Abstract
The use of stereotactic radiosurgery (SRS) expanded to include the treatment of vestibular schwannomas (VSs) in 1969; since then, efforts to increase tumour control and to reduce cranial neuropathy have continued. Using the currently recommended marginal dose of 12–13 Gy, long-term reported outcomes after SRS include not only excellent tumour control rates of 92–100 % but also outstanding functional preservation of the trigeminal and facial nerves, with values of 92–100 % and 94–100 %, respectively. Nonetheless, hearing preservation remains in the range of 32–81 %. Previous studies have suggested possible prognostic factors of hearing preservation such as the Gardner-Robertson grade, radiation dose to the cochlea, transient volume expansion (TVE) after SRS, length of irradiated cochlear nerve, marginal dose to the tumour, and age. However, we still do not clearly understand why patients lose their hearing after SRS for VS.
Relevant to these considerations, one study recently reported that the auditory brainstem response (ABR) wave V latency and waves I and V interval (IL_I–V) correlated well with intracanalicular pressure values and even with hearing level. The demonstration that ABR values, especially wave V latency and IL_I–V, correlate well with intracanalicular pressure suggests that patients with previously elevated intracanalicular pressure might have an increased chance of hearing loss on development of TVE, which has been recognised as a common phenomenon after SRS or stereotactic radiotherapy (SRT) for intracranial schwannomas.
In our experience, the ABR IL_I–V increased during the first 12 months after SRS for VSs in patients who lost their serviceable hearing. The effect of increased ABR IL_I–V on hearing outcome also became significant over time, especially at 12 months after SRS, and was more prominent in patients with poor initial pure-tone average (PTA) and/or ABR values. We hypothesise that patients with considerable intracanalicular pressure at the time of SRS are prone to lose their serviceable hearing due to the added intracanalicular pressure induced by TVE, which usually occurs within the first 12 months after SRS for VSs. Using these findings, we suggested a classification system for the prediction of hearing outcomes after SRS for VSs. This classification system could be useful in the proper selection of management modalities for hearing preservation, especially in patients with only hearing ear schwannoma or neurofibromatosis type 2.
Advances in diagnostic tools, treatment modalities, and optimisation of radiosurgical dose have improved clinical outcomes, including tumour control and cranial neuropathies, in patients with VSs. However, the preservation of hearing function still falls short of our expectation. A prediction model for hearing preservation after each treatment modality will guide the proper selection of treatment modalities and permit the appropriate timing of active treatment, which will lead to the preservation of hearing function in patients with VSs.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Stereotactic radiosurgery
- Vestibular schwannoma
- Hearing preservation
- Intracanalicular pressure
- Transient volume expansion
- Auditory brainstem response
Introduction
It was Harvey Cushing who, recognising the dire problems of haemorrhage during vestibular schwannoma (VS) surgery, compared the cerebellopontine angle with the corner fence at the Battle of Gettysburg and suggested that it might well be called the ‘bloody angle’ [93]. In his monograph ‘Tumors of the Nervus Acusticus and the Syndrome of the Cerebellopontine Angle’, published in 1917, he demonstrated how he was able to reduce the perioperative mortality associated with this surgery from 72 %–84 % to 35 % and then to 10 % by employing the technique of intracapsular debulking [93]. Cushing’s results were a dramatic improvement on those of all of his predecessors. Nevertheless, his method of subtotal removal by intracapsular debulking inevitably resulted in a high recurrence rate, and his great rival of the time, Walter Dandy, soon espoused the philosophy of total tumour removal through a unilateral suboccipital approach [24].
Despite these advances in VS surgery by two great surgeons, most of the neurosurgeons at that time were reluctant to recommend surgery until the tumours became very large. This approach, of course, resulted in a self-perpetuating cycle of poor clinical outcome. The operative results were indeed extremely poor when the tumours become large. Perioperative mortality was 20 % in a series of 130 cases, all of which had rather large tumours at the time of surgery [88]. In one series of partial removals, 60 % of the patients died of tumour recurrence within 4 years [34]. In Northfield’s 1970 series, the average tumour size was 3–4 cm at the time of surgery, and the perioperative death rate was as high as 16 % [83]. He recommended total extirpation of the tumours without procrastination at the first attempt after review of his early experience of partial removal and secondary extirpation. However, the facial and auditory nerves incorporated into the tumour capsule were usually ignored, and the contiguity of the lower cranial nerves, which could consequently be damaged, rendered patients prone to disturbances of swallowing and to respiratory infections [83]. Preservation of the facial nerve was a matter of concern in only a few cases with small tumours, but auditory function was not of interest. Hearing preservation surgery for VSs has only recently become a popular concept on the strength of modern neurosurgical and neurophysiological advances. However, the functional hearing preservation rates are approximately 50–70 %, even if small VSs are in the safe hands of the experts [98, 115].
In these circumstances, the use of stereotactic radiosurgery (SRS) has expanded to include the management of tumours in the ‘bloody angle’ using a bloodless treatment method [64, 93]. Since the first operation performed in 1951 by Lars Leksell [63], SRS, which originated from his idea of replacing the needle electrode with narrow beams of radiant energy, has been an effective alternative to conventional surgery in the management of inoperable intracranial tumours [39]. In his 1971 article [64], Leksell wrote, ‘the lower marginal tumour dose and a rapid dose fall-off using the 50 % isodose line would make serious injury to the neighbouring structures unlikely’. Since the publication of his report, efforts to chase two hares in the management of VSs, namely, to increase the tumour control rate and reduce the risk of cranial nerve injury, including hearing preservation, have continued. The results were in part as Leksell expected, however. Even with a lower tumour marginal dose of 12–13 Gy, numerous reports of long-term outcomes after SRS show not only excellent progression-free survival of 92–100 % but also outstanding preservation of the trigeminal and facial nerves in 92–100 % and 94–100 % of cases, respectively [18, 22, 45, 50, 59].
The efficacy of SRS, especially in relatively small tumours, created interest in hearing function. Nonetheless, hearing preservation still falls short of our expectations. The range of hearing preservation after SRS with long-term follow-up has been reported as 32–81 % [18, 22, 33, 45, 49, 57, 81]. Furthermore, the exact mechanisms of hearing loss after SRS for VSs are not yet understood. Thus, we will review the possible factors associated with hearing deterioration or preservation after SRS for patients with VSs and will suggest the hypothetical causes of hearing loss in patients with VSs after SRS based on our experience. We will also discuss possible methods to preserve hearing in patients with VSs after SRS.
Natural History of Vestibular Schwannomas and Hearing Outcomes
According to a population-based cohort study, the number of diagnosed VSs gradually increased from 7.8 to 23 VSs per one million population per year over approximately 30 years since 1976; since then, the incidence has gradually decreased to 19 VSs per one million individuals [103]. After several decades during which the incidence of diagnosed tumours increased due to easy access to neuroimaging and heightened symptom awareness among the general population, a peak seems to have been reached [103]. This levelling may reflect an approximation of the true incidence of VSs in the general population [103].
