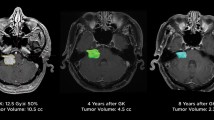
Abstract
The treatment strategy for patients with vestibular schwannoma (VS) is controversial, and data concerning the long-term hearing outcomes > 5 years after gamma knife surgery (GKS) are limited. The long-term hearing outcomes after GKS were evaluated in VS patients with hearing preservation. Ninety-two VS patients with a pure tone average (PTA) ≤ 50 dB were evaluated. The median age was 54 years; the median tumor volume was 1.5 cm3. The tumors were treated with a median margin dose of 12 Gy and a median mean cochlear dose of 4.0 Gy. At the time of GKS, 65 patients retained a PTA of 0–30 dB, and 27 had a PTA of 31–50 dB. The median follow-up period was 106 months. At the final follow-up, 2 (2%) developed tumor progression. During the median audiogram follow-up of 83 months, the PTA was ≤ 30 dB in 22 patients (24%) and 31–50 dB in 27 patients (29%); 43 patients (47%) worsened to a PTA > 50 dB. Hearing preservation rates were 66, 57, and 44% at 3, 5, and 10 years, respectively. In multivariate analysis, the mean cochlear dose (P < 0.001) and pre-GKS PTA (P = 0.045) were significant for hearing preservation. GKS was an effective treatment option for VS patients with a PTA ≤ 50 dB. As a lower cochlear dose and better pre-GKS PTA contributed to long-term hearing preservation, prophylactic GKS before hearing deterioration or tumor growth would be a treatment of choice if patients provided informed consent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gamma knife surgery (GKS) has been proven to be safe and effective for patients with small- to medium-sized vestibular schwannomas (VSs) [1,2,3,4,5,6,7,8,9]. Although good tumor control and a low risk of facial palsy after GKS are important advantages, it remains difficult to preserve hearing function. As VSs are histologically benign, the treatment goal must be retention of a better quality of life with tumor control while preserving neurological function as long as possible. When an optimum dose of 12–13 Gy is applied to the tumor margin, the risk of permanent facial palsy after GKS is approximately zero [4]. Additionally, GKS is quite safe even for older patients or those with medical comorbidities. However, the best strategy to preserve hearing function in VS patients who retained hearing remains controversial; options include the “wait-and-see” approach, surgical resection, or stereotactic radiosurgery (SRS)/radiotherapy. At present, data concerning detailed long-term hearing function > 5 years after GKS are scarce. The purpose of the present study was to evaluate the long-term hearing preservation rate and the factors associated with hearing preservation in patients treated with GKS, focusing on patients who retained a PTA ≤ 50 dB at the time of treatment. Hearing preservation used in this study was defined as a PTA ≤ 50 dB.
Methods and materials
Patient characteristics
The data on patients with VS who were treated with GKS at our institute were obtained from a single institutional review board-approved database. The inclusion criteria of this study were as follows: (1) unilateral VS (excluding neurofibromatosis type 2), (2) patients who had retained hearing function, defined as a pure tone average (PTA) ≤ 50 dB on the tumor side as measured by a pre-GKS audiogram, and (3) patients who had a pre-GKS audiogram and hearing follow-up period of 3 years or longer after GKS. Between May 1991 and December 2013, 872 VS patients were treated with GKS at our institute. Among them, 311 patients (36%) had a PTA ≤ 50 dB at the time of treatment. Of those, 219 were excluded due to the unavailability of a serial audiogram or to neurofibromatosis type 2; most patients followed by referring doctors were excluded because of the unavailability of pure tone audiometry after GKS, regardless of hearing preservation. Accordingly, limited patients followed at our institution were evaluated. A serial audiogram continued to be checked to avoid patient selection bias, even if the patients lost serviceable hearing within 3 years after GKS. Finally, 92 patients were eligible for evaluation of long-term hearing outcomes and were retrospectively analyzed. All patients provided informed consent. Patient characteristics are summarized in Table 1. All patients received their treatment of choice, including the wait-and-see approach, surgical resection, or radiosurgery. Of the 92 patients, GKS was selected on the basis of patient preference in 66 patients; Koos grade 4 tumors with a PTA ≤ 50 dB in 10; tumor growth in 9; and neurological deterioration, such as deterioration of hearing, facial sensation, or gait disturbance during observation in 7. The patients who required intervention were those who had refused or were unsuitable for craniotomy because of advanced age or comorbidity. On the pre-GKS audiogram, 65 patients (71%) were found to have a PTA of ≤ 30 dB on the tumor side, and 27 patients (29%) were found to have a PTA of 31–50 dB. The mean PTAs on the tumor and normal sides were 26.0 and 14.2 dB, respectively. One patient (1%) had House and Brackmann grade 2 facial palsy and 4 (4%) had trigeminal nerve dysfunction.
