Abstract
The purpose of this study is to compare the safety and efficacy of single fraction radiosurgery (SFR) with hypofractionated radiosurgery (HR) for the adjuvant treatment of large, surgically resected brain metastases. Seventy-five patients with 76 resection cavities ≥ 3 cm received 15 Gray (Gy) × 1 SFR (n = 40) or 5–8 Gy × 3–5 HR (n = 36). Cumulative incidence of local failure (LF) and radiation necrosis (RN) was estimated accounting for death as a competing risk and compared with Gray’s test. The effect of multiple covariates was evaluated with the Fine-Gray proportional hazards model. The most common HR dose-fractionation schedules were 6 Gy × 5 (44 %), 7–8 Gy × 3 (36 %), and 6 Gy × 4 (8 %). The median follow-up was 11 months (range 2–71). HR patients had larger median resection cavity volumes (24.0 vs. 13.3 cc, p < 0.001), planning target volumes (PTV) (37.7 vs. 20.5 cc, p < 0.001), and cavity to PTV expansion margins (2 vs. 1.5 mm, p = 0.002) than SFR patients. Cumulative incidence of LF (95 % CI) at 6 and 12-months for HR versus SFR was 18.9 % (0.07–0.34) versus 15.9 % (0.06–0.29), and 25.6 % (0.12–0.42) versus 27.2 % (0.14–0.42), p = 0.80. Cumulative incidence of RN (95 % CI) at 6 and 12 months for HR vs. SFR was 3.3 % (0.00–0.15) versus 10.7 % (0.03–0.23), and 10.3 % (0.02–0.25) versus 19.2 % (0.08–0.34), p = 0.28. On multivariable analysis, SFR was significantly associated with an increased risk of RN, with a HR of 3.81 (95 % CI 1.04–13.93, p = 0.043). Hypofractionated radiosurgery may be the more favorable treatment approach for radiosurgery of cavities 3–4 cm in size and greater.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adjuvant radiation therapy is critical to providing local control of surgically resected brain metastases. The use of whole brain radiation therapy (WBRT) following surgical resection is considered standard of care, as two prospective randomized trials have demonstrated a significant reduction in local tumor recurrence from 46–59 % with observation to 10–28 % with WBRT [1, 2]. However, given the recognized neurocognitive sequelae of WBRT [3] and the lack of a survival benefit with its use [1, 2], tumor bed radiosurgery alone has been increasingly utilized to provide local control while sparing patients the toxicities of WBRT.
Multiple retrospective series reporting excellent local tumor control support the use of radiosurgery to the tumor bed [4, 5]. Most commonly, these series have employed single fraction radiosurgery (SFR) techniques with dosing based on tumor bed size in accordance with Radiation Therapy Oncology Group (RTOG) 90-05 [6]. RTOG 90-05 was limited to tumors ≤4 cm in diameter, and established the maximum tolerated dose of 15 Gy for lesions 3.1–4.0 cm in size. Even with this radiosurgery dose, the risk of unacceptable central nervous system toxicity for tumors >3 cm was found to be 16 times that of lesions ≤2 cm in size in this prospective study. Due to this report and others similarly demonstrating an increased risk of post-treatment edema with SFR for large intracranial lesions [6–9], hypofractionated radiation dose schedules have been suggested as an alternative to improve the toxicity profile.
Multiple institutions have reported favorable outcomes with the use of hypofractionated radiosurgery (HR) for cavities 3–4 cm in size and greater [10–14]. However, the safety and efficacy of HR has not previously been directly compared with SFR for resected brain metastases of this size. The purpose of this analysis is to compare local tumor control (LC) and the incidence and severity of radiation necrosis (RN), among patients treated with either SFR or HR for brain metastasis resection cavities ≥ 3 cm in diameter.
Methods and materials
Patient selection
The use of tumor bed radiosurgery alone was generally reserved for patients with favorable characteristics, including Karnofsky performance status (KPS) ≥70 %, recursive partitioning analysis (RPA) Class I or II [15], and one to three brain metastases, or for patients with progressive local disease following previous WBRT. Initially, SFR was used for the majority of all cavities up to 4 cm in diameter, and HR was reserved for patients who were not considered acceptable candidates for SFR due to cavity size > 4 cm, close proximity to critical structures, or history of previous radiation treatment that was considered to put the patient at increased risk of treatment toxicity. Beginning in July 2011 and then September 2012, respectively, patients were offered enrollment in one of two prospective institutional trials of either SFR (applicable to cavities up to 4 cm in size, NCT01395407) or HR (applicable to 3 cm in size and greater, NCT01705548).