Microsurgery or SRS for VSs has been in use for decades with reasonably convincing evidence of efficacy albeit in the absence of randomised trials comparing these treatments with other treatment modalities, particularly the ‘wait-and-see’ strategy. One argument against the conservative management of VSs is the risk of progressive hearing deterioration over time, which cannot be reversed by any treatment [104]. Advocates of hearing preservation microsurgery insist that if patients with small VSs and good hearing are not operated upon, their hearing could deteriorate and they might lose the opportunity to have their hearing preserved by microsurgery [104]. However, long gone are the days when the diagnosis of VS was followed by indiscriminate surgery on the assumption that the presence of the tumour represented a risk to life and function [11]. The initial enthusiasm for SRS or hearing preservation microsurgery for VSs and the assertions for efficacy without toxicity have been replaced by a more sophisticated approach that combines a better understanding of the natural history of untreated VSs with clear recognition of what the treatment can achieve.
Tumour size at diagnosis has decreased from a mean extrameatal diameter of approximately 30 mm in the mid-1970s to a mean diameter of 10 mm over the last several decades. The level of auditory function has also been improved from a mean pure-tone average (PTA) of 70 dB and a mean speech discrimination (SD) of 30 %–48 dB and 60 %, respectively [103]. The improved hearing at diagnosis is even more evident when considering patients with 100 % SD at diagnosis. In 1976, 3 % of the patients had modified word recognition scoring class 0 compared with 21 % in 2008 [103]. Auditory function has become a matter of concern, and various methods for hearing preservation have been developed and advanced in the management of patients with VSs.
Most vestibular schwannoma ears have deteriorated hearing compared with the normal side; one study reported at least a 10-dB PTA difference in over 90 % of patients and at least 10 %-SD difference in 74 % of patients [104]. Of note, patients with even minor loss of SD at diagnosis (1–10 %) are more prone to lose good hearing over the observation period than those with normal hearing function. The actual good hearing preservation rates of patients with even a minimal loss were 72 %, 60 %, and 38 % after 1, 5, and 10 years, respectively, of the wait-and-see strategy [103]. In patients with modified word recognition who scored class 0 hearing at diagnosis (SD = 100 %), 3 % had lost class 1 hearing at the first year of observation, and 12 % and 31 % had lost class 1 hearing after 5 and 10 years of observation, respectively [103]. Therefore, patients in whom the process of hearing deterioration in VS ears has already begun at the time of diagnosis are prone to lose their hearing during the period of observation [5, 37, 105].
In this context, the outcome of proactive treatment in patients with minimal hearing deficits at diagnosis should be compared with that of the wait-and-see strategy, especially in terms of whether proactive treatment can preserve good hearing or prolong the duration of good hearing in vestibular schwannoma ears. A recent meta-analysis showed better hearing preservation in patients treated with SRS compared with conservative management. However, that study did not provide evidence strong enough to permit clear conclusions, especially in the case of patients with small VSs [75], and further studies are mandatory in the near future. Reported hearing outcomes after the wait-and-see strategy for VSs are summarised in Table 1.
Prognostic Factors Related to Hearing Outcome After Stereotactic Radiosurgery
Despite the many suggested possible prognostic factors related to hearing preservation, such as radiation dose to the cochlea and its structures, the occurrence of transient volume expansion (TVE) after SRS, the length of the irradiated cochlear nerve, marginal dose to the tumour itself, and age, it is not precisely known why patients often lose their hearing after SRS for VSs. This uncertainty regarding the exact mechanism of hearing loss after SRS may be one of the principal causes of unsatisfactory hearing preservation. In the following section, the possible factors affecting hearing outcome are reviewed, and we suggest several hypothetical causes of hearing loss after SRS for patients with VSs based on our experience.
Tumour Control and Dose Prescription
Tumour control is the initial goal of SRS for VSs, and the efficacy of SRS in tumour control has been proven over several decades even in the absence of randomised controlled studies [43, 76]. After achieving good tumour control, cranial nerve preservation, especially hearing preservation, became an important issue. The relationship between tumour control and hearing preservation only received minor attention because of the very high rates of tumour control achieved using contemporary techniques and dose guidelines for SRS. However, failure of tumour control after SRS directly results in hearing deterioration. In fact, all patients who experienced tumour recurrence during the follow-up period after SRS or stereotactic radiotherapy lost their initial useful hearing with tumour progression [23]. In most series of the natural history of VSs, tumour growth is also associated with an increased risk of hearing deterioration [36, 38, 73, 106]. However, hearing deterioration can occur in the absence of tumour growth when the wait-and-see strategy is followed [94, 104]. Failure of tumour control after SRS may lead directly to ipsilateral hearing loss; however, tumour control does not guarantee preservation of the patient’s initial or useful hearing.
In the first report by Leksell [64], the tumour control rate of SRS for VS was reported to be approximately 80 % using tumour marginal doses of 18–20 Gy at a 50 % isodose line, and approximately 20 % of patients developed cranial neuropathies. Thereafter, the dose prescribed to the tumour margin gradually decreased to 12–13 Gy in an effort to avoid cranial neuropathies; now, with the aid of modern technology, tumour control rates have improved significantly to 92–100 % [18, 45, 50].
By decreasing the tumour marginal dose to 12–13 Gy, hearing outcomes have improved, but not to the same extent as other cranial neuropathies [18, 22, 33, 45, 49, 57, 81]. Preservation of trigeminal and facial nerve function has improved to 92–100 % and 94–100 % of cases, respectively [18, 22, 45, 50, 59]. However, improvement in hearing outcome after SRS with long-term follow-up still falls short of expectations; the range of post-radiosurgery hearing preservation has been reported as 32–81 % [18, 22, 33, 45, 49, 57, 81].
In 2007, a useful hearing preservation rate of 74–77 % using a tumour marginal dose of 12–13 Gy was reported [18]. However, the authors of that study excluded patients who were followed up for less than 3 years. If these patients had been included in the analysis, the reported hearing preservation rate might be lower considering that most patients experience hearing deterioration within 2 or 3 years after SRS for VSs, as described by the same group [52]. Another study by the same group showed a useful hearing preservation rate of 71.4 %; however, the median follow-up duration was only 20 months [52]. Therefore, useful hearing preservation rates after long-term follow-up of more than 5 years are estimated to be approximately 50 % or less [14, 22, 57], and rates differ according to follow-up duration and tumour parameters. The fact that hearing outcomes have not considerably improved, even with excellent tumour control rates, suggests that there may be underlying pathophysiological mechanisms of hearing deterioration after SRS for VSs that differ from those in conservatively managed cases.
Radiation to the Temporal Bone Structures
Among the temporal bone structures that are known to be related to hearing function, the cochlea is at the centre of the dispute over hearing preservation after SRS for VSs. The first attempt to link cochlear dose with hearing preservation outcomes after SRS for VSs was made by our Seoul National University Hospital (SNUH) Gamma Knife group in 2005; however, we could not find any statistically significant correlation [67, 86]. The first report to demonstrate a significant relationship between hearing preservation and cochlear dose was published in 2007 [77]. Thereafter, many studies reported similar findings. The cochlear threshold dose most likely lies somewhere in the range of 4–5.33 Gy (Table 2) [9, 44, 52].
