Abstract
Purpose
The aim of this study was to determine whether a higher biological effective dose (BED) would result in improved local control in patients treated with fractionated stereotactic radiotherapy (FSRT) for their resected brain metastases.
Methods
Patients with newly diagnosed brain metastases without previous brain radiotherapy were retrospectively reviewed. Patients underwent surgical resection of at least one brain metastasis and were treated with adjuvant FSRT, delivering 25–36 Gy in 5–6 fractions. Outcomes were computed using Kaplan–Meier survival analysis and univariate analysis.
Results
Fifty-four patients with 63 post-operative cavities were included. Median follow-up was 16 months (3–60). Median metastasis size at diagnosis was 2.9 cm (0.6–8.1) and median planning target volume was 19.7 cm3 (6.3–68.1). Two-year local control (LC) was 83%. When stratified by dose, 2 years LC rate was 95.1% in those treated with 30–36 Gy in 5–6 fractions (BED10 of 48–57.6 Gy10) versus 59.1% lesions treated with 25 Gy in 5 fractions (BED10 of 37.5 Gy10) (p < 0.001). LC was not associated with resection cavity size. One year overall survival was 68.7%, and was independent of BED10. Symptomatic radiation necrosis occurred in 7.9% of patients and was not associated with dose.
Conclusion
In the post-operative setting, high-dose FSRT (BED10 > 37.5 Gy10) were associated with a significantly higher rate of LC compared to lower BED regimens. Overall, 25 Gy in 5 fractions is not an adequate dose to control microscopic disease. If selecting a 5-fraction regimen, 30 Gy in five fractions appears to provide excellent tumor bed control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
About 30% of patients with solid malignancies develop intracranial metastases during the course of their disease [1]. Optimal management depends upon multiple factors including lesion size, number and location of metastases, symptoms, age, and performance status. Treatment options include whole brain radiotherapy, surgery, stereotactic radiosurgery (SRS), or fractionated stereotactic radiotherapy (FSRT) in various combinations [2].
Surgical resection of brain metastases without adjuvant radiotherapy results in a suboptimal 1-year local control rate of only 43% and a distant brain control rate of 33% [3]. The addition of post-operative whole brain radiation (WBRT) significantly reduces the recurrence rate from 40 to 60% following surgery alone to 10–30% [4]. Given the significant neurotoxicity from WBRT, and the corresponding decrement in quality of life [5, 6], alternative focal radiotherapy regimens followed by regular magnetic resonance imaging (MRI) surveillance to detect salvageable distant brain recurrences are now favored. Two recent randomized controlled trials demonstrated high rates of 1 year local control utilizing stereotactic radiosurgery (SRS) in the post-operative setting (61–72%) [3, 7]. Additional studies have reported 1-year local recurrence free survival rates of 75% in patients treated with post-operative FSRT given in 30–35 Gy in 5 fractions [8]. For large resection cavities, FSRT is a common treatment option, which may offer improved local control with less risk of radionecrosis compared to SRS [9, 10].
In this study, we investigated the efficacy and predictors of outcomes in patients who underwent FSRT for newly diagnosed brain metastases in the post-operative setting. We also evaluated dose response of various FSRT fractionation schedules stratified by biological effective dose with using an alpha/beta ratio of 10 (BED10).
Methods
Patients who received adjuvant FSRT at the University of Wisconsin for resected brain metastases between December 2002 and June 2018 were included in this study. This study was approved by our Institutional Review Board. Exclusion criteria included radiosensitive tumors such as lymphoma, germ cell tumors, and small cell lung cancer, or if they previously had WBRT. Electronic medical records and radiotherapy treatment plans were reviewed to extract relevant clinical and dosimetric data.
Treatment
FSRT was prescribed based on treating physician preference but was typically recommended over single fraction SRS for large resection cavities or because of the tumor's location near or within a critical structure. Following immobilization with an aquaplast head mask, a computed tomography (CT) scan was acquired with intravenous contrast for radiotherapy planning. CT images were fused with a T1 weighted post gadolinium MRI scan that was acquired within two weeks of treatment planning. Any residual disease was contoured as gross tumor volume (GTV) and postoperative cavity was contoured as clinical target volume (CTV). A 2–5 mm isotropic margin was added to CTV to obtain a planning target volume (PTV). Depending upon the planning system used, a mix of coplanar and non-coplanar beams were used to obtain an optimal plan that would meet department-specified dose constraints for each fractionation scheme. Patients were prescribed 25 Gy in 5 fractions or 30–36 Gy in 5–6 fractions to the PTV based on individual physician preference. Prescription isodose line varied for individual patients, but generally plans were normalized such that ≥ 95% of the PTV received ≥ 95–98% of the prescription dose. Dose was generally prescribed to the 80–90% isodose line (IDL). Treatment was delivered on alternating weekdays with daily CT guidance.
