Abstract
Purpose
The outcomes of five fraction stereotactic radiotherapy (hfSRT) following brain metastasectomy were evaluated and compared with published series.
Methods
30 Gy in 5 fractions HfSRT prescribed to the surgical cavity was reduced to 25 Gy if the volume of ‘brain−GTV’ receiving 20 Gy exceeded 20 cm3. Endpoints were local recurrence, nodular leptomeningeal recurrence, new brain metastases and radionecrosis. The literature was searched for reports of clinical and dosimetric outcomes following postoperative hfSRT in 3–5 fractions.
Results
39 patients with 40 surgical cavities were analyzed. Cavity local control rate at 1 year was 33/40 (82.5%). 3 local failures followed 30 Gy/5 fractions and 4 with 25 Gy/5 fractions. The incidence of leptomeningeal disease (LMD) was 7/40 (17.5%). No grade 3–4 toxicities, particularly no radionecrosis, were reported. The incidence of distant brain metastases was 15/40 (37.5%). The median overall survival was 15 months. Across 13 published series, the weighted mean local control was 83.1% (adjusted for sample size), the mean incidence of LMD was 14.9% (7–34%) and the mean rate of radionecrosis was 10.3% (0–20.6%).
Conclusion
Postoperative hfSRT can be delivered with 25–30 Gy in 5 fractions with efficacy in excess of 82% and no significant toxicity when the dose to ‘brain−GTV’ does not exceed 20 cm3.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Resection of brain metastases (BM) is indicated to relieve raised intracranial pressure, to relieve symptoms that have not responded to steroid therapy and to acquire tissue for histological diagnosis [1]. Postoperative irradiation sterilizes residual microscopic disease and reduces local recurrence [2]. Postoperative whole brain radiotherapy (WBRT) was standard practice however is associated with neurocognitive impairment and a lack of survival benefit [3]. Analagous to primary radiosurgery, targeted irradiation of the surgical cavity has now been widely adopted. Postoperative stereotactic radiosurgery (SRS) following brain metastasectomy reduces local recurrence by 50% as compared with MRI-based follow-up [4] and is neuroprotective as compared with WBRT [3]. Therefore postoperative SRS has become a standard of care. This study presents the outcomes of a uniform series of patients treated with postoperative hfSRT according to a prospective standardized protocol to evaluate efficacy and toxicity. Similar published series were evaluated with the aim of guiding the future practice of postoperative hfSRT.
Methods
Patient selection and eligibility criteria
Consecutive patients who received postoperative hfSRT between 01/2016 and 02/2020 were identified from the institutional database. Up to three additional metastases were treated with primary SRS/hfSRT according to volume and location. Patients who had previously received WBRT, < 5 Gy per fraction, planning margins > 2 mm or who declined consent to participate were not included. Median interval between diagnosis on MRI and metastasectomy was 5.5 days (2–80 days).
Radiotherapy planning technique
A planning CT scan with 0.6 mm slice thickness in a custom-made radiosurgery mask (Brainlab, Germany) and a gadolinium-enhanced T1 MPR MRI (1 mm slice, no gap) were performed on the same day. Image fusion, autosegmentation and contouring of the surgical cavity were undertaken (Brainlab Elements). The CTV was the cavity with extension along the dura or sinus in case of preoperative contact and any residual tumor and was expanded by 2 mm to create the PTV [5]. Treatment planning used inversely optimized, modulated, non-coplanar arcs (Cranial SRS, Brainlab Elements) or VMAT (Eclipse, Varian, USA).
Dose prescription
30 Gray (Gy) in 5 fractions (biological equivalent dose (BED) for α/β ratios of 10 for tumor control (BED10 = 48 Gy) and of 2 for late effects (BED2 = 120 Gy)) was prescribed to 98–99% of the PTV, with maximum dose between 125 and 143% (equivalent to prescribing to the 70–80% isodose surface (%IDS) when normalized to the maximum point dose). The structure ‘brain minus GTV’ was created and if more than 20 cm3 of this ‘organ at risk’ (OAR) received 20 Gy [6], the dose was reduced to 25 Gy in 5 fractions (BED10 = 37.5 Gy and BED2 = 87.5 Gy).