Several issues should be considered when interpreting these results. First, cochlear threshold doses were evaluated in different parts of the cochlea or using different methods in various studies. For example, dose was evaluated either at a point within the cochlea or for the whole cochlea and as either the maximum dose or the mean dose to the cochlea [9, 52, 77, 109]; these differences in methodology have led to some debate concerning the results [59, 69].
Second, a relationship between cochlear dose and hearing preservation has not been demonstrated in cases involving other intracranial benign tumours near the internal auditory canal (IAC), even when these tumours were treated with the same radiosurgical protocol [12, 58]. We recently determined that the useful hearing preservation rate after SRS for meningiomas near or extending into the IAC (para-IAC meningiomas) was as high as 97.6 % (41 of 42 patients preserved their useful hearing) during the median follow-up duration of 48 months, even with a considerable radiation dose to the ipsilateral cochlea (the average values of maximal and mean cochlear dose were 6.3 ± 0.4 Gy [range, 3.1–13.1] and 4.6 ± 0.2 Gy [range, 2.2–9.6], respectively) [58]. Actually, one patient with para-IAC meningioma that was treated with maximal and mean cochlear doses of 6.9 Gy and 5.5 Gy, respectively, experienced an improvement in hearing from 30 dB of PTA and 80 % of SD to 26 dB of PTA and 90 % of SD at 12 months after radiosurgery (Fig. 1) [58]. In another patient treated with maximal and mean cochlear doses of 11.5 Gy and 7.5 Gy, respectively, the initial useful hearing was preserved at 72 months after SRS for para-IAC meningioma [58]. A similar finding was presented by an American group in patients with glomus jugulare tumours treated with SRS. Only one of eight patients with clinically serviceable hearing before SRS experienced hearing deterioration after SRS during the mean follow-up duration of 26 months, and three of the four patients who received a mean cochlear dose greater than 8 Gy suffered no hearing deterioration [12].
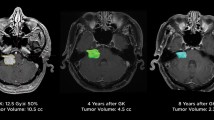
A magnetic resonance imaging of a 47-year-old patient with a meningioma extending into the right internal auditory canal. The tumour volume was 5.6 cm3, and the pure-tone audiometry (PTA) threshold and speech discrimination scores (SDS) were 40 dB and 12 %, respectively, corresponding to Gardner-Robertson (G-R) class 3 (a and e). Gamma Knife radiosurgery was performed. The maximal and mean cochlear radiation doses were 6.9 and 5.5 Gy, respectively. Six months later, the tumour volume was stable (b), and hearing function had improved to 30 dB of PTA and 80 % of SDS. Twelve months after radiosurgery, the tumour volume was 5.2 cm3, and the PTA threshold and SDS were 26 dB and 90 %, respectively (c and f). Twenty-four months after radiosurgery, the tumour had continued to shrink to 4.4 cm3 (d) (Adapted from Kim et al. [58])
Third, the radiation sensitivity of the cochlea was determined in a study that proved the efficacy and safety of intensity-modulated radiotherapy for paediatric patients with medulloblastoma [48]. The threshold cochlear dose in standard fractionated radiotherapy was approximately 35 Gy, which represents approximately 8 Gy of an equivalent radiosurgery dose. This indicates that the recently suggested guideline for the lowest threshold of 4 Gy may be too low [9, 44, 52, 109]. Therefore, the association between the cochlear dose and hearing outcomes after SRS for VSs most likely reflects the dose-volume relationship between the radiosurgical dose and the intracanalicular tumour volume [28, 78].
Transient Volume Expansion, Intracanalicular Pressure, and Auditory Brainstem Response
Hearing outcomes after SRS for VSs differ from those after SRS for other tumours near the IAC, as mentioned above [12, 41, 58]. The cochlear threshold dose has no effect or a minimal effect on hearing outcomes after SRS for tumours near the IAC except VSs. The features of response after SRS for VSs could differ from those after SRS for other tumours near the IAC. One such response could be a transient volume increase, i.e. TVE, which has been recognised as a common phenomenon after SRS and after stereotactic radiotherapy (SRT) for intracranial schwannomas [3, 59, 89]. TVE has also been reported, but not as frequently as in schwannomas, after radiotherapy for other intracranial benign tumours [16, 62]. Infiltration by foamy macrophages, myxoid degeneration, and/or necrosis has been suggested as the underlying histopathological causes of TVE after SRS for VSs [51]. TVE has been reported to occur in 14–74 % of patients 6–16 months after SRS or SRT for VSs [3, 46, 53, 74, 80, 91]. The wide range of its incidence could be caused by differences in the definition of TVE [59], the fractionation scheme of radiation therapy [111], and the method and timing of follow-up tumour measurements in various studies [59, 113, 114].
TVE has been regarded as a remarkable feature of VSs after SRS because it often causes neurological aggravation [40, 91]. In addition, TVE has been considered one of the principal factors that is significantly related to hearing deterioration after SRS for VSs [3, 59, 80], because the period of serviceable hearing loss after SRS often overlaps with that of the development of TVE after SRS for VSs [22, 52, 56]. TVE usually reaches its peak at 6 months after SRS and regresses 12–24 months after surgery. It is also known that most cases of post-SRS auditory neuropathy occur within 24 months, with a median onset of 6 months after SRS (Fig. 2). This finding suggests a possible relationship between post-radiosurgery hearing deterioration and TVE.
This is a Kaplan-Meier plot of survival with serviceable hearing in patients with vestibular schwannoma. Until the last clinical follow-up, in total, 51 patients (42.9 %) lost their serviceable hearing. The median survival with serviceable hearing was 67 months after stereotactic radiosurgery (SRS). The actuarial rates of hearing preservation were 79.7 %, 68.5 %, 62.5 %, 59.9 %, and 56.2 % at 6 months, 12 months, 24 months, 36 months, and 60 months, respectively, after SRS. The patients usually lose their serviceable hearing within 24 months after stereotactic radiosurgery for vestibular schwannomas (Adapted from Han et al. [41])
The mechanism of hearing deterioration caused by TVE might be increased intracanalicular pressure resulting in compression of the intracanalicular path of the cochlear nerve, vascular compromise of the auditory apparatus, or accumulation of intralabyrinthine protein by obstruction of the cochlear pore [5, 61]. However, the association between TVE and hearing outcomes after SRS for VSs has been controversial [59, 80, 112, 120], possibly due to differences in the definition of TVE. To exclude measurement error in the assessment of TVE, the increased volume should exceed the initial volume by at least 13–15 % for area-based manual volume measurements, especially in small-volume tumours [113, 114]. In one study in which TVE was defined as ≥10 % increase in volume, TVE was not related to hearing outcomes [112]. However, in another study in which TVE was defined as ≥30 % increase in volume, TVE was significantly associated with hearing deterioration after SRS for VSs [120]. TVE could therefore be correlated with hearing deterioration after SRS for small VSs, given a sufficient threshold of ≥20 %.
Nonetheless, to directly prove a relationship between TVE and hearing outcomes after SRS for VSs, several issues should be resolved. The first is whether pressure in the IAC can affect auditory function in the initial state before any treatment for VSs. The second is whether a change in pressure in the IAC actually occurs after SRS for VSs, especially during the period of TVE. The last issue is whether the increased intracanalicular pressure is correlated with hearing deterioration after SRS for VSs.