Follow-up
Following FSRT, patients generally had follow-up MRI scans within 6–8 weeks post-treatment, and then every 3 months thereafter. Local failure was defined by the presence of nodular enhancement detected on T1 contrast-enhanced MRI along the resection cavity. Any MRI scans concerning for local failure were discussed at our neuro-oncology tumor board, and further investigations including MRI with perfusion and/or tumor biopsy were performed when recommended. Salvage surgery was considered in patients with isolated large symptomatic in-field or symptomatic distant brain recurrences, whereas repeat FSRT or SRS was typically employed for smaller asymptomatic recurrences. In patients with multiple distant brain metastases, WBRT was recommended.
Study endpoints
The primary endpoint was local control (LC) and was defined as absence of recurrence within the treatment target, determined using methods discussed above. Secondary endpoints included distant brain control (DBC) and overall survival (OS). DBC was defined as absence of new intracranial lesions outside the treatment target. These outcomes were measured from the last day of radiotherapy until most recent follow-up, or the date of the event being analyzed.
Statistical analysis
Descriptive analysis was summarized as median and range for continuous variables, and as proportions for categorical variables. For the purpose of survival analysis, FSRT was divided into two different BED10 groups: high-dose FSRT defined as 30 Gy in 5 fractions or 30–36 Gy in 6 fractions (BED10 of 45 Gy10–57.6 Gy10) compared with low-dose FSRT defined as 25 Gy in 5 fractions (BED10 of 37.5 Gy10). Patients were also dichotomized by median PTV size. Actuarial LC, DBC, and OS were computed by the Kaplan–Meier method and survival curves were compared by the Log-Rank test. Hazard ratios for local progression were computed using Cox regression analysis.
Results
Fifty-four patients with 63 post-operative cavities were included in this study. Baseline clinical characteristics for FSRT patients are shown in Table 1. Median follow-up was 16 months (range 3–60 months). Non-small cell lung cancer was the most common primary site histology. Recursive partitioning analysis (RPA) class 2 comprised 81% of the cohort. A majority (83.3%) of the patients had a single resected intracranial metastasis. The median maximum diameter of the metastasis at diagnosis across all patients was 2.9 cm (0.6–8.1). The median resection cavity diameter was 3.6 cm (0.6–6.1 cm) and the median PTV volume was 19.7 cm3 (6.3–68.1 cm3). The majority of cavities (86%) were treated with a 2–3 mm margin, while the remainder (14%) were treated with a 4–5 mm margin.
Twenty-two cavities (35%) were treated with 25 Gy in 5 fractions (BED10 37.5 Gy10) and the remaining forty-one were treated to a dose of 30–36 Gy in 5–6 fractions (BED10 48–57.6 Gy10). Ten patients with 11 cavities had documented local recurrence, resulting in a LC rate of 82.5% at last follow up.
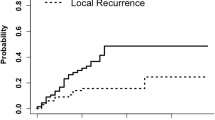
Local recurrence was detected in 9 out of 22 cavities in the group that received a BED10 37.5 Gy10 group and 2 out of 41 cavities in the BED10 48–57.6 Gy10 group. Local control was significantly lower in the BED10 37.5 Gy10 group compared to the BED10 48–57.6 Gy10 group, with a 2 years actuarial LC rate of 95.1% versus 59.1% (p < 0.001, Fig. 1). One year DBC and OS were 44.4% and 68.7%, respectively. At last follow up, 61% of deaths were related to neurologic cause, while the remaining 39% of deaths were due to other causes. Neurologic death was not statistically different between BED10 groups. Stratifying by BED10 did not result in statistically significant differences in either 1-year DBC or OS.
The only significant factor associated with local failure on univariate analysis (UVA) was BED10. (BED10 of 37.5 Gy10 versus BED10 > 48–57.6 Gy10 (HR 9.58, 95% CI 2.07–44.40, p < 0.01) (Table 2). Other factors including age, gender, time from diagnosis until development of brain metastases, histology, number of brain metastases, size, lesions > 2.0 cm versus < 2.0 cm, lesions > 3.0 cm versus < 3.0 cm, RPA class, diagnosis-specific graded prognostic assessment (DS-GPA), presence of extracranial metastasis at diagnosis, and time from surgery to the start of radiotherapy did not predict for local failure on UVA. As BED10 was the only statistically significant factor on UVA, multivariate analysis was not performed. A similar UVA for overall survival did not identify any factors predictive of OS.
