRT and tumor volume has not been previously reported, it may be that the values derived from volumetric analysis provides a more accurate representation of tumor size. The present study offers additional data to support the efficacy of FSRT delivered in 3 or 5 fractions in providing local control. However, further studies comparing FSRT to SRS and other conventional radiotherapy modalities are warranted to determine the applicability of this therapy to various patient and tumor characteristics.
Conclusion
In patients receiving surgical resection for brain metastases, adjuvant SRS has been shown to preserve local control without contributing to neurocognitive decline. However, the risk of radiotoxicity from single-fraction SRS precludes its use for large tumors adjacent to critical neuroanatomic structures. Our results indicate that FSRT may be effective, particularly for patients with larger tumors not amenable to SRS with less risk for radiotoxic effects. Additional studies are needed to establish a role for FSRT in this patient population.
Abbreviations
- WBRT:
-
Whole-brain radiotherapy
- SRS:
-
Stereotactic radiosurgery
- FSRT:
-
Fractionated stereotactic radiotherapy
- MRI:
-
Magnetic resonance imaging
- CT:
-
Computed tomography
- KPS:
-
Karnofsky performance score
- GPA:
-
Graded prognostic assessment
- IRB:
-
Institutional review board
- OS:
-
Overall survival
- T1C+:
-
T1-weighted post-contrast
- BED10 :
-
Biologically effective dose
- EQD2:
-
equivalent dose in 2 Gy
- NSCLC:
-
Non-small cell lung cancer
- CI:
-
Confidence interval
- HR:
-
Hazard ratio
- IORT:
-
Intraoperative radiotherapy
References
Nayak L, Lee EQ, Wen PY (2012) Epidemiology of brain metastases. Curr Oncol Rep 14:48–54. https://doi.org/10.1007/s11912-011-0203-y
Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, Werner-Wasik M, Demas W, Ryu J, Bahary JP, Souhami L, Rotman M, Mehta MP, Curran WJ Jr (2004) Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet (London, England) 363:1665–1672. https://doi.org/10.1016/s0140-6736(04)16250-8
Choi CY, Chang SD, Gibbs IC, Adler JR, Harsh GRT, Lieberson RE, Soltys SG, (2012) Stereotactic radiosurgery of the postoperative resection cavity for brain metastases: prospective evaluation of target margin on tumor control. Int J Radiat Oncol Biol Phys 84:336–342. https://doi.org/10.1016/j.ijrobp.2011.12.009
Luther N, Kondziolka D, Kano H, Mousavi SH, Engh JA, Niranjan A, Flickinger JC, Lunsford LD (2013) Predicting tumor control after resection bed radiosurgery of brain metastases. Neurosurgery 73:1001–1006. https://doi.org/10.1227/neu.0000000000000148
Badiyan SN, Regine WF, Mehta M (2016) Stereotactic radiosurgery for treatment of brain metastases. J Oncol Pract 12:703–712. https://doi.org/10.1200/JOP.2016.012922
Brown PD, Ballman KV, Cerhan JH, Anderson SK, Carrero XW, Whitton AC, Greenspoon J, Parney IF, Laack NNI, Ashman JB, Bahary JP, Hadjipanayis CG, Urbanic JJ, Barker FG 2nd, Farace E, Khuntia D, Giannini C, Buckner JC, Galanis E, Roberge D (2017) Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC.3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol 18:1049–1060. https://doi.org/10.1016/s1470-2045(17)30441-2
Mahajan A, Ahmed S, McAleer MF, Weinberg JS, Li J, Brown P, Settle S, Prabhu SS, Lang FF, Levine N, McGovern S, Sulman E, McCutcheon IE, Azeem S, Cahill D, Tatsui C, Heimberger AB, Ferguson S, Ghia A, Demonte F, Raza S, Guha-Thakurta N, Yang J, Sawaya R, Hess KR, Rao G (2017) Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol 18:1040–1048. https://doi.org/10.1016/S1470-2045(17)30414-X
Korytko T, Radivoyevitch T, Colussi V, Wessels BW, Pillai K, Maciunas RJ, Einstein DB (2006) 12 Gy gamma knife radiosurgical volume is a predictor for radiation necrosis in non-AVM intracranial tumors. Int J Radiat Oncol Biol Phys 64:419–424. https://doi.org/10.1016/j.ijrobp.2005.07.980
Blonigen BJ, Steinmetz RD, Levin L, Lamba MA, Warnick RE, Breneman JC (2010) Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 77:996–1001. https://doi.org/10.1016/j.ijrobp.2009.06.006
Kwon AK, Dibiase SJ, Wang B, Hughes SL, Milcarek B, Zhu Y (2009) Hypofractionated stereotactic radiotherapy for the treatment of brain metastases. Cancer 115:890–898. https://doi.org/10.1002/cncr.24082
