Abstract
Background
Stereotactic radiotherapy (RT) has been established as a valid treatment alternative in patients with vestibular schwannoma (VS). There is ongoing controversy regarding the optimal fractionation. Hearing preservation may be the primary goal for patients with VS, followed by maintenance of quality of life (QoL).
Methods
From 2002 to 2015, 184 patients with VS were treated with radiosurgery (RS) or fractionated stereotactic radiotherapy (FSRT). A survey on current symptoms and QoL was conducted between February and June 2016.
Results
Median follow-up after RT was 7.5 years (range 0–14.4 years). Mean overall survival (OS) after RT was 31.1 years, with 94 and 87% survival at 5 and 10 years, respectively. Mean progression-free survival (PFS) was 13.3 years, with 5‑ and 10-year PFS of 92%. Hearing could be preserved in RS patients for a median of 36.3 months (range 2.3–13.7 years). Hearing worsened in 17 (30%) cases. Median hearing preservation for FSRT was 48.7 months (range 0.0–13.8 years); 29 (23%) showed hearing deterioration. The difference in hearing preservation was not significant between RS and FSRT (p = 0.3). A total of 123/162 patients participated in the patient survey (return rate 76%). The results correlate well with the information documented in the patient files for tinnitus and facial and trigeminal nerve toxicity. Significant differences appeared regarding hearing impairment, gait uncertainty, and imbalance.
Conclusion
These data confirm that RS and FSRT are comparable in terms of local control for VS. RS should be reserved for smaller lesions, while FSRT can be offered independently of tumor size. Patient self-reported outcome during follow-up is of high value. The established questionnaire could be validated in the independent cohort.
Zusammenfassung
Hintergrund
Die stereotaktische Radiotherapie (RT) wurde als gültige Behandlungsalternative bei Patienten mit Vestibularisschwannom (VS) etabliert. Diskussionen über die optimale Fraktionierung laufen jedoch. Der Erhalt von Hörvermögen und Lebensqualität (QoL) sind Hauptziele für Patienten.
Methoden
Von 2002 bis 2015 wurden 184 VS-Patienten mit Radiochirurgie (RS) oder fraktionierter stereotaktischer Radiotherapie (FSRT) behandelt und aktuelle Nebenwirkungen und QoL zwischen Februar und Juni 2016 bewertet.
Ergebnisse
Das mediane Follow-up nach RT betrug 7,5 Jahre (Spanne 0–14,4 Jahre), das mittlere Gesamtüberleben (OS) nach RT 31,1 Jahre, mit Überlebensraten von 94 und 87% nach 5 und 10 Jahren und das mittlere progressionsfreie Überleben (PFS) 13,3 Jahre, mit einem 5‑ und 10-Jahres-PFS von 92%. Patienten mit RS behielten ihr Hörvermögen im Median für 36,3 Monate (2,3–13,7 Jahre). In 17 (30%) Fällen verschlechterte sich das Hörvermögen. Der mediane Gehörerhalt für FSRT betrug 48,7 Monate (Spanne 0,0–13,8 Jahre); 29 (23%) Patienten zeigten eine Verschlechterung. Der Unterschied im Erhalt des Hörvermögens war zwischen RS und FSRT nicht signifikant (p = 0,3). Insgesamt nahmen 123/162 Patienten an der Umfrage teil (Rücklaufquote 76%). Die Ergebnisse korrelieren gut mit den Informationen aus den Patientenakten für Tinnitus, Fazialis- und Trigeminus-Nebenwirkungen. Signifikante Unterschiede gibt es in Bezug auf Hörschädigung, Gangunsicherheit und Gleichgewichtsstörung.
Schlussfolgerung
Unsere Daten bestätigen, dass sowohl RS als auch FSRT bezüglich lokaler Kontrolle vergleichbar sind. RS sollte für kleinere Läsionen angewendet werden, während sich FSRT unabhängig von der Tumorgröße eignet. Der Patientenselbstbericht während des Follow-up-Zeitraums ist von hohem Wert. Der etablierte Fragebogen konnte in dieser unabhängigen Kohorte validiert werden.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Stereotactic radiotherapy (RT) has been established as a valid treatment alternative in patients with vestibular schwannoma (VS). There is ongoing controversy regarding the optimal fractionation: while some authors clearly prefer short treatment times and report data from radiosurgery (RS) approaches, others favor a fractionated regimen on the basis of a radiobiological benefit of fractionation which is associated with a promising risk–benefit profile for smaller and large VS alike.