In cavities with local failure, only 36.4% had isolated local failure, whereas 63.6% also had distant brain failure at 12 months. In patients with local failure, salvage surgery with or without post-operative FSRT was performed in two patients (3.6%), salvage FSRT or SRS alone was administered in three patients (5.5%), and WBRT was given in four patients (7.3%). In the entire cohort, five patients (9.1%) developed leptomeningeal disease (LMD) and 4 (7.3%) developed dural-based metastatic lesions. Symptomatic radiation-induced necrosis occurred from treatment in five cavities (7.9%) in five patients. These patients were all treated with steroids, and three of these patients ultimately required surgery for symptom relief. No specific factors predicted for radiation necrosis on UVA, including BED10 (HR 1.06, 95% CI 0.30–3.78, p = 0.93) or cavity size (HR 1.01, 95% CI 0.96–1.07, p = 0.70).
Discussion
Historically, WBRT has been the mainstay of treatment following resection of brain metastases. Given the decline in quality of life after WBRT, the use of SRS and FSRT is increasingly implemented in lieu of WBRT as adjuvant therapy following surgery. These highly conformal radiation techniques limit therapeutic dose to smaller volumes, decreasing the risk of neurocognitive side effects. As systemic therapies continue to improve, and patients with metastatic disease live longer after their diagnosis, the ability to spare quality of life-limiting toxicity from WBRT grows increasingly important.
While there have been no completed randomized controlled trials comparing FSRT and SRS for intracranial metastases of resection cavities, retrospective series have suggested better LC and decreased risk of necrosis with FSRT in large cavities and metastases [9,10,11]. Minniti et al. analyzed 289 consecutive patients with intact metastases > 2 cm and found the 1-year cumulative LC rates to be higher in those treated with multi-fraction SRS (91%), compared to single-fraction SRS (77%). The 1-year cumulative incidence rate of radionecrosis was also lower with FRST (9%) compared to SRS (18%) [10]. In a separate analysis, Minniti and colleagues examined 101 patients with resection cavities treated with FSRT (9 Gy × 3), and reported 1-year and 2-year LC rates of 93% and 84%, with 9% developing radionecrosis [11]. Eaton et al. reported on 76 resection cavities larger than 3 cm treated with either SRS or FSRT, and while no difference in local failure was demonstrated, FSRT (administered mainly in 30 Gy over 5 fractions) resulted in dramatically less incidence of radionecrosis [9].
Although multiple institutions utilize FSRT for resection cavities, the optimal dose and fractionation schedule is still up for debate. The most common dosing schemes are 27 Gy in 3 fractions [10, 11], 30 Gy in 5 fractions [12] and 25 Gy in 5 fractions [13]. In our current study, we report on a cohort of patients with a median cavity size of 19.7 cm3 (median diameter of 3.5 cm) treated with post-operative FSRT. We show that higher BED10 > 48 Gy10 (30–36 Gy in 5–6 fractions) FSRT regimens result in an elevated 2-year LC rate of 95% compared to 59% in cavities treated to a lower BED10 of 37.5 Gy10 (25 Gy in 5 fractions). We did not observe a difference in control rate or necrosis based on cavity size. Our results are similar to other reports [12, 14, 15].
Kumar et al. reported on 43 cavities that were treated with 3–5 fractions of FSRT, and found those treated with higher BED10 > 48 Gy10 (30 Gy in 5 fractions or 27 Gy in 3 fractions) had better LC compared to those treated with BED10 < 48 Gy10 (25–27.5 Gy in 5 fractions or 24 Gy in 3 fractions) [12]. In their study, this difference in LC according to BED10 was more pronounced in post-operative cavities larger than 2.4 cm. Moreover, the Memorial Sloan Kettering Cancer Center observed 1-year LC rates of 84% with minimal toxicity in resection cavities treated to 30 Gy in 5 fractions [14]. In contrast, Rajakesari et al. reported a much lower 1-year LC rate of 56% in 70 patients with resection cavities treated with 25 Gy in 5 fractions [15]. This rate appears similar to our reported LC rate in patients treated with 25 Gy in 5 fractions. Traylor et al. also found BED10 > 48 Gy10 to be predictive of longer freedom from local recurrence in their series of 66 patients who received FSRT for resected brain metastases treated in 3 or 5 fractions. Collectively, these studies, including our own, illustrate how radiation dose potentially influences the rate of tumor bed control for resected intracranial metastases, with a BED10 > 48 Gy10 (30 Gy in 5 fraction or 27 Gy in 3 fractions) needed for optimal tumor control.