For the purpose of this analysis, outcomes among patients treated either on or off trial with SFR for cavities 3–4 cm in diameter are retrospectively compared to outcomes for patients treated with HR for cavities 3 cm in size or greater. After institutional review board approval, patient records were searched to identify patients treated at Emory University with either SFR or HR (in 2–5 fractions) for a diagnosis of brain metastases between January 2007 and June 2014. Patients who were planned to receive combined WBRT and radiosurgery boost were not included, nor were patients with radiosensitive tumors such as small-cell cancer, lymphoma, or seminoma. Only patients treated following surgical resection with cavities at least 3 cm in the largest dimension and with at least 1 month of follow-up surveillance imaging were included for analysis.
Treatment technique
SFR and HR were delivered according to the radiosurgery techniques previously described [5, 10]. A high-resolution MRI with and without contrast was acquired for treatment planning immediately before CT simulation for each patient, unless there was a contraindication to undergoing MRI (n = 1). At the time of simulation, patients were immobilized with a stereotactic head frame (Brainlab, Feldkirchen, Germany) placed by the neurosurgical team, or with an Aquaplast mask (WFR/Aquaplast Corporation, Wyckoff, NJ) if a frameless technique was used. A high-resolution, thin-slice (0.625-mm slice thickness) CT scan without contrast was obtained for treatment planning on the day of radiosurgery for framed procedures and up to 4 days prior for frameless procedures. The treatment planning MRI was registered to the simulation CT using BrainScan or iPlan (Brainlab) or Velocity AI (Varian, Palo Alto, CA) software. Delineation of gross tumor volume (GTV) was made to include the entire resection cavity plus any remaining enhancing tumor.
For SFR, the planning target volume (PTV) included the GTV plus 0–2 mm margins, per physician judgment, to account for patient setup and target motion uncertainty. Since 2008, 2 mm margins were made standard to account for uncertainty in resection cavity delineation. The prescribed dose of 15 Gy was used for SFR according to RTOG protocol 90-05 [6].
For HR, the PTV included the GTV plus a 1–10 mm margin, per physician judgment. Most often, a GTV to PTV margin of 2 mm was used, though 10 mm was used in one case to account for additional uncertainty for a patient who could not undergo MRI. Radiation dose and fractionation were prescribed per the treating physician’s discretion prior to 2012, after which 6 Gy × 5 became the standard departmental hypofractionation schedule. HR patients uniformly were treated with a frameless radiosurgery technique.
For both SFR and HR, treatment was delivered with either intensity-modulated static fields (IMRS), volumetric modulated arc therapy (VMAT) or dynamic conformal arcs (DCA). IMRS or VMAT was prescribed so that at least 95 % of the PTV received at least 100 % of the prescribed dose, and DCA therapy was prescribed at the 80 % isodose line. All SFR and HR procedures were carried out with either the TrilogyTM or Novalis TxTM linear accelerators (Varian Medical Systems, Palo Alto, CA). For frameless single fraction or HR treatments, Cone beam CT (CBCT) scans with 6° of freedom registration allowed for precise positioning prior to treatment [16, 17].
For comparison of single and multiple dose regimens, radiation dose was converted to biological equivalent dose (BED) [18] and single fraction equivalent dose (SFED) [19] according to the formulas below, assuming an α/β ratio of 10 for tumor control, and where D is total dose, d is dose per fraction and Dq is 1.8 [19].
Follow-up and outcomes analysis
Patients were followed with history, physical examination, and MRI imaging (when feasible) with and without contrast, initially 4–6 weeks after radiation treatment completion, and then every 3 months thereafter. Local failure and distant intracranial failure were defined by MRI-detected intracranial tumor recurrence or progression. Local failure was defined as greater than 90 % of tumor recurrence within the prescription isodose volume, whereas recurrence outside of that volume was defined as distant intracranial failure. Local and distant intracranial failures were recorded irrespective of first site of failure. RN was diagnosed by increased enhancement on T1 post-contrast MRI with or without abnormal T2/FLAIR signal abnormality that did not rapidly progress on serial imaging, was associated with hypoperfusion on dynamic susceptibility contrast (DSC) enhanced MRI, or was confirmed by surgical resection and pathologic analysis. RN was graded according to the Common Terminology Criteria for Adverse Events version 3.0 [20]. For patients with uncertainty regarding the diagnosis of tumor progression or RN, cases were frequently reviewed at Adult Brain Tumor Conference meetings to arrive at a consensus diagnosis.