During the period of the Second World War, several tests of auditory function, including Fowler’s alternate loudness balance test, Carhart’s test for tone decay, Bekesy audiometry, the short increment sensitivity index, and speech audiometry, were introduced, thus enabling the differentiation of neural and sensory deafness [93]. Currently, the most accurate auditory function test is the auditory brainstem response (ABR), with a quoted sensitivity of 98 %. It is certainly true that abnormalities of the ABR are found in almost all proven cases of vestibular schwannoma [93].
The mechanism by which the ABR is altered in the presence of VSs has been suggested to be compression of the auditory nerve in the internal auditory canal. Such compression has been shown to occur in an experimental animal model [17, 37] and was also recently demonstrated in humans. The intracanalicular pressure was significantly associated with the size of the tumour, which is confined only in the IAC [6, 61], and the intracanalicular pressure correlated well with the ABR values, especially wave V latency and the wave I and V interval. Overall, tumour growth has no significant relationship to changes in ABR latency during conservative management [95]. Changes in intracanalicular pressure caused by changes in tumour features such as TVE, especially in the IAC, could be indirectly identified using the ABR test after SRS for VSs.
Intracanalicular Pressure and Hearing Function
To test whether pressure in the IAC can affect auditory function, the initial state before any treatment for VSs should be evaluated. In one study [61], the intracanalicular pressure was directly measured by insertion of a microsensor into the IAC before any tumour manipulation during VS surgery via a retromastoid suboccipital approach. And then, the authors evaluated a possible relation between the intracanalicular pressure and the preoperative and intraoperative baseline ABR values.
The range of intracanalicular pressure in VS ears was 0–45 mmHg with a mean value of 15.4 mmHg, and 63 % of VS ears had intracanalicular pressure greater than or equal to 10 mmHg. These results indicate that most patients had a significant pressure difference between the IAC and the cerebellopontine angle cistern.
Patients with class A hearing as defined by the American Academy of Otolaryngology-Head and Neck Surgery [1] (AAO-HNS) (PTA < 30 dB, SD > 70 %) tend to have lower intracanalicular pressure than patients who have AAO-HNS class B hearing (PTA 30–50 dB, SD ≥ 50 %), although this difference did not reach statistical significance [61]. However, the intracanalicular pressure of patients with class A hearing was significantly lower than that of patients with AAO-HNS class C and D hearing (PTA > 50 dB, SD < 50 %) (Fig. 3) [61].
Bar graph demonstrating the correlation of preoperative auditory function and intracanalicular pressure in 33 patients with intact hearing. Preoperative hearing was classified based on American Academy of Otolaryngology-Head and Neck Surgery criteria. Patients with Class A hearing had lower intracanalicular pressures. Although the difference between Class A and Class B groups was not statistically significant (p = 0.16), 12 patients with Class A hearing had significantly lower intracanalicular pressures compared with 12 patients in the combined Class C and D group (p = 0.03). Bars represent the standard error of the mean (Adapted from Lapsiwala et al. [61])
As expected, the ABR wave V latency and wave I and V interval (IL_I–V) also correlated well with intracanalicular pressure values (Fig. 4) [61]. This finding suggests that intracanalicular pressure can be indirectly estimated as a function of the ABR value in VS patients with testable hearing and especially in those with serviceable hearing.
Graph demonstrating the correlation of intracanalicular pressure (ICaP) measurements and the preoperative absolute latency of wave V in 20 patients with VS (p = 0.0001, r = 0.75) (Adapted from Lapsiwala et al. [61])
Pre-radiosurgery Auditory Brainstem Response and Hearing Outcome
Recently, we summarised our experience in the management of patients with VSs who were treated with SRS in our institute between 1997 and 2011. Our studies are divided into two parts. The first is based on data obtained between 1997 and 2009 and addresses whether pressure in the IAC affects auditory function in the initial state before any treatment for VSs has been performed. The second assesses data collected between 1997 and 2011 and addresses whether a change in pressure in the IAC after SRS for VSs actually occurs, especially during the period of TVE.
Among 728 VS patients treated with SRS in our institute between 1997 and 2009, 119 (16.3 %) patients with unilateral sporadic VSs who had serviceable hearing underwent SRS as primary treatment and were given a pre-radiosurgery ABR test [41]. When ABR testing showed no response regardless of the level of hearing, the maximum values of the baseline ABR tests obtained in our cohort were coded for the ABR IL_I–V value. The median tumour marginal dose was 12 Gy, and the mean tumour volume was 1.95 cm3. The mean follow-up duration was 55 months. The actual rates of serviceable hearing preservation were 68.5 %, 62.5 %, 59.9 %, and 56.2 % at 12, 24, 36, and 60 months after SRS, respectively [41].
According to a report by Lapsiwala et al. [61], pre-radiosurgery ABR IL_I–V values did not differ between Gardner-Robertson (G-R) class 1 and class 2 [32]; they were 4.79 ± 0.62 mS and 4.82 ± 0.60 mS, respectively (p = 0.743) (SNUH series, unpublished data). However, the initial ABR IL_I–V values of the ‘post-radiosurgery serviceable hearing preservation group’ were significantly different from those of the ‘post-radiosurgery serviceable hearing loss group’ (4.67 ± 0.49 and 4.97 ± 0.71, respectively [p = 0.007]) (SNUH series, unpublished data). The results of multivariate analysis of prognostic factors of serviceable hearing preservation indicated that the initial PTA score and the ABR IL_I–V value remain significant and independent factors (HR, 1.072 [95 % CI, 1.046–1.098; p < 0.001] and 1.534 [95 % CI, 1.008–2.336; p = 0.046], respectively) [41].
The above findings suggest that the intracanalicular pressure in some regions of schwannoma ears with a serviceable hearing level on the PTA exam is already increased. This increased intracanalicular pressure at the time of SRS could play a role in the development of hearing deterioration after SRS for VSs by adding additional pressure to the intracanalicular pressure caused by TVE. Thus, it may be possible to predict hearing outcomes after SRS for VSs according to the presence of increased intracanalicular pressure, indirectly determined by the ABR value, at the time of SRS.
Post-radiosurgery Auditory Brainstem Response Changes and Hearing Outcome
We continued the analysis of the data to determine whether a change in the intracanalicular pressure after SRS for VSs actually occurs during the period of TVE. Changes in intracanalicular pressure were measured by follow-up ABR tests after SRS. Of 936 patients treated with SRS for VSs between 1997 and 2011, 141 (15.1 %) with unilateral sporadic VSs who had serviceable hearing underwent SRS as a primary treatment and were also given pre-radiosurgery and one or more follow-up ABR tests between 1997 and 2011 (SNUH unpublished data). For patients in whom ABR testing produced no response regardless of the level of hearing, the ABR IL_I–V values were coded in the same manner as mentioned previously. The mean tumour marginal dose was 12.1 Gy, and the mean tumour volume was 1.94 cm3. The mean follow-up duration was 57.6 months. The mean initial PTA of the patients was 25.6 dB. The analyses were performed using the follow-up data obtained within the first 12 months after SRS because TVE usually occurs within 12 months after SRS and because tumour volume increases with TVE, usually reaching a peak at 6 months after SRS and regressing during the 12–24 month period thereafter.