Treatment delivery
Treatment was delivered on alternate days with the Truebeam STx with Novalis Radiosurgery platform (Brainlab/Varian) with high definition MLC leaves (2.5 mm) without steroids unless SRS/hfSRT was delivered to intact metastases.
Outcome parameters
MRIs were performed 3-monthly and time to local recurrence, nodular leptomeningeal recurrence, new brain metastases and radionecrosis were calculated from the date of the last fraction of postoperative radiotherapy. Patient follow-up was censored at death or last follow-up until 05.04.2021.
Second-look radiology review
Given the overlap in appearance of tumour recurrence and radionecrosis and the potential for interobserver variability, MRIs reported to show local failure (LF) or nodular leptomeningeal disease (nLMD) underwent a ‘second look’ by a board-certified neuroradiologist. Features to differentiate recurrence from radionecrosis included new contrast enhancement in the surgical cavity, tumor progression in the case of residual tumor [7], low apparent diffusion coefficient (ADC) values [8] and ratio [9], ‘lesion quotient’ (ratio of maximal cross sectional area on T2 weighted to T1 weighted sequences) [10] as well as time elapsed following hfSRT [11].
Statistical analyses
Kaplan–Meier analysis was utilized to calculate the actuarial local control rate, otherwise descriptive statistics were applied. Ethics approval was granted (EKNZ 2091-01705).
Terms for the literature search in Pubmed with no time limit were “hypofractionated”, “stereotactic”, “radiotherapy”, “radiosurgery”, “metastasis”, “adjuvant”, “resection” and “surgery” and “brain” and a hand search of the references was performed.
Results
Patient characteristics
39 patients with 40 surgical cavities were eligible (Table 1). 5-ALA fluorescence was used to facilitate ‘en bloc’ resection and ultrasonic tissue ablation was used (CUSA, Integra Life Sciences, USA) where necessary. 97% of patients had a postoperative MRI within 24 h of surgery which showed suspected residual tumour in 10/40 cavities (25%). 100% of patients had a planning MRI within 6 days of radiotherapy. Median interval between resection and completion of hfSRT was 31 days (7–64 days). 22/40 (55%) cavities were treated with 30 Gy/5 fractions and 18/40 cavities (45%) received 25 Gy/5 fractions. The median follow-up was 11.7 months (2.7–40.1 months).
Treatment outcomes: local and leptomeningeal failure and toxicity
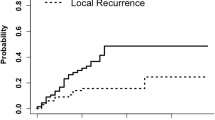
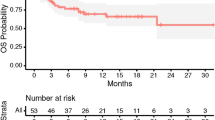
LF occurred in 7/40 cavities (17.5%) at a median time of 7 months (2.4–25.8 months), thus actuarial local control at last follow up was 82.5% (Fig. 1). Three patients with local failure had non-small cell lung cancer (NSCLC) and 4 had gastro-intestinal tumors (3 colorectal, 1 esophageal). Similarly, 7/40 cavities (17.5%), developed LMD at a median of 3 months (0.6–17.9), and 3/40 cavities developed both LF and LMD (Table 2), 1 following 30 Gy and two after 25 Gy in 5 fractions. The new contrast-enhancement which developed around the seven cavities was reported as recurrence rather than radionecrosis or postoperative change after independent re-evaluation. New brain metastases developed in 15/39 (39%) patients and median overall survival was 15 months (0.8–43.3 months).