Statistical analysis
Patient’s characteristics were summarized and compared between those treated with SFR or HR by Wilcoxon rank-sum test for numerical covariates and chi-square test or Fisher’s exact test for categorical covariates, where appropriate. Covariates analyzed included age at treatment, gender, primary tumor histology, RPA class [15], gross-total resection (GTR) versus sub-total resection (STR), interval between surgery and RT, GTV volume, PTV volume, GTV-to-PTV margins, total radiation dose, SFED, BED10, and history of prior or salvage WBRT.
Survival functions were estimated by the Kaplan–Meier method [21] and a log-rank test is used to assess the difference in OS for patients treated with HR and SFR. OS was censored at last follow-up for living patients. The cumulative incidence functions of LF, RN and symptomatic RN were estimated using death without the event of interest as a competing risk. The difference in cumulative incidence rates between patients treated with HR and SFR was examined with the Gray’s test [22]. The Cox [23] or Fine-Gray [24] proportional hazards model was employed, as appropriate, to estimate the effect of HR vs. SFR and other covariates on the outcome variables. Multivariable survival analysis was carried out by entering all covariates of interest in a model and using a backward variable selection method with an alpha level of removal of 0.2. SFR vs. HR was forced in the model. All analyses are done using SAS 9.3 (SAS Institute, Inc., Cary, North Carolina) with a significant level of 0.05.
Results
Patient and tumor characteristics
Analysis included 75 patients with 76 resection cavities treated with 15 Gy × 1 fraction SFR (n = 40) or 5–8 Gy × 3–5 fractions HR (n = 36). Patient, tumor and treatment characteristics are listed in Table 1. HR patients were more likely to have a larger median resection cavity volume (24.0 vs. 13.3cc, p < 0.001), PTV volume (37.7 vs. 20.5cc, p < 0.001), and cavity to PTV expansion margins (2 vs. 1.5 mm, p = 0.002) than SFR patients. The median SFED and BED10 were significantly greater with HR as compared with SFR; 21.6 vs. 15 (p < 0.001) and 45.6 versus 37.5 (p = 0.006), respectively. The most common HR dose-fractionation schedules used were 6 Gy × 5 (44 %), 7–8 Gy × 3 (36 %), and 6 Gy × 4 (8 %).
Intracranial tumor control and patters of failure
Follow-up time was similar in both HR and SFR cohorts (p = 0.651), with a median (range) total follow-up of 11 months (2–71) for all patients, and a median MRI surveillance follow up time of 8 months (1–64). There was no difference in local control according to whether HR or SFR were used. Cumulative incidence of LF (95 % CI) at 6 and 12 months for HR vs. SFR was 18.9 % (0.07–0.34) versus 15.9 % (0.06–0.29), and 25.6 % (0.12–0.42) versus 27.2 % (0.14–0.42), p = 0.80 (Fig. 1). On univariable analysis, only increasing GTV volume was significantly associated with LF (p = 0.014), though there was also a trend for an association between increasing PTV volume (p = 0.084) and RPA class 3 patients (p = 0.079). After accounting for other prognostic variables on multivariable analysis, increasing GTV volume remained significantly associated with local failure (p = 0.024), as did the history of previous or salvage WBRT (p = 0.031). Additionally, there was a trend between higher RT dose and decreased LF (p = 0.078). All variables included in the multivariable analysis are listed in Table 2.
Local failure was a component of first failure in 11 (27.5 %) SFR patients and 8 (22.2 %) HR patients at a median time of 4.1 months (0.2–14.3) and 4.8 months (0.8–7.4), respectively. Salvage cranial RT was received by 16 (69.6 %) HR patients with intracranial progression, and included WBRT in 6 patients and SFR to distant sites in 10 patients. Among the SFR cohort, 15 (57.7 %) patients received salvage cranial RT, including WBRT in 9 patients and SFR to distant sites in 6 patients.