At 6 months post-radiosurgery, 33 (23.7 %, excluding 2 missing data points) patients lost their serviceable hearing (the ‘6-month hearing loss group’). G-R class 1 or class 2 was regarded as serviceable hearing. The mean value of the initial PTA in this group was higher than that of the patients whose serviceable hearing was preserved at 6 months post-radiosurgery (the ‘6-month hearing preservation group’) (34.73 dB vs. 22.59 dB, p < 0.001). The mean values of PTA decreased over time; they were 56.61 dB and 58.22 dB at 6 and 12 months after SRS, respectively, in the ‘6-month hearing loss group’. The mean of the initial ABR IL_I–V values in the ‘6-month hearing loss group’ was also higher than that in the ‘6-month hearing preservation group’ (4.9242 mS vs. 4.7883 mS, p = 0.291); however, the difference did not reach statistical significance. Interestingly, the mean ABR IL_I–V value in the ‘6-month hearing loss group’ increased over time, while that of the ‘6-month hearing preservation group’ increased at 6 months and then decreased to close to the initial level at 12 months after SRS. The difference in the mean ABR IL_I–V values of the two groups reached statistical significance at 12 months (5.2875 mS vs. 4.9427 mS at 6 months, p = 0.060; 5.2905 mS vs. 4.7032 mS at 12 months, p = 0.001). A representative case is illustrated in Table 3 and Fig. 5.
Gamma Knife radiosurgery was performed using a tumour marginal dose of 12 Gy at 50 % isodose line for a 30-year-old woman with a vestibular schwannoma in the right side. The tumour volume of was 1.3 cm3, and the pure-tone audiometry (PTA) threshold and speech discrimination scores (SDS) were 36 dB and 80 %, respectively, corresponding to Gardner-Robertson (G-R) class 2 (a). The interlatency of waves I and V on the auditory brainstem response (ABR IL_I–V) was 4.87 mS before stereotactic radiosurgery (e). Six months later, the tumour volume increased to 1.8 cm3 (b) and the ABR IL_I–V also increased to 5.08 mS (f). However, her hearing function was slightly improved to G-R class 1. Twelve months after radiosurgery, the tumour volume decreased to 1.6 cm3 (c) and interestingly the ABR IL_I–V also decreased to 4.79 mS (g). The hearing function was stable in the improved state as G-R class 1 at post-radiosurgery 12 months. And then the tumour volume gradually decreased until 24 months after radiosurgery to 0.9 cm3 (d)
At 12 months after SRS, 41 (33.6 %, excluding 19 missing data points) patients reached an unserviceable hearing state. Changes in the ABR IL_I–V values of these patients over time showed similar patterns to those found at 6 months. The mean of the initial ABR IL_I–V values in the ‘12-month hearing loss group’ was significantly higher than in the ‘12-month hearing preservation group’ (5.0300 mS vs. 4.7095 mS, p = 0.008). Similar to the trend observed at 6 months, the mean of the ABR IL_I–V values in the ‘12-month hearing loss group’ increased over time; however, that of the ‘12-month hearing preservation group’ increased at 6 months and then decreased below the initial level at 12 months after SRS (5.2413 mS vs. 4.9146 mS at 6 months, p = 0.065; 5.3425 mS vs. 4.6344 mS at 12 months, p < 0.001). The results are summarised in Table 4.
Prognostic factors of hearing outcomes were analysed separately according to the patients’ hearing status at 6 months and at 12 months to determine the most significant time-dependent factors within the first 12 months after SRS. In the repeated analyses, p < 0.01 was regarded as significant. Based on the results of multivariate analysis of the 6-month data, only the initial PTA value was a significant and independent factor for hearing loss (OR = 1.130; 95 % CI, 1.068–1.195; p < 0.001) (SNUH unpublished data). Changes in ABR IL_I–V between the initial and 6-month values also tended to increase the risk of hearing deterioration (OR = 2.038; 95 % CI, 0.952–4.361; p = 0.067); however, this association did not reach statistical significance. The results from the multivariate analysis of 12-month data, the initial PTA (OR = 1.114; 95 % CI, 1.057–1.174; p < 0.001), the initial ABR IL_I–V value (OR = 8.799; 95 % CI, 2.678–28.91; p < 0.001), and the change of ABR IL_I–V between the initial value and the 12-month value (OR = 3.784; 95 % CI, 1.532–9.347; p = 0.004) were all significant and independent factors associated with hearing outcome. The cochlear dose and overall tumour volume did not reach statistical significance in the above two analyses.
In summary, ABR IL_I–V increased during the first 12 months after SRS for VSs in patients who lost their serviceable hearing. Its effect on hearing outcome also became significant over time, especially at 12 months after SRS, and was more prominent in patients with poor initial PTA and/or ABR values. We hypothesise that patients with considerable intracanalicular pressure at the time of SRS are prone to lose their serviceable hearing due to the added intracanalicular pressure resulting from TVE, which usually occurs within the first 12 months after SRS for VSs.
Seoul National University Hospital (SNUH) Classification for Prediction of Hearing Preservation After Stereotactic Radiosurgery for Vestibular Schwannomas
Based on our findings that initial intracanalicular pressure and added pressure during the period of TVE are two principal factors associated with the possible mechanism of hearing loss in patients treated with SRS for VSs, we suggested a classification system for the prediction of hearing preservation after SRS for VSs [36].
The most commonly used hearing level classification systems are the G-R classification and the AAO-HNS guidelines [1, 32], which are based on PTA values and SD score. However, these two classification systems are deficient in the ability to predict the exact rates of hearing preservation among patients treated with SRS for VSs. In fact, 10.9–39.2 % of patients with hearing levels of G-R class 1 lose their hearing after such treatment. Conversely, hearing can be preserved in approximately 50 % of patients who have a hearing level of G-R class 2 after SRS for VSs [52, 55, 108]. These findings cannot be explained only by the patients’ initial hearing levels, which has, however, interestingly been regarded as the most important factor related to hearing outcome after SRS for VSs. In addition, we could not predict which patients with G-R class 1 hearing will lose their hearing or which patients with G-R class 2 hearing can preserve their serviceable hearing after SRS. This suggests that the underlying pathophysiology of hearing deterioration after SRS for VSs may depend on factors other than or in addition to those that determine baseline auditory function [41, 49, 52, 55].
In these circumstances, we determined another prognostic factor, the initial intracanalicular pressure measured as the initial ABR IL_I–V. Based on the two prognostic factors, the initial hearing level and ABR IL_I–V, we could propose a new classification model to predict hearing outcomes after SRS for VSs using classification and regression tree analysis [41].
Three nodes were identified, the first by an initial PTA score of 20 dB, the second by an initial ABR IL_I–V value of 5.225 mS, and the last by an initial PTA score of 30 dB. On the basis of the terminal nodes, we categorised the patients into four groups (Table 5) [41]. The ratios of patients with serviceable hearing at distant follow-up were 89.6 %, 64 %, 25.8 %, and 6.7 % for groups A–D, respectively. These results suggest that VS ears of patients with minimally deteriorated hearing before SRS have adequate space in the IAC for TVE and that the presence of this space can minimise increases in intracanalicular pressure. However, VS ears with considerable initial pressure before SRS, regardless of the hearing level, may not tolerate the increased pressure caused by TVE and may eventually lose their function.