Hearing preservation may be the primary goal for VS patients, followed by maintenance of quality of life (QoL). In turn, QoL is influenced by the typical side effects of impaired facial and trigeminal nerve function, tinnitus, imbalance, dizziness, and gait uncertainty. Current treatment options are wait-and-see, microsurgery, RS, and fractionated stereotactic radiotherapy (FSRT), all equally effective depending on the size and location of the tumor [1, 2].
Often the treatment decision depends on the physician’s experience and patients’ preferences. For large tumors, surgical resection is often preferred, with preservation of facial nerve function in about 85% of cases, while the rate of hearing preservation is about 50% [3–5]. Better outcome and QoL are associated with smaller tumor size.
For small and medium-sized VS, all strategies are possible, with comparable control rates. While surgery is possible, it bears more risks than noninvasive RS or FSRT. For smaller tumors, preservation rates of 90% for facial nerve function and of 80% for hearing can be achieved [6–8].
Recent studies examine very little data on QoL. However, it has been shown that the physician-reported outcome can vary substantially from the patient-reported outcome, and this information should therefore be taken into account when comparing treatment modalities. Particularly for patients with long-term survival, the patient-reported outcome is of high value for treatment evaluation. In the past, the authors of the current paper developed a questionnaire addressing patient-reported outcome. In the present work, the authors sought to validate the questionnaire previously developed by Combs et al. in 2013 [9] in an independent cohort of VS patients, and differentially report on outcome and hearing preservation after single-fraction or fractionated stereotactic radiotherapy (FSRT).
Patients and methods
Patients
From 2002 to 2015, 184 patients with VS were consecutively treated at the Department of Radiation Oncology at the Klinikum rechts der Isar, Munich, Germany. Patient data were collected prospectively in the institutional database directly after therapy or follow-up appointment in a standardized form. The cutoff date for data collection was June 2016. The local ethics committee of the Medical Faculty of the Technische Universität München (TUM) approved the study with vote number 257/16S. Primary endpoints were local control and facial and trigeminal neuropathy, as well as QoL and patient-reported outcome.
Of all patients, 43% were male (80/184) and 57% (104/184) female. Median age at treatment was 60 years (range 16–85 years). The Gardner–Robertson Scale was used to determine hearing status before and after RT; the House–Brackmann Facial Weakness Scale for the facial nerve status.
Of all patients, 8 had received previous surgery within 6 months before RT. Of these, 4 patients suffered from neurofibromatosis type 2 (NF2). The remaining 3 patients with NF2 had undergone surgery more than 4.5 years before RT.
Radiotherapy
In 56 patients, RT was performed as RS with a median dose of 12 Gy. In the remaining 128 patients, RT was performed as FSRT with a median dose of 54 Gy and a median single dose of 1.8 Gy. Dose was applied with a Varian Trilogy linear accelerator (LINAC; Varian, Palo Alto, CA, USA). Treatment planning for RS and FSRT was performed using a stereotactic treatment setup with a thermoplastic mask system (Brainlab, Feldkirchen, Germany) and daily image-guided RT (IGRT) by robotic ExacTrac positioning (Brainlab). On contrast-enhanced T1-weighted MRI imaging, the gross tumor volume (GTV) was defined. The planning target volume (PTV) resulted from the GTV with an additional margin of 1–2 mm. The dose was described on the 80% isodose for RS and on the 95% isodose for FSRT. Median PTV volume was 1.96 ml (range 0.09–41.1 ml). For patient characteristics, see Table 1.
Follow-up
Patients were enrolled into a follow-up regimen with assessment 6 months after RT and yearly thereafter, including contrast-enhanced MRI as well as clinical assessment. Additional examinations were scheduled if clinically needed. All decisions regarding further treatments were made on an interdisciplinary basis.
Patient survey
The survey was conducted between February and June 2016. The questionnaire developed by Combs et al. in 2013 [9] was sent to 162 of all 184 patients. Patients living outside Germany and already deceased patients were not included. The survey on patient self-reported outcome focuses mainly on the following aspects: questions regarding symptoms, items assessing overall hearing and QoL before and after RT treatment, post-RT treatments, and status of last (external) follow-up.
Statistics
Statistical calculations were performed using SPSS Statistics v. 23 (IBM, Armonk, NY, USA). For all patients, overall survival (OS) was calculated from the last day of RT until death or last follow-up; progression-free survival (PFS) from the last day of RT until the date of progression or death or last follow-up. Survival analyses were based on the Kaplan–Meier method; PFS and hearing preservation were calculated with the Cox regression method. For univariate analyses of different patient groups, the log-rank test was used. Frequency distributions in the RT groups were tested with the chi-squared test. Comparison of patient-reported side effects with documented information in the patient file was performed using the McNemar test for related samples. A p-value ≤0.05 was considered statistically significant.