Minniti G, D'Angelillo RM, Scaringi C, Trodella LE, Clarke E, Matteucci P, Osti MF, Ramella S, Enrici RM, Trodella L (2014) Fractionated stereotactic radiosurgery for patients with brain metastases. J Neuro-oncol 117:295–301. https://doi.org/10.1007/s11060-014-1388-3
Matsuyama T, Kogo K, Oya N (2013) Clinical outcomes of biological effective dose-based fractionated stereotactic radiation therapy for metastatic brain tumors from non-small cell lung cancer. Int J Radiat Oncol Biol Phys 85:984–990. https://doi.org/10.1016/j.ijrobp.2012.09.008
Lischalk JW, Oermann E, Collins SP, Nair MN, Nayar VV, Bhasin R, Voyadzis JM, Rudra S, Unger K, Collins BT (2015) Five-fraction stereotactic radiosurgery (SRS) for single inoperable high-risk non-small cell lung cancer (NSCLC) brain metastases. Radiat Oncol (London, England) 10:216. https://doi.org/10.1186/s13014-015-0525-2
Ishihara T, Yamada K, Harada A, Isogai K, Tonosaki Y, Demizu Y, Miyawaki D, Yoshida K, Ejima Y, Sasaki R (2016) Hypofractionated stereotactic radiotherapy for brain metastases from lung cancer: Evaluation of indications and predictors of local control. Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft [et al] 192:386–393. https://doi.org/10.1007/s00066-016-0963-2
Ahmed KA, Sarangkasiri S, Chinnaiyan P, Sahebjam S, Yu HH, Etame AB, Rao NG (2016) Outcomes following hypofractionated stereotactic radiotherapy in the management of brain metastases. Am J Clin Oncol 39:379–383. https://doi.org/10.1097/coc.0000000000000076
Jeong WJ, Park JH, Lee EJ, Kim JH, Kim CJ, Cho YH (2015) Efficacy and safety of fractionated stereotactic radiosurgery for large brain metastases. J Korean Neurosurg Soc 58:217–224. https://doi.org/10.3340/jkns.2015.58.3.217
Steinmann D, Maertens B, Janssen S, Werner M, Fruhauf J, Nakamura M, Christiansen H, Bremer M (2012) Hypofractionated stereotactic radiotherapy (hfSRT) after tumour resection of a single brain metastasis: report of a single-centre individualized treatment approach. J Cancer Res Clin Oncol 138:1523–1529. https://doi.org/10.1007/s00432-012-1227-x
Dore M, Martin S, Delpon G, Clement K, Campion L, Thillays F (2017) Stereotactic radiotherapy following surgery for brain metastasis: predictive factors for local control and radionecrosis. Cancer Radiother 21:4–9. https://doi.org/10.1016/j.canrad.2016.06.010
Keller A, Dore M, Cebula H, Thillays F, Proust F, Darie I, Martin SA, Delpon G, Lefebvre F, Noel G, Antoni D (2017) Hypofractionated stereotactic radiation therapy to the resection bed for intracranial metastases. Int J Radiat Oncol Biol Phys 99:1179–1189. https://doi.org/10.1016/j.ijrobp.2017.08.014
Kumar AMS, Miller J, Hoffer SA, Mansur DB, Coffey M, Lo SS, Sloan AE, Machtay M (2018) Postoperative hypofractionated stereotactic brain radiation (HSRT) for resected brain metastases: improved local control with higher BED10. J Neuro-oncol 139:449–454. https://doi.org/10.1007/s11060-018-2885-6
Shibamoto Y, Miyakawa A, Otsuka S, Iwata H (2016) Radiobiology of hypofractionated stereotactic radiotherapy: what are the optimal fractionation schedules? J Radiat Research 57(Suppl 1):i76–i82. https://doi.org/10.1093/jrr/rrw015
Ahmed KA, Freilich JM, Abuodeh Y, Figura N, Patel N, Sarangkasiri S, Chinnaiyan P, Yu HH, Etame AB, Rao NG (2014) Fractionated stereotactic radiotherapy to the post-operative cavity for radioresistant and radiosensitive brain metastases. J Neuro-oncol 118:179–186. https://doi.org/10.1007/s11060-014-1417-2
Eaton BR, LaRiviere MJ, Kim S, Prabhu RS, Patel K, Kandula S, Oyesiku N, Olson J, Curran W, Shu HK, Crocker I (2015) Hypofractionated radiosurgery has a better safety profile than single fraction radiosurgery for large resected brain metastases. J Neuro-oncol 123:103–111. https://doi.org/10.1007/s11060-015-1767-4
Lima LC, Sharim J, Levin-Epstein R, Tenn S, Teles AR, Kaprealian T, Pouratian N (2017) Hypofractionated stereotactic radiosurgery and radiotherapy to large resection cavity of metastatic brain tumors. World Neurosurg 97:571–579. https://doi.org/10.1016/j.wneu.2016.10.076
Keller A, Dore M, Antoni D, Menoux I, Thillays F, Clavier JB, Delpon G, Jarnet D, Bourrier C, Lefebvre F, Chibbaro S, Darie I, Proust F, Noel G (2017) Risk of radionecrosis after hypofractionated stereotactic radiotherapy targeting the postoperative resection cavity of brain metastases. Cancer Radiother 21:377–388. https://doi.org/10.1016/j.canrad.2017.01.017