Radiosurgical techniques
Detailed radiosurgical treatment data are shown in Table 2. The median tumor volume was 1.5 cm3. The median maximum dose, marginal dose, and D95 (irradiation dose that included 95% of the planning target volume) were 24, 12, and 11 Gy, respectively.
Dose planning
When dose-planning for GKS, the KULA system (Elekta, Stockholm, Sweden) was used until August 1996. Thereafter, GammaPlan software (Elekta) was used. On the basis of the dose-planning methods, the patients were classified into early or recent treatment method groups. In 47 patients (51%) treated with a recent treatment method that matched our current treatment method, dose-planning was conducted with 4-mm collimators applied to the intracanalicular portion based on thin-slice, axial, three-dimensional spoiled gradient echo recalled images and heavy T2-weighted images with gadolinium enhancement. The other 45 patients were classified into the early treatment method group, including 14 patients treated with the KULA system.
Follow-up evaluation
Clinical follow-up data were obtained when the patients visited our hospital, or from the referring doctors if the patients lived a considerable distance from our institution. Magnetic resonance (MR) imaging scans and audiograms were requested at 3-month intervals for the first year after GKS, at 6-month intervals for the second and third years, and then annually thereafter. On the basis of the radiological follow-up studies with thin-sliced T1-weighted axial images with gadolinium enhancement, tumor regression was defined as a volume reduction of ≥ 25%, no change was defined as a volume reduction or increase of < 25%, and tumor progression was defined as a volume increase of ≥ 25%. In addition, transient expansion was defined as the occurrence of any enlargement before tumor shrinkage. Hearing function was evaluated based on the PTA as measured by serial audiograms before and after GKS. The PTA was calculated with the following formula: PTA = (a + 2b + c)/4, where “a” had a threshold at 500 Hz, “b” had a threshold at 1000 Hz, and “c” had a threshold at 2000 Hz. To evaluate hearing deterioration due to GKS, the PTA on the normal side was used as a control. In addition, the difference in the PTA before and after GKS was calculated for each patient. Hearing preservation was defined as retention of a PTA of ≤ 50 dB. In all patients, serial audiograms according to the follow-up protocol were obtained until the patients lost hearing function.
Statistical analysis
Tumor control and hearing preservation rates were calculated using Kaplan and Meier methods. To analyze the factors that were correlated with hearing preservation, the following were assessed: age, sex, tumor nature, dose-planning group, pre-GKS PTA, difference between bilateral pre-GKS PTAs, Koos grade [10], transient expansion, tumor volume, treatment dose (maximum, marginal, and D95), mean cochlear dose, use of a 4-mm collimator in the intracanalicular portion, fundus obliteration, and distance from the meatal fundus to the tumor end. As the mean cochlear dose and D95 were calculated using GammaPlan software, these data were not available in 14 patients treated with the KULA system. The factors affecting hearing preservation were assessed with a log-rank test in a univariate analysis and a Cox proportional regression model in a multivariate analysis in which the continuous variables were age, dose, distance, tumor volume, and pre-GKS PTA. A final multivariate analysis was calculated with a stepwise forward selection method. Hazard ratios were reported with 95% confidence intervals (CIs). The statistical analyses were performed with SPSS Statistics for Windows, Version 21.0 (IBM Corporation, Armonk, NY, USA). P values < 0.05 were considered significant.