Radiation necrosis
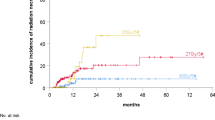
The cumulative incidence of RN (95 % CI) at 6 and 12 months for HR vs. SFR was 3.3 % (0.00–0.15) versus 10.7 % (0.03–0.23), and 10.3 % (0.02–0.25) versus 19.2 % (0.08–0.34), p = 0.28 (Fig. 2). There were no significant predictors of RN on univariable analysis. However, on multivariable analysis, SFR was associated with a significantly increased risk of RN, with a hazard ration of 3.81 (95 % CI 1.04–13.93, p = 0.043). Female gender (p = 0.030) and older age at treatment (p = 0.046) were also independent predictors of radiation necrosis (Table 2).
In total, 12 (30 %) SFR patients experienced RN at a median (range) time of 9.0 months (1.1–44.9) after treatment, compared with only 5 (14 %) HR patients at a median time of 7.4 months (5.2–40.9). Among the 5 HR patients with RN, 3 patients received 7 Gy × 3 fractions and 2 patients received 6 Gy × 5 fractions. The incidence and severity of RN experienced by patients treated with HR versus SFR is listed in Table 3. A higher proportion of patients treated with SFR experienced symptomatic (≥grade 2) or severe (grade 3–4) RN as compared to patients treated with HR (20 vs. 6 %, and 10 vs. 0 %, respectively). Both HR patients with grade 2 RN had received prior WBRT 15 and 11 months before HR. Among the SFR cohort, one patient with grade 1 RN had received salvage WBRT 8 months after SFR and 5.4 months prior to RN diagnosis. An additional 2 patients with grade 2 RN had received salvage WBRT 6–18 months after SFR and 2–7.6 months prior to RN diagnosis. Among the 4 patients with grade 3–4 RN in the SFR cohort, two required advanced medical management such as bevacizumab or hyperbaric oxygen plus pentoxifylline and vitamin E for severe symptoms (grade 3), one required hospitalization and high-dose dexamethasone (grade 3), and one required intensive care unit admission and intubation (grade 4). None of the patients with grade 3–4 RN had received prior or salvage WBRT. In comparison, no HR patients experienced RN greater than grade 2.
The cumulative incidence of symptomatic RN at 6 and 12 months for HR vs. SFR was 3.3 % (0.00–0.14) versus 10.7 % (0.03–0.23) and 6.8 % (0.01–0.02) versus 16.4 % (0.06–0.30), p = 0.116 (Fig. 2). On univariable analysis, there were no significant predictors of symptomatic RN, though there was a trend for an increased risk with SFR (p = 0.106). On multivariable analysis, only female gender was significantly associated with an increased risk of symptomatic RN (p = 0.041), though the trend with SFR persisted (p = 0.084).
Overall survival
Median OS time in all patients was 11.2 months (95 % CI 8.9–7.4). There was no significant difference in OS among the two cohorts, with 6 and 12 month KM estimates (95 % CI) of OS for HR versus SFR of 71.6 % (0.52–0.84) versus 66.7 % (0.49–0.79), and 54.9 % (0.36–0.70) versus 41.1 % (0.26–0.56), p = 0.308.
Discussion
The results of this study demonstrate that the use of SFR for resected brain metastases 3–4 cm in size is associated with an increased risk of radiation necrosis compared with the use of HR, delivered in 3–5 fractions, for lesions ≥3 cm. No difference in local control among patients treated with either modality was found. HR appears may be the more favorable treatment modality with a reduced toxicity profile, despite the fact that patients in the HR group tended to have larger GTV and PTV volumes, which could be expected to predispose the HR cohort to an increased risk of local failure and treatment toxicity [9, 10, 25].