Combs SE, Bilger A, Diehl C, Bretzinger E, Lorenz H, Oehlke O, Specht HM, Kirstein A, Grosu A-L (2018) Multicenter analysis of stereotactic radiotherapy of the resection cavity in patients with brain metastases. Cancer Med 7:2319–2327. https://doi.org/10.1002/cam4.1477
Brown PD, Brown CA, Pollock BE, Gorman DA, Foote RL (2002) Stereotactic radiosurgery for patients with "radioresistant" brain metastases. Neurosurgery 51:656–665; discussion 665–657
Sawaya R, Hammoud M, Schoppa D, Hess KR, Wu SZ, Shi WM, Wildrick DM (1998) Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery 42:1044–1055; discussion 1055–1046
Lin NU, Lee EQ, Aoyama H, Barani IJ, Barboriak DP, Baumert BG, Bendszus M, Brown PD, Camidge DR, Chang SM, Dancey J, de Vries EG, Gaspar LE, Harris GJ, Hodi FS, Kalkanis SN, Linskey ME, Macdonald DR, Margolin K, Mehta MP, Schiff D, Soffietti R, Suh JH, van den Bent MJ, Vogelbaum MA, Wen PY (2015) Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol 16:e270–e278. https://doi.org/10.1016/s1470-2045(15)70057-4
Pawlik TM, Keyomarsi K (2004) Role of cell cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol Biol Phys 59:928–942. https://doi.org/10.1016/j.ijrobp.2004.03.005
Vargo JA, Sparks KM, Singh R, Jacobson GM, Hack JD, Cifarelli CP (2018) Feasibility of dose escalation using intraoperative radiotherapy following resection of large brain metastases compared to post-operative stereotactic radiosurgery. J Neuro-oncol 140:413–420. https://doi.org/10.1007/s11060-018-2968-4
Wernicke AG, Hirschfeld CB, Smith AW, Taube S, Yondorf MZ, Parashar B, Nedialkova L, Kulidzhanov F, Trichter S, Sabbas A, Ramakrishna R, Pannullo S, Schwartz TH (2017) Clinical outcomes of large brain metastases treated with neurosurgical resection and intraoperative cesium-131 brachytherapy: results of a prospective trial. Int J Radiat Oncol Biol Phys 98:1059–1068. https://doi.org/10.1016/j.ijrobp.2017.03.044
Mayo C, Martel MK, Marks LB, Flickinger J, Nam J, Kirkpatrick J (2010) Radiation dose-volume effects of opticnerves and chiasm. Int J Radiat Oncol Biol Phys 76:S28–S35. https://doi.org/10.1016/j.ijrobp.2009.07.1753
Mayo C, Yorke E, Merchant TE (2010) Radiation associated brainstem injury. Int J Radiat Oncol Biol Phys 76:S36–S41. https://doi.org/10.1016/j.ijrobp.2009.08.078
Ruzevick J, Kleinberg L, Rigamonti D (2014) Imaging changes following stereotactic radiosurgery for metastatic intracranial tumors: differentiating pseudoprogression from tumor progression and its effect on clinical practice. Neurosurg Rev 37:193–201. https://doi.org/10.1007/s10143-013-0504-8; discussion 201
Aoki M, Abe Y, Hatayama Y, Kondo H, Basaki K (2006) Clinical outcome of hypofractionated conventional conformation radiotherapy for patients with single and no more than three metastatic brain tumors, with noninvasive fixation of the skull without whole brain irradiation. Int J Radiat Oncol Biol Phys 64:414–418. https://doi.org/10.1016/j.ijrobp.2005.03.017
Do L, Pezner R, Radany E, Liu A, Staud C, Badie B (2009) Resection followed by stereotactic radiosurgery to resection cavity for intracranial metastases. Int J Radiat Oncol Biol Phys 73:486–491. https://doi.org/10.1016/j.ijrobp.2008.04.070
Baliga S, Garg MK, Fox J, Kalnicki S, Lasala PA, Welch MR, Tome WA, Ohri N (2017) Fractionated stereotactic radiation therapy for brain metastases: a systematic review with tumour control probability modelling. Br J Radiol 90:20160666. https://doi.org/10.1259/bjr.20160666
Murata R, Shibamoto Y, Sasai K, Oya N, Shibata T, Takagi T, Abe M (1996) Reoxygenation after single irradiation in rodent tumors of different types and sizes. Int J Radiat Oncol Biol Phys 34:859–865
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (University of Texas MD Anderson Cancer Center) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Traylor, J.I., Habib, A., Patel, R. et al. Fractionated stereotactic radiotherapy for local control of resected brain metastases. J Neurooncol 144, 343–350 (2019). https://doi.org/10.1007/s11060-019-03233-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-019-03233-9