Of the ten patients with residual tumor, two developed a recurrence; one received 30 Gy, the other 25 Gy in 5 fractions. Of the eight patients with residual tumor who did not develop a local recurrence, three received 30 Gy and five received 25 Gy. Of those who developed nodular leptomeningeal recurrence (nLMD), 5 patients had NSCLC (3 adenocarcinoma, 2 non-adenocarcinoma), 1 had melanoma and 1 pancreatic adenocarcinoma. Of the three patients with both LF and nLMD, 2 had the large cell neuroendocrine adenocarcinoma subtype of NSCLC and the third had squamous cell lung cancer.
Data from the literature
The literature search identified 24 retrospective publications reporting hfSRT. Five that overlapped with others were excluded [12,13,14,15,16], as were series with multiple fractionation schedules. One series did not report details of the planning technique [17] and another presented outcomes at 6 months [18], thus 13 series were included. The 82.5% local control (LC) observed in the KSA series (Fig. 1) is similar to the weighted mean LC of 85.3% computed from the 13 published series (Table 3). However, we report a 0% incidence of radiological radionecrosis as compared with a mean of 10.3% (0–19%) radiological or histological radionecrosis. The 17.5% incidence of LMD in this series is comparable to the mean of 14.4% (7–34%) in the 13 publications.
Two additional series stating the volume of irradiated normal brain which resulted in histological or radiological radionecrosis (V xGy) following 5 fraction hfSRT were identified [13, 19]. Three data points were reported in a postoperative series [26] and two were derived in the setting of primary hfSRT [6, 19] applied to cavity hfSRT: [24] and this series. A plot of brain volume against dose in five fractions associated with radionecrosis yielded a linear inverse relationship, R2 = 0.59 (Fig. 2).
From the literature, a PTV margin > 2–3 mm did not increase local control rate and the rate of radionecrosis was not higher with margins in excess of 3 mm (Supplementary Fig. 2), and there may be a higher incidence of radionecrosis with the 3 fraction schedules as compared with 5 fraction schedules (Supplementary Fig. 3).
Discussion
In the absence of published dose recommendations for 5 fraction postoperative hfSRT, we adopted those from a phase II trial published in the setting of primary hfSRT [6] as did another group who increased the fractionation to 10 × 4 Gy if more than 25 cm3 of brain received more than 20 Gy [20]. They reported neither severe toxicity other than alopecia nor radionecrosis, however the LC rate at 1 year was only 71%. None of the other publications described their dose volume constraints, however several detailed the V xGy, which can represent total brain, ‘brain−PTV’ [13] or ‘brain−GTV’ [21]. Given the range of fractionation schemes, the ‘radionecrosis dose’ was converted to BED2 to enable comparison and the V xGy was plotted against the respective BED2 (Fig. 2). The line of best fit requires prospective validation but might form the basis for a future nomogram for the isotoxic prescription [22] of postoperative hfSRT.
A meta-analysis of 50 studies evaluated 3458 patients treated with SRS as well as hfSRT, yielding rates of cavity LC at 12 months of 83.7%, radionecrosis of 6.9% and LMD of 13% [23]. A review of predominantly postoperative SRS publications developed practice guidelines without recommended dose volume constraints [24] and a comprehensive review focusing on hfSRT again did not conclude with any recommendations. The aim of the current work was to compare and contrast with the most similar series, hence only the literature pertaining to postoperative hfSRT was included. None used an identical methodology but outcomes closely approximated those reported here.
Local control
The LC rate in this study was consistent with the median and weighted mean of the 13 published hfSRT series and the weighted mean LC of 83.7% across 50 SRS/hfSRT studies [23], where hfSRT achieved higher LC rates (87.3%) than SRS (80%) (p = 0.021) [23]. Unlike in the meta-analysis, in the current series prescribed dose from the 13 published and the current hfSRT series were converted to BED to allow comparison. As there does not appear to be a dose response above 5 × 6 Gy (Supplementary Fig. 1), and may well be an increase in radionecrosis above the corresponding BED2 of 120 Gy (Fig. 2), this endorses the 5 × 6 Gy schedule for postoperative hfSRT [17, 25].