This classification system could be useful in the proper selection of management modalities, especially in patients with only hearing ear schwannoma or neurofibromatosis type 2, because the predictive value is superior to those of previous classification systems. However, further studies should be performed to validate this classification system in a large population (Fig. 6).
The chart illustrates the rates of serviceable hearing preservation in the SNUH classification four groups based on a classification and regression tree analysis of the groups (Adapted from Han et al. [41])
How to Preserve and Aid Hearing After Stereotactic Radiosurgery
The improved hearing level at diagnosis of VSs due to the advancement and easy accessibility of neuroimaging makes preservation of auditory function a matter of concern in the management of patients with VSs. Prediction of hearing preservation in each individual with VSs becomes very important. Especially in cases of neurofibromatosis type 2, hearing preservation is one of the important goals of treatment. Therefore, we should be well aware of the rescue therapies for VS ears that have begun to lose their auditory function.
It is truly a blessing that the incidence of patients with poor hearing levels in the contralateral ear compared with the VS ear is not high; its incidence is approximately 1 % among patients with unilateral sporadic VSs [104], and neurofibromatosis type 2 is not common [85]. Unilateral hearing deterioration has been considered unlikely to contribute to a severe reduction in the quality of life of ordinary people with VSs [23, 35, 47]. However, based on our experience in the management of patients with VSs, most patients with unilateral hearing loss suffer from difficulties in sound localisation and verbal communication under reverberation and background noise due to loss of the benefits of binaural hearing [65, 116]. Therefore, serviceable hearing preservation should be attempted in the management of patients with VSs.
Patient Selection
Proactive treatment of small VSs in patients with good hearing should be considered carefully because of the possibility of deterioration of serviceable hearing in the early period after treatment [103]. From this point of view, the wait-and-see strategy seems to be rational, but its outcome is often not desirable. Because most patients have some degree of hearing deterioration at the diagnosis of VSs, tumour growth during the observation period may directly result in hearing loss [103, 118]. Moreover, data from studies addressing various management approaches, including the wait-and-see strategy, hearing preservation microsurgery, and even SRS for VSs, show that the better the hearing at diagnosis, the greater the chance of hearing preservation [10, 99, 104].
Two important decisions that affect hearing preservation in patients with VSs are the duration for which the wait-and-see strategy is continued and assessment of which patients are good candidates for proactive treatment. Tumour growth has typically been the endpoint of the wait-and-see strategy [8, 118]; however, it is not an ideal endpoint in terms of hearing preservation due to the deterioration of hearing that may occur regardless of tumour growth [104]. Tumour growth should be an indicator of active treatment only in patients whose hearing is already lost at diagnosis.
Less than 20 % of patients with VSs belong to AAO-HNS class 1 (PTA less than 30 dB and SD score more than 70 %), and more than 90 % of such patients already show deteriorated hearing of more than 10-dB PTA difference compared with the normal side [103]. This indicates that most VSs patients are classified as SNUH groups B–D. The tendency to hearing deterioration in VSs patients with minimal hearing loss at diagnosis is a very critical point because the chance of hearing preservation decreases from approximately 90 % to 26 %–64 % once the PTA score increases to over 20 dB, i.e. SNUH classification B–D, at the time of SRS [41]. A hearing level of PTA 20 dB may be used as an indicator of proactive treatment and as a clinical guideline to determine whether to actively treat after the wait-and-see period.
Another possible indicator of hearing outcome is the ABR value before SRS. Hearing outcome is generally very poor in patients treated with SRS for VSs who have ABR IL_I–V values over the threshold of 5.225 mS [41]. This value seems to be a threshold for proactive treatment because proactive treatment in patients with ABR IL_I–V values over 5.225 mS could shorten the duration of serviceable hearing rather than preserve hearing.
In patients with small to large VSs with serviceable hearing, hearing preservation rates after hearing preservation microsurgery range widely (2–93 %) in recent studies depending on a number of parameters, including surgical approach, pretreatment hearing level, tumour size, and nerve of origin [54]. One of the most important points of hearing preservation microsurgery is the need to shorten the learning curve of an individual neurosurgeon or neuro-otologist [99]. Hearing preservation microsurgery is not easy in some cases, especially with gross total resection, because the cranial nerves are often intermingled with the tumour capsule or with the tumour itself [82, 99].
SRS might be a superior tool for hearing preservation considering the shorter learning curve of the modality and the lower chance of damage to the cranial nerves compared to hearing preservation microsurgery. In addition, a recent meta-analysis supports the superiority of SRS in hearing preservation in patients with VSs [76]. Each physician responsible for patients with VSs should apply the most suitable treatment before patients lose their good hearing of 20 dB or less. For patients who already have a hearing level of ≥21 dB, i.e. SNUH class B – D, multimodal or stepwise approaches should be integrated to preserve their serviceable hearing.
Radiosurgical Planning
With respect to radiosurgical planning for VSs, several issues should be considered including tumour marginal dose, distortion of magnetic resonance imaging, and the use of fractionation. The recommended dose to the tumour margin has been decreased to 12–13 Gy to help avoid cranial neuropathies and produce improved tumour control rates [18, 45, 50]. However, one should keep in mind that use of a decreased radiation dose coupled with the possible distortion of magnetic resonance images could cause some VSs to receive less than the optimal dose [92], leading to a failure of tumour control and to hearing loss. The importance of the accuracy and conformity of the prescribed dose is also shown by the fact that tumour control rates have improved with advances in the neuroimaging techniques used in radiosurgical planning, even with a decrease in the tumour marginal dose to approximately half of the dose initially used by Leksell [64]. To avoid the effect of distortion of magnetic resonance imaging on accuracy and conformity, it might be essential to use fused images of magnetic resonance images obtained at high imaging resolution and computed tomography, which has minimal imaging distortion, in radiosurgical planning [60].
Schwannomas are late-responding tissues with low proliferative indices and a low α/β ratio for the application of the linear quadratic formula [30, 66]. From a radiobiological point of view, better tumour control may be achieved with a single high dose of radiation than with multiple smaller doses [67], and normal brain sparing also favours single-fraction treatment for lesions with a low α/β ratio [72]. However, high-dose irradiation using a single fraction may result in a higher incidence of TVE, which may lead to poor hearing outcomes compared with SRT performed using multiple fractionations [111]. Therefore, fractionation techniques for VSs may yield equivalent or improved hearing preservation rates by reducing the chance of TVE [23, 29]. However, no comparative studies of hypo-fractionated SRS using 2–5 fractions vs. low-dose single-fraction SRS have been performed.
How to Manage Transient Volume Expansion and Tumour Growth
TVE is a common phenomenon after SRS for VSs [3, 59, 89]; it is caused by tumour cell necrosis or apoptosis and vascular damage [107]. Loss of central enhancement on magnetic resonance imaging has been regarded as a representative neuroimaging feature of TVE and as a reliable indicator of tumour control [20, 70]. However, this is not always the case [111]. It is important to differentiate TVE from real tumour growth. The apparent diffusion coefficient values may be useful in differentiating TVE from tumour growth; however, their use is limited in VSs in the IAC [19].