Results
Tumor control
The median radiological follow-up period was 106 months (range 36–262 months). At the final follow-up, 71 patients (77%) had tumor regression, 19 (21%) had stable tumors, and 2 (2%) had experienced tumor progression. Of those two patients, one required a craniotomy 41 months after GKS, followed by a second GKS for further progression. The other was observed without any additional treatment due to lack of symptomatic deterioration. The overall tumor control rate with the Kaplan and Meier method was 98%. During the follow-up period, transient expansion was found in 20 patients (22%). The median intervals to transient expansion was 6 months (range 3–60 months).
Hearing results
The median follow-up period for the audiogram was 83 months (range 36–262 months) and the median number of audiometric evaluation was 10 (range 2–27). At the final follow-up audiogram, 22 patients (24%) retained a PTA of ≤ 30 dB, 27 (29%) retained a PTA of 31–50 dB, and 43 (47%) lost hearing with a PTA of > 50 dB. Detailed hearing outcomes depending on pre-GKS PTA are shown in Table 3. Time courses of the mean PTA on the tumor and contralateral sides are demonstrated in Fig. 1a; time courses of the mean differences between the pre- and post-GKS PTA are shown in Fig. 1b. Compared with the pre-GKS PTAs, the post-GKS PTAs showed mean declines of 15.7 and 26.6 dB at 5 and 10 years, respectively, on the tumor side and 1.2 and 2.2 dB at 5 and 10 years, respectively, on the contralateral side. The actuarial 3-, 5-, 8- and 10-year hearing preservation rates were 66, 57, 47, and 44%, respectively (Fig. 2a). Depending on the pre-GKS PTA, the actuarial 3-, 5-, and 10-year hearing preservation rates in 65 patients with a pre-GKS PTA of ≤ 30 dB were 73, 67, and 55%, respectively, whereas those of 27 patients with a pre-GKS PTA of 31–50 dB were 48, 35, and 23%, respectively (log rank test: P = 0.004; Fig. 2b).
Graphs showing time courses for mean pure tone averages on the tumor side and the normal side, and the difference between mean pure tone averages on the tumor and the normal side (a), and time courses for the differences between pre- and post-GKS pure tone averages on the tumor side and the normal side (b). Error bars represent standard error of the mean
Factors associated with hearing preservation
Factors affecting hearing preservation are shown in Table 3. In univariate analysis, the pre-GKS PTA (P < 0.001), difference between bilateral pre-GKS PTA (P < 0.001), mean cochlear dose (P < 0.001), fundus obliteration (P = 0.011), use of a 4-mm collimator to the intracanalicular portion (P = 0.032), and distance from the fundus and the tumor end (P = 0.038) were significant for hearing preservation. By multivariate analysis, the mean cochlear dose and pre-GKS PTA were significantly correlated with hearing preservation, with hazard ratios of 1.366 (95% CI 1.181–1.580; P < 0.001) and 1.036 (95% CI 1.001–1.072; P = 0.045), respectively. In a limited number of 41 patients with a pre-GKS PTA ≤ 30 dB who were treated with a mean cochlear dose of ≤ 5 Gy, the 3-, 5-, and 10-year hearing preservation rates increased to 88, 76, and 69%, respectively.
Other neurological function
During the follow-up period, no newly developed neurological deterioration other than hearing deterioration was found. At the last follow-up, pre-GKS mild facial palsy was resolved in one patient, and trigeminal dysfunction was improved in three patients and stable in one.
Discussion
Currently, the best treatment strategy for VS patients with serviceable hearing remains controversial. Due to the tumors being histologically benign, true long-term results of both tumor control and hearing preservation are essential for decision-making strategies.