Although intuitive that a higher dose might be needed to achieve local control in radioresistant histologies, only one series reported a correlation between histology and local control [26]. Further, better LC rates were reported with postoperative rather than primary hfSRT for melanoma [27] but not lung cancer brain metastases [28]. Consistent with Shi et al. [29], four of the seven patients who developed LF in this series had a GI primary tumor.
Common to many hfSRT series was an increase in fractionation with increasing cavity size [20], for example 3 × 8 Gy for 10–19.9 cm3 and 5 × 6 Gy for 20–30 cm3 [19, 30]. In the current series, the median PTV of cavities with recurrence approximated the median PTV for all 40 cavities but was indeed larger than the median PTV of the 33 without recurrence (Table 2). Reduction in cavity control rates have been reported for PTVs > 11.7 cm3 [31], > 17 cm3 [26] and > 23 cm3 [32], however there was no such correlation when 3 × 8 Gy was increased to 5 × 6 Gy for PTVs > 20 cm3 [18] as biological efficacy was maintained through fractionation [28, 30, 33,34,35].
Putative risk factors for local recurrence are residual tumor at the time of hfSRT and a prolonged interval between neurosurgery and radiotherapy. As 8 of 10 cavities with suspected residual tumor were controlled at 1 year, 5 × 5–6 Gy with Dmax 140% appears sufficient. The aim in this series was to start hfSRT 30 days postoperatively. The median interval to start of hfSRT was 31 days but recurrence was not observed in the few patients who started after 60 days due to delayed wound healing or other patient factors. Similarly, a start more than 30 days postoperatively did not affect LC rates on meta-analysis [23].
Radionecrosis
Following observation that SRS plans with a lower conformity index (CI) were associated with better local control rates, a 2 mm rather than 0 mm planning margin has been recommended [5] however no benefit was shown on meta-analysis (LC 2 mm 84.3% vs 0 mm 83.1%, p = 0.71) [23]. A 2 mm margin in this series achieved LC rates equivalent to or in excess of series using 5 mm expansion (Supplementary Fig. 2) which does not support the need for larger margins and the consequent risk of radionecrosis [36]. The smaller margins and radiosurgical dose prescription employed in this series reduce the volume of irradiated normal brain and the dose reduction above 20 cm3 of ‘brain−GTV’ to 20 Gy may also have been beneficial.
The maximum volumes of brain that can be safely irradiated with SRS have been identified [37, 38] and these data can be applied to hfSRT by calculating the single dose equivalent (SDE) [39], which has been reported to correlate with incidence of radionecrosis [29]. 3 × 7.4 Gy to the 70% isodose with Dmax 100%, daily was associated with a radionecrosis rate of 20% [32, 40]. 3 × 9 Gy (BED2 148.5 Gy) daily is associated with radionecrosis rates between 9 and 15% [27, 28, 33]. 10 Gy per fraction (BED2 = 180) achieved an 89.9% 1 year LC offset by a 25% incidence of radionecrosis [41]. The exception to the higher rates of radionecrosis with the three fraction schedule was 3 × 8 Gy daily to cavities > 3 cm diameter with only 2.9% incidence of radionecrosis [18]. This schedule equates to BED2 of 120 Gy, the same as 5 × 6 Gy without consideration of the overall treatment time. Symptomatic radionecrosis has been reported in patients treated with three rather than five fractions [42], matching our observations (Supplementary Fig. 3) and putatively due to the immunogenicity of this schedule [43].
On multivariate analysis, a V18 Gy in 3 fractions of 30–32 cm3 normal brain was significantly associated with increased risk [27] and in an earlier evaluation, V24Gy in 3 fractions of 16.8 cm3 was a significant predictor of radionecrosis [33]. ‘Brain−GTV’ used here and by others [44], is more conservative than ‘brain−PTV’ and may contribute to the lack of observed toxicity. The risk of radionecrosis reported in 36 of 50 studies was 6.9% and is generally thought to be acceptable [23].