The present study adds to the current literature by specifically reporting both radiographic and symptomatic radiation necrosis rates among patients treated with SFR and HR for cavities ≥3 cm in diameter, as this data is currently limited. Previously reported series of tumor bed radiosurgery have most commonly included smaller target volumes and have not reported toxicity according to tumor size. One recently published series from Brennan et al., which did separately analyzed outcomes with SFR for cavities >3 cm (n = 23), reported a 13 % incidence of pathologically proven radiation necrosis, though total radiation necrosis rates was not reported or graded 24. In the only other study, to our knowledge, to have previously compared the use of SFR and HR among resection cavities, Broemme et al. evaluated 41 cavities treated with either technique and reported only one case of histologically proven RN in the SFR cohort [26]. The target volumes included in this study were of smaller median size than the present analysis (median PTVs for SFR and HR of 17cc and 21 cc versus 21 cc and 37 cc in the current study), and rates of radiographic and clinically significant symptomatic RN were again not reported [26]. The 30 % total incidence, of radiographic (10 %) and symptomatic (20 %) RN among the SFR cohort in this analysis is an important finding that has not previously been reported in the literature for resection cavities of this size.
It is important to note that patients were included in this analysis regardless of whether they received radiosurgery only or radiosurgery plus prior or salvage WBRT. The authors felt it was important to include patients who received WBRT as this is a common scenario with the use of radiosurgery in clinical practice. However, the increased risk of radiation necrosis in the SFR cohort compared to the HR cohort cannot be explained by the addition of WBRT. A greater proportion of HR patients received prior or salvage WBRT than did the SFR patients (31 vs. 18 %). Furthermore, the addition of prior or salvage of WBRT was not found to be associated with an increased risk of radiation necrosis on either univariable or multivariable analysis.
The local control among the SFR and HR cohorts in this series is comparable with the published literature. The cumulative incidence of local failure at 1 year was 26 and 27 % for HR and SFR patients, respectively, in this series. Brennan et al. reported a similar LF rate of 31 % at 12 months [27] with the use of SFR among cavities >3 cm, and an even higher LF rate of 54 % was found when looking specifically at superficial cavities >3 cm [27]. While local failure rates with the use of SFR for smaller size cavities treated to higher doses have ranged from 0–26 % at 1 year [4, 5], our results are not surprising given the known poor prognostic factor of increasing tumor size [28]. Similarly, past HR series have reported 1 year LF ranging from 7–29 % [11, 13, 29, 30]. These results are also comparable to the 10–28 % local failure rates observed after WBRT [1, 2], and continue to support the use of focal radiation therapy for these large lesions. In this study, increasing GTV size was also a significant independent predictor for increases local control, which highlights the need for optimization of local control with large intracranial lesions. Given the increased toxicity of SFR for large resection cavities demonstrated here, there seems more opportunity to improve local control without excessive toxicity by dose escalating the HR treatment. Indeed, a trend between increased RT dose and reduced incidence of local failure was demonstrated on multivariable analysis in this report. A phase I dose escalation study is currently underway at Emory University (NCT01705548), using the most common dose-fractionation scheme in this study, 6 Gy × 5, as the entry dose level.
This study is limited by the retrospective study design and unknown bias in treatment selection. However, considering HR was more often used in patients with larger resection cavities and among more patients who had previously received radiation therapy to the treatment site, one may expect bias to favor better outcomes in SFR group rather than the HR group. Additional limitations exist regarding the diagnosis of radiation necrosis by imaging characteristics alone, attributing symptoms to radiation necrosis in patients with existing intracranial pathology, and the limited follow-up time of 11 months, as radiation necrosis may occur even later. Further, the heterogeneity of dose-fractionation schedules used within the HR cohort may limit the ability to draw conclusions about the safety and efficacy of a particular fractionation schedule. However, an important finding was the high proportion of patients with radiation necrosis who experienced severe grade 3–4 symptoms in the SFR cohort (4 of 12, 33 %), while no patients in the HR group experienced more than mild symptoms requiring short-term medical management. These results should be interpreted with caution given the small number of events in each cohort and the limited patient sample size.
In conclusion, hypofractionated radiosurgery appears to be better tolerated that single fraction radiosurgery for resected large brain metastases ≥3 cm in size. Hypofractionated radiosurgery is associated with a reduced risk of symptomatic radiation necrosis while providing equivalent tumor control. The investigators therefore recommend the use of HR for resection cavities of this size to minimize toxicity risk with local therapy. Dose optimization of HR is warranted in a prospective fashion, given the variable dose and fractionation schedules used in this series and in others.