Results
Outcome
Median follow-up after RT was 7.5 years (range 0–14.4 years, including foreign patients who were lost to follow-up). During the period of analysis, 17 patients died and 12 developed a local recurrence. Mean OS after RT was 13.1 years, with 94 and 87% survival at 5 and 10 years, respectively. For PFS, the mean was 13.3 years; with 5 and 10 year PFS of 92%. Neither OS nor PFS differed significantly between the RS and FSRT groups (OS: p = 0.9, PFS: p = 0.3; hazard ratio, HR = 2.11; confidence interval, CI = 0.46–9.64; Fig. 1).
Hearing preservation and tinnitus
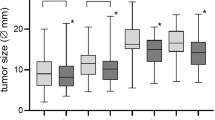
For the RS group, 19 (34%) patients had useful hearing (grade I/II) before RT, see Table 2. Hearing could be preserved for a median of 36.3 months (range 2.3–13.7 years). Hearing worsened in 17 (30%) cases. Median hearing preservation in the FSRT group was 48.7 months (range 0.0–13.8 years). Of all FSRT patients, 29 (23%) showed a hearing deterioration. Development of hearing impairment during the follow-up period of each patient is shown in Fig. 2. For all patients with useful hearing (n = 65) before RT, the differences in hearing preservation were not significant (Fig. 3; p = 0.3, HR = 0.66, CI = 0.29–1.50) between RS and FSRT. Out of 59 patients without tinnitus, 15 reported post-RT symptoms (8.5%).
Facial and trigeminal nerve toxicity
Of all patients without facial nerve toxicity, 5 developed new symptoms after RT. Facial nerve function could be preserved in 93.5% of all patients. Trigeminal nerve function remained without interference for 89.7%. The distribution was not significantly different between the RS and FSRT groups (facial nerve p = 0.5, trigeminal nerve p = 0.1).
Dizziness, imbalance, gait uncertainty
Documentation accuracy of dizziness, imbalance, and gait uncertainty was poor, since it is hard for patients and even doctors to distinguish between the three. Rates of new occurrences were recorded between 14 and 20% after RT; however, symptoms improved in most cases during long-term follow-up. Differences between RS and FSRT were not significant for gait uncertainty (p = 0.5), dizziness (p = 0.2), or imbalance (p = 0.4).
Quality of life questionnaire
A total of 123 out of 162 patients participated in the survey, which represents a return rate of 76%. Median follow-up of this subgroup was 9.6 years (1.8 months–14.3 years). The results of the self-reported typical side effects are listed in Fig. 4. Considering the responses of 76% of patients, the results correlate well with the information documented in the patient files for tinnitus, facial nerve toxicity (grade II–VI), and trigeminal nerve toxicity before and after RT (Table 3). Significant differences between patient-reported side effects and documented information in the patient file appeared regarding hearing impairment (only before RT), gait uncertainty, and imbalance.
As self-assessed by the patients, side effects improved after RT in 32% (Fig. 5a). The improvement differed between the two RT methods and was significantly better for patients treated with RS compared to FSRT (p = 0.03). This result corresponds with the answers to the survey questions “How do you feel after RT?” and “How is your quality of life after RT” (Fig. 5b, c), with 27 and 30% also reporting an improvement in these items, respectively. Overall, QoL was unchanged or improved for about 80% of patients (Fig. 5c), and no significant differences could be observed between the RS and FSRT groups (p = 0.8). Of the 17% reporting a reduction in QoL, a total or severe hearing impairment was most often mentioned; three cases were related to secondary illnesses, i. e., stroke, leukemia, and age-related macular degeneration (AMD).
Discussion
Long-term follow-up confirms that both FSRT and RS remain safe and effective in terms of local control and cranial nerve toxicity. Besides objective clinical follow-up, patient-reported outcome underlines the overall beneficial treatment of VS with noninvasive high-precision RT. The questionnaire developed by Combs et al. [9] was validated in this present project in an independent patient population and reveals comparative results. This supports the value of the present questionnaire during follow-up in addition to standard clinical and imaging examination.
For VS, treatment decision-making depends on the size of the lesion as well the clinical presentation of patients. For small and asymptomatic tumors, a wait-and-scan strategy might be followed; generally, average growth ranges between 1 and 3 mm per year, and close clinical and radiological follow-up will be able to capture early recurrence, enabling safe and effective treatment at such a time. Considering hearing development throughout the clinical course, natural hearing deterioration is also present independently of VS, particularly in the aging population. With VS, natural hearing impairment without any treatment is reported to be 56% [10].