As mentioned above, TVE may result in increased intracanalicular pressure and compression of the cochlear nerve, which can ultimately result in hearing deterioration, especially in patients in whom the IAC of the schwannoma ear does not have adequate space to buffer the increased intracanalicular pressure, i.e. SNUH class D patients. Currently, there are no proven rescue management approaches for VS patients showing hearing deterioration after SRS. Nonetheless, several possible methods to preserve or aid hearing in such patients are reviewed and discussed.
Corticosteroids
Although the specific action of steroids on the auditory apparatus is uncertain, their use has been based on their ability to decrease inflammatory reactions and neural oedema [4, 96, 117]. Thus, steroids might reduce compression of the acoustic apparatus and vascular structures by TVE after SRS for VSs [4, 56, 78, 96]. Notably, Sakamoto et al. [96] reported that hearing recovery occurred in all 8 patients who had experienced a hearing loss within 1 year after SRT for VSs. These patients were treated with a mean 30-mg daily dose of prednisone for approximately 2 weeks, and the degree of hearing recovery was approximately 10 dB.
However, our data reflect a somewhat different finding. Based on our experience, the administration of corticosteroids merely alleviates the deterioration in hearing compared with the historical control group [56]; in our study, the mean PTA score of the treated patients was significantly lower than that of the control group at distant follow-up. The difference in the mean PTA was approximately 12 dB; nonetheless, no benefit was gained in terms of serviceable hearing preservation. These conflicting results might be caused by differences in the initial PTA scores and in the patients’ ages in the Hokkaido University series and our series; mean values for these parameters were 24 dB vs. 30 dB and 39 years vs. 49 years, respectively.
Two possibly more important points relevant to the different findings of these two studies are the method and timing of detection of hearing deterioration after SRS. The Hokkaido University group did not use corticosteroids in patients who already had practically useless hearing levels; in these patients, very low hearing levels were regularly found at every 3-month-interval follow-up evaluation. In contrast, we advised patients to immediately visit our clinic when they experienced a decrease in hearing level, and corticosteroids were given to all patients regardless of their hearing levels. The range of the PTA scores at visiting our clinic was wide, from 32 to 60 dB. The administration of corticosteroids might play a role in hearing preservation if it is performed for the appropriate patients (those with relatively good hearing) at the appropriate time, when hearing deterioration just begins. Recently developed smartphone-based ear-level pure-tone hearing test applications will make future studies more reliable and efficient [42].
According to our data on post-radiosurgery ABR changes, the mean of the ABR IL_I–V values in the post-radiosurgery 12-month unserviceable hearing group increased over time; however, that of the post-radiosurgery 12-month serviceable hearing group increased at 6 months and then decreased below the initial level at 12 months after SRS (5.2413 mS vs. 4.9146 mS at 6 months, p = 0.065; 5.3425 mS vs. 4.6344 mS at 12 months, p < 0.001). A similar finding was obtained in the analysis of the post-radiosurgery 6-month unserviceable and serviceable groups. This suggests that there might be a threshold of intracanalicular pressure as measured by the ABR value over which the hearing level cannot be restored; based on our data, this threshold could be near an ABR IL_I–V value of 5.000–5.225 mS.
Another point is whether a short-term (usually 2 weeks) use of corticosteroids can render the auditory apparatus able to tolerate a nearly 1-year period of standing pressure caused by TVE [4, 78, 96]. The dose of corticosteroids also should be evaluated and further compared with the doses used in patients with idiopathic sudden hearing loss [7, 117].
Decompression of the IAC
In terms of rescue therapy for hearing preservation after TVE, decompression of the IAC is another possible option. Slattery et al. [102] reported successful clinical outcomes of middle fossa decompression for hearing preservation in patients with only hearing ear schwannomas, including more than 90 % of neurofibromatosis type 2 patients. The use of this procedure preserved the hearing level in patients who had exhibited significant declines in hearing for a mean duration of approximately 2 years before being considered for middle fossa decompression. In addition, decompression can delay the need for further treatments that could result in the loss of hearing for over 40 months [31, 102]. Additionally, it is of great significance that most of the patients maintained serviceable hearing, though clinically insignificant loss of hearing developed, seemingly caused by surgical injury to the auditory apparatus.
Of note, patients who failed to preserve their preoperative hearing class apparently had poor hearing levels (≥50 dB) compared with a mean PTA of 40 dB in other patients [102]. A close reading of our SNUH classification reveals that the difference in hearing preservation rates between class B and C is large [41], which suggests a probable threshold PTA score between 30 and 50 dB. ABR values could provide additional information on decisions regarding the timing of intervention.
The effect of decompression of the IAC, which leaves the tumour itself, eventually abates in approximately 2 years because pathologies related to hearing loss, as well as tumour growth, progress over time after surgery [95, 106]. TVE has been known to persist for 1 or 2 years after its development [3, 46, 53, 74, 80, 91]. Therefore, the effect of decompression of the IAC for increased pressure due to TVE after SRS might be sufficient to produce a semipermanent effect.
In cases that show no response to corticosteroids for hearing deterioration due to TVE, decompression of the IAC could be considered. The middle fossa approach is safe and offers a better quality of postoperative hearing after resection of VSs than other approaches [84]; however, selection of the approach primarily depends on individual anatomical considerations and the surgeons’ experience.
Chemotherapy
In patients whose tumour size increases not due to TVE but by recurrence, there are not many management options for hearing preservation. Decompression of the IAC with or without internal debulking of growing tumours is one, and systemic chemotherapy might be another. Recently, the expression of vascular endothelial growth factor (VEGF) in schwannoma cells has been demonstrated especially in neurofibromatosis type 2 cases, and morphometric analysis has revealed a greater microvascular density, a larger vessel diameter, and a larger perimeter in schwannomas than in normal nerves [90]. Anti-VEGF therapy normalises the vasculature of schwannomas and successfully controls the growth of these tumours in an animal model, most likely by re-establishing a natural balance between VEGF and semaphorin 3 signalling [119]. In humans, anti-VEGF therapy reduced the volume of growing VSs in nine of ten neurofibromatosis type 2 patients, improved hearing function in four patients, and stabilised it in two of seven patients [25, 90].
Patients with a hearing response who were on anti-VEGF therapy showed progressive improvement in word recognition; this improvement usually began approximately 8 weeks after the initiation of chemotherapy and continued to improve for as long as 16 months [90]. The improved hearing was robust for 11–16 months with a median duration of treatment of 12 months (range, 3–19). The mechanism of improvement seems to be reduction in intraneural oedema as well as tumour shrinkage. Of 9 tumours that shrank after anti-VEGF treatment, six had an imaging response. Interestingly, a strong correlation was observed between the mean apparent diffusion coefficient at baseline within tumours and the per cent decrease in tumour volume at 3 months, which could provide a potential neuroimaging marker for volumetric response to anti-VEGF therapy [90]. However, VEGF-mediated angiogenesis in schwannomas is not yet fully understood, and drawbacks to this therapy are that it is limited in neurofibromatosis type 2 patients and lengthy and expensive until now.