References
Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, Markesbery WR, Macdonald JS, Young B (1990) A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 322(8):494–500. doi:10.1056/NEJM199002223220802
Kocher M, Soffietti R, Abacioglu U, Villa S, Fauchon F, Baumert BG, Fariselli L, Tzuk-Shina T, Kortmann RD, Carrie C, Ben Hassel M, Kouri M, Valeinis E, Van Den Berge D, Collette S, Collette L, Mueller RP (2011) Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the eortc 22952-26001 study. J Clin Oncol 29 (2):134–141. doi:10.1200/JCO.2010.30.1655
Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, Arbuckle RB, Swint JM, Shiu AS, Maor MH, Meyers CA (2009) Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 10(11):1037–1044. doi:10.1016/S1470-2045(09)70263-3
Roberge D, Parney I, Brown PD (2012) Radiosurgery to the postoperative surgical cavity: who needs evidence? Int J Radiat Oncol Biol Phys 83(2):486–493. doi:10.1016/J.IJROBP.2011.09.032
Prabhu R, Shu HK, Hadjipanayis C, Dhabaan A, Hall W, Raore B, Olson J, Curran W, Oyesiku N, Crocker I (2012) Current dosing paradigm for stereotactic radiosurgery alone after surgical resection of brain metastases needs to be optimized for improved local control. Int J Radiat Oncol Biol Phys 83(1):E61–66. doi:10.1016/J.IJROBP.2011.12.017
Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, Farnan N (2000) Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of rtog protocol 90-05. Int J Radiat Oncol Biol Phys 47(2):291–298
Linskey ME, Andrews DW, Asher AL, Burri SH, Kondziolka D, Robinson PD, Ammirati M, Cobbs CS, Gaspar LE, Loeffler JS, McDermott M, Mehta MP, Mikkelsen T, Olson JJ, Paleologos NA, Patchell RA, Ryken TC, Kalkanis SN (2010) The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neuro-oncol 96(1):45–68. doi:10.1007/S11060-009-0073-4
Adler JR, Cox RS, Kaplan I, Martin DP (1992) Stereotactic radiosurgical treatment of brain metastases. J Neurosurg 76(3):444–449. doi:10.3171/JNS.1992.76.3.0444
Alexander E 3rd, Moriarty TM, Davis RB, Wen PY, Fine HA, Black PM, Kooy HM, Loeffler JS (1995) Stereotactic radiosurgery for the definitive, noninvasive treatment of brain metastases. J Natl Cancer Inst 87 (1):34–40
Eaton BR, Gebhardt B, Prabhu R, Shu HK, Curran WJ, Jr., Crocker I (2013) Hypofractionated radiosurgery for intact or resected brain metastases: defining the optimal dose and fractionation. Radiat Oncol 8:135. doi:10.1186/1748-717X-8-135
Wang CC, Floyd SR, Chang CH, Warnke PC, Chio CC, Kasper EM, Mahadevan A, Wong ET, Chen CC (2012) Cyberknife hypofractionated stereotactic radiosurgery (hsrs) of resection cavity after excision of large cerebral metastasis: efficacy and safety of an 800 cgy × 3 daily fractions regimen. J Neuro-oncol 106(3):601–610. doi:10.1007/S11060-011-0697-Z
Soltys SG, Adler JR, Lipani JD, Jackson PS, Choi CY, Puataweepong P, White S, Gibbs IC, Chang SD (2008) Stereotactic radiosurgery of the postoperative resection cavity for brain metastases. Int J Radiat Oncol Biol Phys 70(1):187–193. doi:10.1016/J.IJROBP.2007.06.068
Minniti G, Esposito V, Clarke E, Scaringi C, Lanzetta G, Salvati M, Raco A, Bozzao A, MAURIZI ENRICI R (2013) Multidose stereotactic radiosurgery (9 gy × 3) of the postoperative resection cavity for treatment of large brain metastases. Int J Radiat Oncol Biol Phys 86(4):623–629. doi:10.1016/J.IJROBP.2013.03.037
Choi CY, Chang SD, Gibbs IC, Adler JR, Harsh GRT, Atalar B, Lieberson RE, Soltys SG (2012) What is the optimal treatment of large brain metastases? An argument for a multidisciplinary approach. Int J Radiat Oncol Biol Phys 84(3):688–693. doi:10.1016/J.IJROBP.2012.01.028
Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, McKenna WG, Byhardt R (1997) Recursive partitioning analysis (Rpa) of prognostic factors in three radiation therapy oncology group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 37(4):745–751