Leptomeningeal recurrence
Nodular leptomeningeal disease (nLMD) is now recognized as a complication of brain metastasectomy [24, 45]. An incidence of 13% was calculated on meta-analysis [23] and factors such as larger cavities [32] and resection of multiple metastases may be risk factors [30] for tumor cell dissemination. More than 50 days between surgery and hfSRT has been reported to be associated with risk of LMD [30] as have breast histology and infratentorial location [46]. Breast cancer is commonly associated with classical LMD independent of neurosurgery [47] however, which might underlie the association with female gender in some reports. Three of seven patients with nLMD in this series had large cell neuroendocrine lung cancer, which may have a greater propensity to disseminate to the brain [48]. Piecemeal resection has also been linked to nodular LMD [49] and the ‘en bloc’ technique is preferred. Sterilisation of tumour cells dispersed in the cerebrospinal fluid at resection is the compelling rationale behind preoperative radiosurgery [50]. Ultimately, many of the factors influencing local recurrence and nLMD, such as dural contact [5], relate to the size of the metastasis.
Strengths and limitations
The strengths of this analysis are the uniform planning technique, protocol-based margins and dose prescription with delivery of image-guided hfSRT on an SRS platform. Contouring was performed by two experienced radiation oncologists previously shown to have only 5% interobserver variability (unpublished data) and MRI review was undertaken by a single neuro-radiologist. The weaknesses of the study are the mix of histologies, lack of histological confirmation of recurrence and that additional specialized imaging, such as metabolic imaging studies, was not performed because either WBRT was indicated or progression of extracranial disease prevented further investigation.
Our aim is efficacy with minimal toxicity and thus we currently favor 5 × 6 Gy even for small cavities as this achieves LC comparable to published series without toxicity other than grade 2 alopecia. Whether the dose should be reduced to 5 × 5 Gy for larger cavities remains unanswered, as the risk of radionecrosis may be three-fold less in the postoperative as opposed to the primary setting [44], but this approach did not compromise efficacy.
Conclusions
Postoperative hfSRT with 5 × 6 Gy (V99%: 100%, Dmax 140%) is an efficacious schedule without significant toxicity if the dose to ‘brain−GTV’ does not exceed 20 Gy to 20 cm3. Prospective investigation of the dose volume constraints for cavities exceeding this guidance is required to further optimize treatment regimens.
Data availability
The raw data will be made available upon reasonable request.
References
Ewend MG, Morris DE, Carey LA, Ladha AM, Brem S (2008) Guidelines for the initial management of metastatic brain tumors: role of surgery, radiosurgery, and radiation therapy. J Natl Compr Cancer Netw 6:505–513 (quiz 14)
Patchell RA, Tibbs PA, Regine WF, Dempsey RJ, Mohiuddin M, Kryscio RJ et al (1998) Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA 280:1485–1489
Brown PD, Ballman KV, Cerhan JH, Anderson SK, Carrero XW, Whitton AC et al (2017) Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC.3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol 18:1049–1060
Mahajan A, Ahmed S, McAleer MF, Weinberg JS, Li J, Brown P et al (2017) Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol 18:1040–1048
Soliman H, Ruschin M, Angelov L, Brown PD, Chiang VLS, Kirkpatrick JP et al (2018) Consensus contouring guidelines for postoperative completely resected cavity stereotactic radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys 100:436–442