Long-term hearing preservation
Many investigators have reported relatively long-term hearing outcomes after SRS [4, 9, 11,12,13], but these results are still unsatisfactory, showing a serviceable hearing preservation rate of < 50% over longer periods. In this study, long-term hearing preservation rates were 57 and 44% at 5 and 10 years, respectively. This result seemed consistent with other radiosurgical reports. One possibility for the retention of hearing function in VS patients is dose reduction. To date, the tumor margin dose has been reduced to an optimum dose of 12 Gy to avoid facial palsy. Although further dose reduction may improve hearing results, it could cause an increased rate of tumor progression. Watanabe et al. [9] reported the long-term hearing results of 183 VS patients with a median audiometric follow-up period of 59 months. The tumors were treated with a median marginal dose of 12 Gy, but the anterior tumor portion facing the cochlear and facial nerves was covered with a 10 Gy isodose gradient. Consequently, despite a dose reduction to the cochlear nerve, serviceable hearing could not be preserved; the hearing preservation rates were 49 and 24% at 5 and 10 years, respectively.
Another possibility for the reduction of damage to the normal tissues is fractionation. There are several papers on the use of fractionated stereotactic radiotherapy for the treatment of VS [14,15,16,17,18,19,20,21,22]. Champ et al. [16] reported the results for 154 VS patients treated with fractionated stereotactic radiotherapy using a reduced dose of 46.8 Gy in 1.8 Gy fractions. The mean tumor volume was 2.4 cm3. At a median follow-up period of 35 months, the actuarial 3- and 5-year serviceable hearing preservation rates were 66 and 54%, respectively, with a median PTA decline of 13 dB. This result was comparable to our results. Currently, there is no evidence showing SRT is superior to SRS in hearing preservation.
Factors affecting hearing preservation
To date, various factors such as patient age, tumor volume, pre-radiosurgical PTA and cochlear dose have been reported as significant factors associated with hearing preservation after radiosurgery [9, 11, 13, 23]. In our study, the mean cochlear dose and pre-GKS PTA were significant for hearing preservation. Considering mean PTA declines of 15.7 and 26.6 dB at 5 and 10 years compared to the pre-GKS PTA, it was not surprising that pre-GS PTA was a prognostic factor for hearing preservation. The mean cochlear dose has been considered to be a common prognostic factor in several articles [23,24,25]. To minimize the risk of hearing loss, dose-planning should include a cochlear dose that is as low as possible. If possible, it is recommended that the mean cochlear dose be reduced to < 4–6 Gy. The tumor extension to the fundus and dose escalation of the anterolateral portion of the tumor would lead to an increased cochlear dose, resulting in a decline of hearing preservation rates. The cause of continuous hearing deterioration beyond 3 years after GKS is unknown. Hearing deterioration within 3 years after GKS could be explained by ischemic or mechanical damage to the cochlear nerve due to high-dose irradiation or nerve compression by transient expansion. However, as has been demonstrated in many articles [4, 6, 9, 11,12,13], hearing acuity continues to deteriorate even beyond 5 years; hearing preservation rates after radiosurgery were 42–50% at 5 years and decreased to 23–24% at 10 years. At present, it is not evident whether long-term continuous deterioration was caused by direct radiation injury of the cochlear nerve, vascular insufficiency due to the cochlear nerve scarring, long-term cochlear nerve compression by a tumor, or cochlear nerve distortion due to tumor shrinkage. From the results of GKS in patients with facial nerve schwannomas [26], most retained serviceable hearing despite treatment with higher mean cochlear doses, indicating that hearing loss does not seem to be related to direct damage of the cochlea by high-dose irradiation.