Surgery has been the mainstay of treatment over the years. Different anatomical approaches are possible, from translabyrinthine to subtemporal, each associated with specific risk–benefit profiles. Large series of VS report a local control rate of about 90% [11, 12], with a rate of hearing impairment of around 60% [6, 12, 13]. Hearing deterioration generally occurs directly after surgery, whereas any hearing impairment after RT develops over time, in most cases within the first 6 months when directly attributable to RT.
RT has become established as a noninvasive and toxicity-minimized treatment alternative. Depending on the volume of the lesion, either single-fraction RS or fractionated regimens [14] are possible. Several reports from single institutions have confirmed excellent long-term local control of over 95%, even after 10 and 20 years [15]. Hearing deterioration depends on the series and is between 85 and 90% after 10 and 20 years [16], although RT rarely leads to complete loss of hearing when performed early. For smaller volumes, RS is comparable to fractionated treatment [14]. Smaller series have reported that fractionated treatment might be safer in terms of cranial nerve toxicity, and this certainly may hold true for larger lesions; only recently, Fong and colleagues reported that hearing preservation was higher after fractionated regimens [8]. In a previously published multicenter analysis, the authors of the current study demonstrated comparable hearing preservation for smaller lesions; however, larger lesions were all treated with fractionated concepts, independent of the treating center [14]. To date, there are no clear data regarding which volume can be considered “large”. Prospective and clearly stratified studies are required to define the border between smaller and large lesions, and thus define the threshold for RS vs. fractionated RT. Independent of size, dose is also a strong prognostic factor: it has been shown in the past that single doses exceeding 13 Gy lead to a significant risk of hearing impairment and facial or trigeminal nerve toxicity [17, 18]. For fractionated regimens, no difference in toxicity profiles between 54 and 57.6 Gy has been reported; however, in the study by Champ et al., even more beneficial toxicity profiles were reported with 46.8 Gy in single fractions [19]. Long-term analysis must confirm that this safety does not compromise long-term local control.
Considering the effectiveness of neurosurgical resection in an experienced team, as well as the beneficial toxicity profile of RT, a combined treatment approach might be the treatment of choice in the future, particularly for larger and brainstem-compressing lesions. Planned partial and maximal safe resection can then be consolidated with highly precise RT to any tumor remnants.
As the validation of the questionnaire by Combs et al. [9] showed reliable results, this is now being implemented in the authors’ daily clinical routine. Patient-reported outcome is a valuable addition to standard clinical treatment documentation and leads to improved accuracy and completeness of follow-up data. Furthermore, patients feel better understood and actively included in management of their disease. Particularly in cases where long-term side effects of treatment are relevant and patients are easily lost to follow-up, regularly performed assessment of patient-reported outcome is a suitable tool to quantify symptomatic improvement or worsening. In addition, the collected data can be used to gain further scientific findings. However, it must be considered that the need for continuous validation of QoL questionnaires is substantial, as controllability is a crucial part of the scientific process and data of nonvalidated questionnaires do not match the criteria of good scientific practice.
Conclusion
These data confirm that RS and FSRT are comparable in terms of local control for VS. RS should be reserved for smaller lesions, while FSRT can be offered independently of tumor size. Particularly for larger volumes, a combined treatment approach comprising neurosurgery and RT might be the treatment of choice in the future. In addition to standard clinical and imaging examinations, patient self-reported outcome during follow-up is of high value. The established questionnaire could be validated in the present independent cohort and provides valuable data on outcome after RS or FSRT.