Ernst-Stecken A, Ganslandt O, Lambrecht U, Sauer R, Grabenbauer G (2006) Phase II trial of hypofractionated stereotactic radiotherapy for brain metastases: results and toxicity. Radiother Oncol 81:18–24
Suh CH, Kim HS, Jung SC, Choi CG, Kim SJ (2018) Comparison of MRI and PET as potential surrogate endpoints for treatment response after stereotactic radiosurgery in patients with brain metastasis. Am J Roentgenol 211:1332–1341
Hein PA, Eskey CJ, Dunn JF, Hug EB (2004) Diffusion-weighted imaging in the follow-up of treated high-grade gliomas: tumor recurrence versus radiation injury. AJNR Am J Neuroradiol 25:201–209
Xu JL, Li YL, Lian JM, Dou SW, Yan FS, Wu H et al (2010) Distinction between postoperative recurrent glioma and radiation injury using MR diffusion tensor imaging. Neuroradiology 52:1193–1199
Dequesada IM, Quisling RG, Yachnis A, Friedman WA (2008) Can standard magnetic resonance imaging reliably distinguish recurrent tumor from radiation necrosis after radiosurgery for brain metastases? A radiographic-pathological study. Neurosurgery 63:898
Kohutek ZA, Yamada Y, Chan TA, Brennan CW, Tabar V, Gutin PH et al (2015) Long-term risk of radionecrosis and imaging changes after stereotactic radiosurgery for brain metastases. J Neurooncol 125:149–156
Ahmed KA, Freilich JM, Abuodeh Y, Figura N, Patel N, Sarangkasiri S et al (2014) Fractionated stereotactic radiotherapy to the post-operative cavity for radioresistant and radiosensitive brain metastases. J Neurooncol 118:179–186
Dore M, Martin S, Delpon G, Clement K, Campion L, Thillays F (2017) Stereotactic radiotherapy following surgery for brain metastasis: predictive factors for local control and radionecrosis. Cancer Radiother 21:4–9
Bilger A, Bretzinger E, Fennell J, Nieder C, Lorenz H, Oehlke O et al (2018) Local control and possibility of tailored salvage after hypofractionated stereotactic radiotherapy of the cavity after brain metastases resection. Cancer Med 7:2350–2359
Scharl S, Kirstein A, Kessel KA, Diehl C, Oechsner M, Straube C et al (2019) Stereotactic irradiation of the resection cavity after surgical resection of brain metastases—when is the right timing? Acta Oncol 58:1714–1719
Specht HM, Kessel KA, Oechsner M, Meyer B, Zimmer C, Combs SE (2016) HFSRT of the resection cavity in patients with brain metastases. Strahlenther Onkol 192:368–376
Kumar AMS, Miller J, Hoffer SA, Mansur DB, Coffey M, Lo SS et al (2018) Postoperative hypofractionated stereotactic brain radiation (HSRT) for resected brain metastases: improved local control with higher BED10. J Neurooncol 139:449–454
Wang CC, Floyd SR, Chang CH, Warnke PC, Chio CC, Kasper EM et al (2012) Cyberknife hypofractionated stereotactic radiosurgery (HSRS) of resection cavity after excision of large cerebral metastasis: efficacy and safety of an 800 cGy x 3 daily fractions regimen. J Neurooncol 106:601–610
Inoue HK, Sato H, Suzuki Y, Saitoh J, Noda SE, Seto K et al (2014) Optimal hypofractionated conformal radiotherapy for large brain metastases in patients with high risk factors: a single-institutional prospective study. Radiat Oncol 9:231
Steinmann D, Maertens B, Janssen S, Werner M, Fruhauf J, Nakamura M et al (2012) Hypofractionated stereotactic radiotherapy (hfSRT) after tumour resection of a single brain metastasis: report of a single-centre individualized treatment approach. J Cancer Res Clin Oncol 138:1523–1529
Hanna GG, Murray L, Patel R, Jain S, Aitken KL, Franks KN et al (2018) UK consensus on normal tissue dose constraints for stereotactic radiotherapy. Clin Oncol (R Coll Radiol) 30:5–14
Bohoudi O, Bruynzeel AM, Lagerwaard FJ, Cuijpers JP, Slotman BJ, Palacios MA (2016) Isotoxic radiosurgery planning for brain metastases. Radiother Oncol 120:253–257