Comparison of hearing outcomes with other treatments
Recently, the long-term follow-up data of 156 intracanalicular VS patients who were managed conservatively were reported in the Danish national database [27]. After a mean follow-up period of 9.5 years, 37% of patients developed tumor progression, defined as a ≥ 2 mm increase in any tumor diameter. During the follow-up period, 15% of patients underwent surgical resection or radiation therapy after a mean follow-up period of 4.2 years. Of 73 evaluated patients with serviceable hearing, 25 retained serviceable hearing at their final audiogram, resulting in a crude serviceable hearing preservation rate of 34%. This result indicates that even if VS patients were observed without any intervention they had a high risk of loss of serviceable hearing, regardless of tumor growth. The hearing function after GKS shown in this study indicated a relatively rapid deterioration in some patients instead of providing tumor control, but the 5- and 10-year hearing preservation rates seemed consistent with those managed conservatively. According to recent surgical series [28,29,30,31,32,33,34], the immediate postoperative hearing results for VS patients with serviceable hearing are improving. However, the durability of hearing function after microsurgery is of great interest. Wang et al. [34] reported in the results of their microsurgical treatment with the middle fossa approach that immediate postoperative serviceable hearing was achieved in 78 of 95 patients (82%) and that 27 of 32 patients with immediate postoperative serviceable hearing (84%) maintained serviceable hearing at 5 years after surgery, meaning the serviceable hearing preservation rate at 5 years was approximately 69%. This seems to be a better hearing outcome than that of radiosurgery, but the higher risk of facial palsy should be considered, as patients showed an immediate postoperative facial palsy of 31% and a persistent House and Brackmann grade III or worse palsy of 9% postoperatively. Although the ambiguity of such microsurgical hearing results dependent on the surgeons’ experience is difficult to comprehend for decision-making purposes, the results of the durability of hearing preservation after microsurgery would encourage patients with serviceable hearing.
Study limitations
This study was a retrospective study at a single institution. Approximately 70% of patients were followed by referring doctors and discontinued serial audiograms within 3 years after GKS regardless of hearing preservation; those patients were excluded from this study. Consequently, limited patients followed at our institution were included. Although it might be possible that some patients discontinued serial audiograms within 3 years because of hearing loss after GKS, this was our limitation in this study. In addition, some biases due to patient selection or radiosurgical techniques at a single institution may have impacted our results. The inclusion of patients treated using older radiosurgical techniques such as the KULA system, an 8-mm collimator applied to the intracanalicular tumor, or higher prescription doses may have led to lower hearing preservation rates. The major weakness in this study was that speech discrimination was not evaluated because long-term follow-up data of speech discrimination were not available in most patients. It has been known that the speech discrimination tends to be worse in patients with retrocochlear lesions such as VSs than in patients with cochlear lesions with similar hearing thresholds. Van Dijk et al. [35] demonstrated that VS patients who retain better hearing function of a PTA ≤ 50 dB rarely have the maximum speech discrimination score < 50%, while those with a PTA > 50 dB are more likely to lose the speech discrimination. However, hearing preservation rates in this study may not have been strictly equivalent to serviceable hearing preservation rates.
Conclusions
GKS was an effective treatment option for VS patients with hearing preservation of a PTA ≤ 50 dB. As a lower cochlear dose and better pre-GKS PTA contributed to long-term hearing preservation, prophylactic GKS before hearing deterioration or tumor growth would be a treatment of choice if patients provided informed consent.