Dhabaan A, Schreibmann E, Siddiqi A, Elder E, Fox T, Ogunleye T, Esiashvili N, Curran W, Crocker I, Shu HK (2012) Six degrees of freedom cbct-based positioning for intracranial targets treated with frameless stereotactic radiosurgery. J Appl Clin Med Phys 13(6):3916. doi:10.1120/JACMP.V13I6.3916
Prabhu RS, Dhabaan A, Hall WA, Ogunleye T, Crocker I, Curran WJ, Shu HK (2013) Clinical outcomes for a novel 6 degrees of freedom image guided localization method for frameless radiosurgery for intracranial brain metastases. J Neurooncol 113(1):93–99. doi:10.1007/S11060-013-1093-7
Hall EJ, Giaccia AJ (2006) Radiobiology for the radiologist, 6th edn. Lippincott Williams & Wilkins, Philadelphia
Park C, Papiez L, Zhang S, Story M, Timmerman RD (2008) Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol Biol Phys 70(3):847–852. doi:10.1016/J.IJROBP.2007.10.059
Cancer Therapy Evaluation Program. Common terminology criteria for adverse events, version 3.0. DCTD, NCI, NIH, DHHS
Kalbfleisch JD, Prentice RL (1980) The statistical analysis of failure time data. wiley series in probability and mathematical statistics, Wiley, New York
Gray R (1988) A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 16:1141–1154
Cox DR (1972) Regression models and life tables. J R Stat Soc B34:187–220
Fine JP, Gray RJ (1999) A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496–509
Kwon AK, Dibiase SJ, Wang B, Hughes SL, Milcarek B, Zhu Y (2009) Hypofractionated stereotactic radiotherapy for the treatment of brain metastases. Cancer 115(4):890–898. doi:10.1002/CNCR.24082
Broemme J, Abu-Isa J, Kottke R, Beck J, Wiest R, Malthaner M, Schmidhalter D, Raabe A, Aebersold DM, Pica A (2013) Adjuvant therapy after resection of brain metastases. Frameless image-guided linac-based radiosurgery and stereotactic hypofractionated radiotherapy. Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft [et al] 189 (9):765–770. doi:10.1007/S00066-013-0409-Z.
Brennan C, Yang TJ, Hilden P, Zhang Z, Chan K, Yamada Y, Chan TA, Lymberis SC, Narayana A, Tabar V, Gutin PH, Ballangrud A, Lis E, Beal K (2014) A phase 2 trial of stereotactic radiosurgery boost after surgical resection for brain metastases. Int J Radiat Oncol Biol Phys 88(1):130–136. doi:10.1016/J.IJROBP.2013.09.051
Hartford AC, Paravati AJ, Spire WJ, Li Z, Jarvis LA, Fadul CE, Rhodes CH, Erkmen K, Friedman J, Gladstone DJ, Hug EB, Roberts DW, Simmons NE (2013) Postoperative stereotactic radiosurgery without whole-brain radiation therapy for brain metastases: potential role of preoperative tumor size. Int J Radiat Oncol Biol Phys 85(3):650–655. doi:10.1016/J.IJROBP.2012.05.027
Ahmed KA, Freilich JM, Abuodeh Y, Figura N, Patel N, Sarangkasiri S, Chinnaiyan P, Yu HH, Etame AB, Rao NG (2014) Fractionated stereotactic radiotherapy to the post-operative cavity for radioresistant and radiosensitive brain metastases. J Neuro-oncol 118(1):179–186. doi:10.1007/S11060-014-1417-2
Steinmann D, Maertens B, Janssen S, Werner M, Fruhauf J, Nakamura M, Christiansen H, Bremer M (2012) Hypofractionated stereotactic radiotherapy (hfsrt) after tumour resection of a single brain metastasis: report of a single-centre individualized treatment approach. J Cancer Res Clin Oncol 138(9):1523–1529. doi:10.1007/S00432-012-1227-X
Conflict of interest
Ian Crocker serves as a consultant for Varian Medical Systems (Varian, Palo Alto, CA).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eaton, B.R., La Riviere, M.J., Kim, S. et al. Hypofractionated radiosurgery has a better safety profile than single fraction radiosurgery for large resected brain metastases. J Neurooncol 123, 103–111 (2015). https://doi.org/10.1007/s11060-015-1767-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-015-1767-4