Akanda ZZ, Hong W, Nahavandi S, Haghighi N, Phillips C, Kok DL (2020) Post-operative stereotactic radiosurgery following excision of brain metastases: a systematic review and meta-analysis. Radiother Oncol 142:27–35
Redmond KJ, De Salles AA, Fariselli L, Levivier M, Ma L, Paddick I et al (2021) Stereotactic radiosurgery for post-operative metastatic surgical cavities: a critical review and International Society of Stereotactic Radiosurgery (ISRS) Practice Guidelines. Int J Radiat Oncol Biol Phys 111:68–80
Eaton BR, LaRiviere MJ, Kim S, Prabhu RS, Patel K, Kandula S et al (2015) Hypofractionated radiosurgery has a better safety profile than single fraction radiosurgery for large resected brain metastases. J Neurooncol 123:103–111
Abuodeh Y, Ahmed KA, Naghavi AO, Venkat PS, Sarangkasiri S, Johnstone PAS et al (2016) Postoperative stereotactic radiosurgery using 5-Gy x 5 sessions in the management of brain metastases. World Neurosurg 90:58–65
Minniti G, Paolini S, D’Andrea G, Lanzetta G, Cicone F, Confaloni V et al (2017) Outcomes of postoperative stereotactic radiosurgery to the resection cavity versus stereotactic radiosurgery alone for melanoma brain metastases. J Neurooncol 132:455–462
Minniti G, Scaringi C, Lanzetta G, Anzellini D, Bianciardi F, Tolu B et al (2019) Comparative effectiveness of multi-fraction stereotactic radiosurgery for surgically resected or intact large brain metastases from non-small-cell lung cancer (NSCLC). Lung Cancer 132:119–125
Shi S, Sandhu N, Jin MC, Wang E, Jaoude JA, Schofield K et al (2020) Stereotactic radiosurgery for resected brain metastases: single-institutional experience of over 500 cavities. Int J Radiat Oncol Biol Phys 106:764–771
Vogel J, Ojerholm E, Hollander A, Briola C, Mooij R, Bieda M et al (2015) Intracranial control after Cyberknife radiosurgery to the resection bed for large brain metastases. Radiat Oncol 10:221
Combs SE, Bilger A, Diehl C, Bretzinger E, Lorenz H, Oehlke O et al (2018) Multicenter analysis of stereotactic radiotherapy of the resection cavity in patients with brain metastases. Cancer Med 7:2319–2327
Eitz KA, Lo SS, Soliman H, Sahgal A, Theriault A, Pinkham MB et al (2020) Multi-institutional analysis of prognostic factors and outcomes after hypofractionated stereotactic radiotherapy to the resection cavity in patients with brain metastases. JAMA Oncol 6:1901
Minniti G, Esposito V, Clarke E, Scaringi C, Lanzetta G, Salvati M et al (2013) Multidose stereotactic radiosurgery (9 Gy × 3) of the postoperative resection cavity for treatment of large brain metastases. Int J Radiat Oncol Biol Phys 86:623–629
Minniti G, Scaringi C, Paolini S, Lanzetta G, Romano A, Cicone F et al (2016) Single-fraction versus multifraction (3 × 9 Gy) stereotactic radiosurgery for large (>2 cm) brain metastases: a comparative analysis of local control and risk of radiation-induced brain necrosis. Int J Radiat Oncol Biol Phys 95:1142–1148
Lima LC, Sharim J, Levin-Epstein R, Tenn S, Teles AR, Kaprealian T et al (2017) Hypofractionated stereotactic radiosurgery and radiotherapy to large resection cavity of metastatic brain tumors. World Neurosurg 97:571–579
Kirkpatrick JP, Wang Z, Sampson JH, McSherry F, Herndon JE 2nd, Allen KJ et al (2015) Defining the optimal planning target volume in image-guided stereotactic radiosurgery of brain metastases: results of a randomized trial. Int J Radiat Oncol Biol Phys 91:100–108
Blonigen BJ, Steinmetz RD, Levin L, Lamba MA, Warnick RE, Breneman JC (2010) Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 77:996–1001
Minniti G, D’Angelillo RM, Scaringi C, Trodella LE, Clarke E, Matteucci P et al (2014) Fractionated stereotactic radiosurgery for patients with brain metastases. J Neurooncol 117:295–301