References
Hasegawa T, Kida Y, Kobayashi T, Yoshimoto M, Mori Y, Yoshida J (2005) Long-term outcomes in patients with vestibular schwannomas treated using gamma knife surgery: 10-year follow up. J Neurosurg 102:10–16
Hasegawa T, Fujitani S, Katsumata S, Kida Y, Yoshimoto M, Koike J (2005) Stereotactic radiosurgery for vestibular schwannomas: analysis of 317 patients followed more than 5 years. Neurosurgery 57:257–265
Hasegawa T, Kida Y, Yoshimoto M, Koike J, Goto K (2006) Evaluation of tumor expansion after stereotactic radiosurgery in patients harboring vestibular schwannomas. Neurosurgery 58:1119–1128
Hasegawa T, Kida Y, Kato T, Iizuka H, Kuramitsu S, Yamamoto T (2013) Long-term safety and efficacy of stereotactic radiosurgery for vestibular schwannomas: evaluation of 440 patients more than 10 years after treatment with Gamma Knife surgery. J Neurosurg 118:557–565
Iwai Y, Yamanaka K, Shiotani M, Uyama T (2003) Radiosurgery for acoustic neuromas: results of low-dose treatment. Neurosurgery 53:282–287
Klijn S, Verheul JB, Beute GN, Leenstra S, Mulder JJS, Kunst HPM, Hanssens PEJ (2016) Gamma Knife radiosurgery for vestibular schwannomas: evaluation of tumor control and its predictors in a large patient cohort in The Netherlands. J Neurosurg 124:1619–1626
Kondziolka D, Lunsford LD, McLaughlin MR, Flickinger JC (1998) Long-term outcomes after radiosurgery for acoustic neuromas. N Engl J Med 339:1426–1433
Lunsford LD, Niranjan A, Flickinger JC, Maitz A, Kondziolka D (2013) Radiosurgery of vestibular schwannomas: summary of experience in 829 cases. J Neurosurg 119:195–199
Watanabe S, Yamamoto M, Kawabe T, Koiso T, Yamamoto T, Matsumura A, Kasuya H (2016) Stereotactic radiosurgery for vestibular schwannomas: average 10-year follow-up results focusing on long-term hearing preservation. J Neurosurg 125(Suppl 1):64–72
Koos WT, Day JD, Matula C, Levy DI (1998) Neurotopographic considerations in the microsurgical treatment of small acoustic neurinomas. J Neurosurg 88:506–512
Carlson ML, Jacob JT, Pollock BE, Neff BA, Tombers NM, Driscoll CLW, Link MJ (2013) Long-term hearing outcomes following stereotactic radiosurgery for vestibular schwannoma: patterns of hearing loss and variables influencing audiometric decline. J Neurosurg 118:579–587
Mousavi SH, Niranjan A, Akpinar B, Huang M, Kano H, Tonetti D, Flickinger JC, Dade Lunsford L (2016) Hearing subclassification may predict long-term auditory outcomes after radiosurgery for vestibular schwannoma patients with good hearing. J Neurosurg 125:845–852
Roos DE, Potter AE, Zacest AC (2011) Hearing preservation after low dose linac radiosurgery for acoustic neuroma depends on initial hearing and time. Radiother Oncol 101:420–424
Puataweepong P, Dhanachai M, Dangprasert S, Narkwong L, Sitathanee C, Sawangsilpa T, Janwityanujit T, Yongvithisatid P (2014) Linac-based stereotactic radiosurgery and fractionated stereotactic radiotherapy for vestibular schwannomas: comparative observations of 139 patients treated at a single institution. J Radiat Res 55:351–358
Woolf DK, Williams M, Goh CL, Henderson DR, Menashy RV, Simpson N, Mastroianni B, Collis CH (2013) Fractionated stereotactic radiotherapy for acoustic neuromas: long-term outcomes. Clin Oncol 25:734–738
Champ CE, Shen X, Shi W et al (2013) Reduced-dose fractionated stereotactic radiotherapy for acoustic neuromas: maintenance of tumor control with improved hearing preservation. Neurosurgery 73:489–496
Hansasuta A, Choi CYH, Gibbs IC et al (2011) Multisession stereotactic radiosurgery for vestibular schwannomas. Neurosurgery 69:1200–1209
Apicella G, Paolini M, Deantonio L, Masini L, Krengli M (2016) Radiotherapy for vestibular schwannoma: review of recent literature results. Rep Pract Oncol Radiother 21:399–406
Combs SE, Engelhard C, Kopp C, Wiedenmann N, Schramm O, Prokic V, Debus J, Molls M, Grosu AL (2015) Long-term outcome after highly advanced single-dose or fractionated radiotherapy in patients with vestibular schwannomas—Pooled results from 3 large German centers. Radiother Oncol 114:378–383