References
Varughese JK, Breivik CN, Wentzel-Larsen T, Lund-Johansen M (2012) Growth of untreated vestibular schwannoma: a prospective study. J Neurosurg 116:706–712. doi:10.3171/2011.12.JNS111662
Golfinos JG, Hill TC, Rokosh R et al (2016) A matched cohort comparison of clinical outcomes following microsurgical resection or stereotactic radiosurgery for patients with small- and medium-sized vestibular schwannomas. J Neurosurg. doi:10.3171/2015.12.JNS151857
Gerganov VM, Giordano M, Samii A, Samii M (2012) Surgical treatment of patients with vestibular schwannomas after failed previous radiosurgery. J Neurosurg 116:713–720. doi:10.3171/2011.12.JNS111682
Kunert P, Dziedzic T, Podgórska A et al (2015) Surgery for sporadic vestibular schwannoma. Part III: Facial and auditory nerve function. Neurol Neurochir Pol 49:373–380. doi:10.1016/j.pjnns.2015.08.008
Monfared A, Corrales E, Theodosopoulos P et al (2016) Facial nerve outcome and tumor control rate as a function of degree of resection in treatment of large acoustic neuromas: preliminary report of the acoustic neuroma subtotal resection study. Neurosurgery. doi:10.1227/NEU.0000000000001162
Anaizi A, DiNapoli V, Pensak M, Theodosopoulos P (2016) Small vestibular Schwannomas: does surgery remain a viable treatment option? J Neurol Surg B Skull Base 77:212–218. doi:10.1055/s-0035-1564591
Kopp C, Fauser C, Müller A et al (2011) Stereotactic fractionated radiotherapy and LINAC radiosurgery in the treatment of vestibular schwannoma-report about both stereotactic methods from a single institution. Int J Radiat Oncol Biol Phys 80:1485–1491. doi:10.1016/j.ijrobp.2010.04.057
Fong BM, Pezeshkian P, Nagasawa DT et al (2012) Hearing preservation after LINAC radiosurgery and LINAC radiotherapy for vestibular schwannoma. J Clin Neurosci 19:1065–1070. doi:10.1016/j.jocn.2012.01.015
Combs SE, Welzel T, Kessel KA et al (2013) Hearing preservation after radiotherapy for vestibular schwannomas is comparable to hearing deterioration in healthy adults and is accompanied by local tumor control and a highly preserved quality of life (QOL) as patients’ self-reported outcome. Radiother Oncol 106:175–180. doi:10.1016/j.radonc.2012.12.004
Liu W, Ni M, Jia W et al (2015) How to address small- and medium-sized acoustic neuromas with hearing: a systematic review and decision analysis. World Neurosurg 84:283–291.e1. doi:10.1016/j.wneu.2015.03.013
Syed-Mohamad S‑M (2009) Development and implementation of a web-based system to study children with malnutrition. Comput Methods Programs Biomed 93:83–92. doi:10.1016/j.cmpb.2008.07.011
Mendelsohn D, Westerberg BD, Dong C, Akagami R (2016) Clinical and radiographic factors predicting hearing preservation rates in large vestibular schwannomas. J Neurol Surg B Skull Base 77:193–198. doi:10.1055/s-0035-1564054
Roessler K, Krawagna M, Bischoff B et al (2016) Improved postoperative facial nerve and hearing function in retrosigmoid vestibular schwannoma surgery significantly associated with semisitting position. World Neurosurg 87:290–297. doi:10.1016/j.wneu.2015.11.089
Combs SE, Engelhard C, Kopp C et al (2015) Long-term outcome after highly advanced single-dose or fractionated radiotherapy in patients with vestibular schwannomas – pooled results from 3 large German centers. Radiother Oncol 114:378–383. doi:10.1016/j.radonc.2015.01.011
Hasegawa T, Kida Y, Kato T et al (2013) Long-term safety and efficacy of stereotactic radiosurgery for vestibular schwannomas: evaluation of 440 patients more than 10 years after treatment with Gamma Knife surgery. J Neurosurg 118:557–565. doi:10.3171/2012.10.JNS12523
Sun S, Liu A (2012) Long-term follow-up studies of Gamma Knife surgery with a low margin dose for vestibular schwannoma. J Neurosurg 117(Suppl):57–62. doi:10.3171/2012.7.GKS12783
Combs SE, Welzel T, Schulz-Ertner D et al (2010) Differences in clinical results after LINAC-based single-dose radiosurgery versus fractionated stereotactic radiotherapy for patients with vestibular schwannomas. Int J Radiat Oncol Biol Phys 76:193–200. doi:10.1016/j.ijrobp.2009.01.064
Flickinger JC, Kondziolka D, Niranjan A, Lunsford LD (2013) Results of acoustic neuroma radiosurgery: an analysis of 5 years’ experience using current methods. J Neurosurg 119(Suppl):1–6
Champ CE, Shen X, Shi W et al (2013) Reduced-dose fractionated stereotactic radiotherapy for acoustic neuromas: maintenance of tumor control with improved hearing preservation. Neurosurgery 73:489–496. doi:10.1227/NEU.0000000000000019
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
K.A. Kessel, H. Fischer, M.M.E. Vogel, M. Oechsner, H. Bier, B. Meyer, and S.E. Combs declare that they have no competing interests.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s00066-016-1087-4.
Rights and permissions
About this article
Cite this article
Kessel, K.A., Fischer, H., Vogel, M.M.E. et al. Fractionated vs. single-fraction stereotactic radiotherapy in patients with vestibular schwannoma. Strahlenther Onkol 193, 192–199 (2017). https://doi.org/10.1007/s00066-016-1070-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-016-1070-0