Inoue HK, Sato H, Seto K, Torikai K, Suzuki Y, Saitoh J et al (2014) Five-fraction CyberKnife radiotherapy for large brain metastases in critical areas: impact on the surrounding brain volumes circumscribed with a single dose equivalent of 14 Gy (V14) to avoid radiation necrosis. J Radiat Res 55:334–342
Keller A, Dore M, Cebula H, Thillays F, Proust F, Darie I et al (2017) Hypofractionated stereotactic radiation therapy to the resection bed for intracranial metastases. Int J Radiat Oncol Biol Phys 99:1179–1189
Navarria P, Pessina F, Clerici E, Franceschini D, Gay LG, De Rose F et al (2019) Surgery followed by hypofractionated radiosurgery on the tumor bed in oligometastatic patients with large brain metastases. Results of a phase 2 study. Int J Radiat Oncol Biol Phys. 105:1095–1105
Garimall S, Shanker M, Johns E, Watkins T, Olson S, Huo M et al (2020) Evidence of dose-response following hypofractionated stereotactic radiotherapy to the cavity after surgery for brain metastases. J Neurooncol 146:357–362
Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC et al (2009) Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res 15:5379–5388
Faruqi S, Ruschin M, Soliman H, Myrehaug S, Zeng KL, Husain Z et al (2020) Adverse radiation effect after hypofractionated stereotactic radiosurgery in 5 daily fractions for surgical cavities and intact brain metastases. Int J Radiat Oncol Biol Phys 106:772–779
Atalar B, Modlin LA, Choi CY, Adler JR, Gibbs IC, Chang SD et al (2013) Risk of leptomeningeal disease in patients treated with stereotactic radiosurgery targeting the postoperative resection cavity for brain metastases. Int J Radiat Oncol Biol Phys 87:713–718
Ojerholm E, Lee JY, Thawani JP, Miller D, O’Rourke DM, Dorsey JF et al (2014) Stereotactic radiosurgery to the resection bed for intracranial metastases and risk of leptomeningeal carcinomatosis. J Neurosurg 121(Suppl):75–83
Franzoi MA, Hortobagyi GN (2019) Leptomeningeal carcinomatosis in patients with breast cancer. Crit Rev Oncol Hematol 135:85–94
Zhao Y, Castonguay M, Wilke D, Xu Z, Plourde M, Mulroy L et al (2019) Treatment outcomes and incidence of brain metastases in pulmonary large cell neuroendocrine carcinoma. Curr Probl Cancer 43:54–65
Ahn JH, Lee SH, Kim S, Joo J, Yoo H, Lee SH et al (2012) Risk for leptomeningeal seeding after resection for brain metastases: implication of tumor location with mode of resection. J Neurosurg 116:984–993
Prabhu RS, Patel KR, Press RH, Soltys SG, Brown PD, Mehta MP et al (2019) Preoperative Vs postoperative radiosurgery for resected brain metastases: a review. Neurosurgery 84:19–29
Do L, Pezner R, Radany E, Liu A, Staud C, Badie B (2009) Resection followed by stereotactic radiosurgery to resection cavity for intracranial metastases. Int J Radiat Oncol Biol Phys 73(2):486–491
Funding
This work was not supported by additional funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by SR, AS, BE, SG, NL, SA, MB, TL, LS and OR. The first draft of the manuscript was written by SR and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest or competing interests. SR has received Speakers’ Honoraria from Brainlab.
Ethical approval
EKNZ 2091-01705.
Consent to participate
Patients who declined consent for participation in clinical studies were not included.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rogers, S., Stauffer, A., Lomax, N. et al. Five fraction stereotactic radiotherapy after brain metastasectomy: a single-institution experience and literature review. J Neurooncol 155, 35–43 (2021). https://doi.org/10.1007/s11060-021-03840-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-021-03840-5