Collen C, Ampe B, Gevaert T, Moens M, Linthout N, De Ridder M, Verellen D, D’Haens J, Storme G (2011) Single fraction versus fractionated linac-based stereotactic radiotherapy for vestibular schwannoma: a single-institution experience. Int J Radiat Oncol Biol Phys 81:e503–e509
Kranzinger M, Zehentmayr F, Fastner G, Oberascher G, Merz F, Nairz O, Rahim H, Sedlmayer F (2014) Hypofractionated stereotactic radiotherapy of acoustic neuroma. Volume changes and hearing results after 89-month median follow-up. Strahlenther Onkol 190:798–805
Kessel KA, Fischer H, Vogel MME, Oechsner M, Bier H, Meyer B, Combs SE (2017) Fractionated vs. single-fraction stereotactic radiotherapy in patients with vestibular schwannoma. Strahlenther Onkol 193:192–199
Hasegawa T, Kida Y, Kato T, Iizuka H, Yamamoto T (2011) Factors associated with hearing preservation after Gamma Knife surgery for vestibular schwannomas in patients who retain serviceable hearing. J Neurosurg 115:1078–1086
Kano H, Kondziolka D, Khan A, Flickinger JC, Lunsford LD (2009) Predictors of hearing preservation after stereotactic radiosurgery for acoustic neuroma. J Neurosurg 111:863–873
Tamura M, Carron R, Yomo S, Arkha Y, Muraciolle X, Porcheron D, Thomassin JM, Roche PH, Régis J (2009) Hearing preservation after gamma knife radiosurgery for vestibular schwannomas presenting with high-level hearing. Neurosurgery 64:289–296
Hasegawa T, Kato T, Kida Y et al (2016) Gamma Knife surgery for patients with facial nerve schwannomas: a multiinstitutional retrospective study in Japan. J Neurosurg 124:403–410
Kirchmann M, Karnov K, Hansen S, Dethloff T, Stangerup S-E, Caye-Thomasen P (2016) Ten-year follow-up on tumor growth and hearing in patients observed with an intracanalicular vestibular schwannoma. Neurosurgery 80:49–56
Scheller C, Wienke A, Tatagiba M et al (2016) Stability of hearing preservation and regeneration capacity of the cochlear nerve following vestibular schwannoma surgery via a retrosigmoid approach. J Neurosurg 125:1277–1282
Golfinos JG, Hill TC, Rokosh R, Choudhry O, Shinseki M, Mansouri A, Friedmann DR, Thomas Roland JJ, Kondziolka D (2016) A matched cohort comparison of clinical outcomes following microsurgical resection or stereotactic radiosurgery for patients with small- and medium-sized vestibular schwannomas. J Neurosurg 125:1472–1482
Quist TS, Givens DJ, Gurgel RK, Chamoun R, Shelton C (2015) Hearing preservation after middle fossa vestibular schwannoma removal. Otolaryngol Neck Surg 152:706–711
Hilton CW, Haines SJ, Agrawal A, Levine SC (2011) Late failure rate of hearing preservation after middle fossa approach for resection of vestibular schwannoma. Otol Neurotol 32:132–135
Betchen SA, Walsh J, Post KD (2005) Long-term hearing preservation after surgery for vestibular schwannoma. J Neurosurg 102:6–9
Nakamizo A, Mori M, Inoue D, Amano T, Mizoguchi M, Yoshimoto K, Sasaki T (2013) Long-term hearing outcome after retrosigmoid removal of vestibular schwannoma. Neurol Med Chir 53:688–694
Wang AC, Chinn SB, Than KD, Arts HA, Telian SA, El-Kashlan HK, Thompson BG (2013) Durability of hearing preservation after microsurgical treatment of vestibular schwannoma using the middle cranial fossa approach. J Neurosurg 119:131–138
Van Dijk JE, Duijndam J, Graamans K (2000) Acoustic neuroma: deterioration of speech discrimination related to thresholds in pure-tone audiometry. Acta Otolaryngol 120:627–632
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Hasegawa, T., Kato, T., Yamamoto, T. et al. Long-term hearing outcomes after gamma knife surgery in patients with vestibular schwannoma with hearing preservation: evaluation in 92 patients with serial audiograms. J Neurooncol 138, 283–290 (2018). https://doi.org/10.1007/s11060-018-2784-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-2784-x