There are limitations to our study, including its retrospective nature, small patient numbers, lack of neurocognitive and quality of life data, and a comparison SRS cohort. Nevertheless, we believe that our study provides useful information about the impact of FSRT dose on LC. Our data in combination with others [12] indicate that 25 Gy in five fractions might not be adequate to reliably control microscopic disease in the post-operative setting. If selecting a 5-fraction regimen, 30 Gy in five fractions appears to provide better tumor bed control without a corresponding increase in toxicity. Future prospective studies, such as the ongoing Alliance study (NCT04114981), comparing cavity SRS versus FSRT using international CTV contouring guidelines [16] will provide further insight into the ideal dose-fractionation schedules, potentially improving LC further while simultaneously maintaining patient quality of life.
References
Andrews DW, Scott CB, Sperduto PW et al (2004) Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. https://doi.org/10.1016/S0140-6736(04)16250-8
Sittenfeld SMC, Suh JH, Murphy ES, Yu JS, Chao ST (2018) Contemporary management of 1–4 brain metastases. Front Oncol 8:385. https://doi.org/10.3389/fonc.2018.00385
Mahajan A, Ahmed S, McAleer MF et al (2017) Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol 18:1040–1048. https://doi.org/10.1016/S1470-2045(17)30414-X
Soon YY, Tham IW, Lim KH, Koh WY, Lu JJ (2014) Surgery or radiosurgery plus whole brain radiotherapy versus surgery or radiosurgery alone for brain metastases. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD009454.pub2
Chang EL, Wefel JS, Hess KR et al (2009) Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 10:1037–1044. https://doi.org/10.1016/S1470-2045(09)70263-3
Brown PD, Jaeckle K, Ballman KV et al (2016) Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA 316:401–409. https://doi.org/10.1001/jama.2016.9839
Brown PD, Ballman KV, Cerhan JH et al (2017) Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC.3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol 18:1049–1060. https://doi.org/10.1016/S1470-2045(17)30441-2
Combs SE, Bilger A, Diehl C et al (2018) Multicenter analysis of stereotactic radiotherapy of the resection cavity in patients with brain metastases. Cancer Med 7:2319–2327. https://doi.org/10.1002/cam4.1477
Eaton BR, LaRiviere MJ, Kim S et al (2015) Hypofractionated radiosurgery has a better safety profile than single fraction radiosurgery for large resected brain metastases. J Neurooncol 123:103–111. https://doi.org/10.1007/s11060-015-1767-4
Minniti G, Scaringi C, Paolini S et al (2016) Single-fraction versus multifraction (3 × 9 Gy) stereotactic radiosurgery for large (> 2 cm) brain metastases: a comparative analysis of local control and risk of radiation-induced brain necrosis. Int J Radiat Oncol Biol Phys 95:1142–1148. https://doi.org/10.1016/j.ijrobp.2016.03.013
Minniti G, Esposito V, Clarke E et al (2013) Multidose stereotactic radiosurgery (9 Gy × 3) of the postoperative resection cavity for treatment of large brain metastases. Int J Radiat Oncol Biol Phys 86:623–629. https://doi.org/10.1016/j.ijrobp.2013.03.037
Kumar AMS, Miller J, Hoffer SA et al (2018) Postoperative hypofractionated stereotactic brain radiation (HSRT) for resected brain metastases: improved local control with higher BED10. J Neurooncol 139:449–454. https://doi.org/10.1007/s11060-018-2885-6
Abuodeh Y, Ahmed KA, Naghavi AO et al (2016) Postoperative stereotactic radiosurgery using 5-Gy x 5 sessions in the management of brain metastases. World Neurosurg 90:58–65. https://doi.org/10.1016/j.wneu.2016.02.007
Lockney NA, Wang DG, Gutin PH et al (2017) Clinical outcomes of patients with limited brain metastases treated with hypofractionated (5x6Gy) conformal radiotherapy. Radiother Oncol 123:203–208. https://doi.org/10.1016/j.radonc.2017.03.018
Rajakesari S, Arvold ND, Jimenez RB et al (2014) Local control after fractionated stereotactic radiation therapy for brain metastases. J Neurooncol 120:339–346. https://doi.org/10.1007/s11060-014-1556-5
Soliman H, Ruschin M, Angelov L, et al (2018) Consensus contouring guidelines for postoperative completely resected cavity stereotactic radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys 100:436–442. https://doi.org/10.1016/j.ijrobp.2017.09.047
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Musunuru, H.B., Witt, J.S., Yadav, P. et al. Impact of adjuvant fractionated stereotactic radiotherapy dose on local control of brain metastases. J Neurooncol 145, 385–390 (2019). https://doi.org/10.1007/s11060-019-03308-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-019-03308-7