Hearing Aids
In patients whose hearing deterioration continues over a possible threshold of the ABR IL_I–V, the use of corticosteroids, decompression of the IAC, and anti-VEGF therapy may rescue their hearing. Notwithstanding these rescue efforts, serviceable hearing may be lost eventually. In these situations, hearing aids could be helpful in improving the quality of daily life. There are several types of hearing aids that can be considered for patients with hearing loss after treatment for VSs, including a bone-anchored hearing aid (BAHA), cochlear implants, and auditory brainstem implants.
BAHA, a new type of bone conduction hearing device, has now become widely accepted for patients with conductive or mixed hearing loss as an alternative to a conventional air-conduction hearing aid [26]. Although directional hearing remains a problem for BAHA patients with unilateral hearing loss, its application has become popular [26, 65].
To place a cochlear implant, VS patients should undergo microsurgery with preservation of the cochlear nerve. However, neurofibromatosis-related schwannomas usually invade and grow within the cochlear nerve [68], and sporadic schwannomas may present the same situation as they grow. Identification of the surgical plane between the tumour and the cochlear nerve is demanding [97, 99, 100]; moreover, cochlear nerve function is not always preserved in spite of anatomical preservation of the nerve [79]. Nonetheless, the results of cochlear implantation in VS patients are promising [13, 15]. Approximately 70 % of patients achieve open-set speech discrimination, many scoring at the ceiling of audiometric testing [13]. This implies that a moderate injury that can cause hearing loss may still allow electrical transmission of the stimulus. Such transmission can be evaluated by electrical promontory stimulation [13, 15]. It is also interesting that cochlear implantation after SRS or SRT showed good results in a select group of patients with VSs [71, 110].
Lack of bilateral auditory function should be considered an indication for auditory brainstem implantation. Especially in neurofibromatosis type 2 patients, auditory brainstem implantation directly after tumour removal is a safe procedure and offers the best means of hearing rehabilitation if the cochlear nerve is not preserved [21, 101]. However, the results in neurofibromatosis type 2 cases in the literature are poor compared with the results of cochlear implantation [2, 15, 21, 87, 101, 110]. If a cochlear implant is possible, it is preferable compared to auditory brainstem implantation. The majority of indicated patients have benefitted from auditory brainstem implantation during daily life, particularly in combination with lip reading [101].
Conclusion
Advances in diagnostic tools and treatment modalities and the optimisation of radiosurgical dose have improved clinical outcomes, including tumour control and cranial neuropathies, in patients with VSs. However, preservation of hearing function still falls short of our expectations. A prediction model for the hearing preservation potential of each treatment modality will guide the proper selection of treatment modalities and the appropriate timing of active treatment, which will lead to improved hearing function in patients with VSs. In particular, patients with pre-radiosurgery good hearing levels and favourable initial values of the ABR test may have excellent hearing outcomes after SRS for VSs.
Notwithstanding these findings, most neurosurgeons and neurologists are still reluctant to recommend treatment until hearing deteriorates or is lost. This approach, of course, could result in a self-perpetuating cycle of poor clinical hearing outcome. Most studies, including natural history data, hearing preservation microsurgery data, and hearing outcome data after SRS, have shown that early management of VSs in patients with good hearing level can result in good hearing outcomes.
References
Committee on Hearing and Equilibrium guidelines for the evaluation of hearing preservation in acoustic neuroma (vestibular schwannoma). American Academy of Otolaryngology-Head and Neck Surgery Foundation, INC (1995) Otolaryngol Head Neck Surg 113:179–180
Amoodi HA, Makki FM, Cavanagh J, Maessen H, Bance M (2012) Cochlear implant rehabilitation for patients with vestibular schwannoma: report of two cases. Cochlear Implants Int 13:124–127
Aoyama H, Onodera S, Takeichi N, Onimaru R, Terasaka S, Sawamura Y, Shirato H (2013) Symptomatic outcomes in relation to tumor expansion after fractionated stereotactic radiation therapy for vestibular schwannomas: single-institutional long-term experience. Int J Radiat Oncol Biol Phys 85:329–334
Aronzon A, Ruckenstein MJ, Bigelow DC (2003) The efficacy of corticosteroids in restoring hearing in patients undergoing conservative management of acoustic neuromas. Otol Neurotol 24:465–468
Asthagiri AR, Vasquez RA, Butman JA, Wu T, Morgan K, Brewer CC, King K, Zalewski C, Kim HJ, Lonser RR (2012) Mechanisms of hearing loss in neurofibromatosis type 2. PLoS One 7, e46132
Badie B, Pyle GM, Nguyen PH, Hadar EJ (2001) Elevation of internal auditory canal pressure by vestibular schwannomas. Otol Neurotol 22:696–700
Bae SC, Noh HI, Jun BC, Jeon EJ, Seo JH, Park SY, Kim JK, Lee DH, Oh JH, Park SN, Yeo SW (2013) Efficacy of intratympanic steroid therapy for idiopathic sudden sensorineural hearing loss: comparison with systemic steroid therapy and combined therapy. Acta Otolaryngol 133:428–433
Bakkouri WE, Kania RE, Guichard JP, Lot G, Herman P, Huy PT (2009) Conservative management of 386 cases of unilateral vestibular schwannoma: tumor growth and consequences for treatment. J Neurosurg 110:662–669
Baschnagel AM, Chen PY, Bojrab D, Pieper D, Kartush J, Didyuk O, Naumann IC, Maitz A, Grills IS (2013) Hearing preservation in patients with vestibular schwannoma treated with Gamma Knife surgery. J Neurosurg 118:571–578
Brackmann DE, Owens RM, Friedman RA, Hitselberger WE, De la Cruz A, House JW, Nelson RA, Luxford WM, Slattery WH 3rd, Fayad JN (2000) Prognostic factors for hearing preservation in vestibular schwannoma surgery. Am J Otol 21:417–424
Brada M (2013) Radiotherapy for benign brain tumours coming of age; example of vestibular schwannoma. Radiother Oncol 106:157–160
Bradley Lega JS, Michael Ruckinstein, Douglas Bigelow, Jay Dorsey, Michele Alonso-Basanta, John YK Lee (2012) Cochlear radiation dose does not predict hearing loss after gamma knife for glomus jugulare tumors. The 16th International Leksell Gamma Knife Society meeting Sydney
Carlson ML, Breen JT, Driscoll CL, Link MJ, Neff BA, Gifford RH, Beatty CW (2012) Cochlear implantation in patients with neurofibromatosis type 2: variables affecting auditory performance. Otol Neurotol 33:853–862
Carlson ML, Jacob JT, Pollock BE, Neff BA, Tombers NM, Driscoll CL, Link MJ (2013) Long-term hearing outcomes following stereotactic radiosurgery for vestibular schwannoma: patterns of hearing loss and variables influencing audiometric decline. J Neurosurg 118:579–587
Celis-Aguilar E, Lassaletta L, Gavilan J (2012) Cochlear implantation in patients with neurofibromatosis type 2 and patients with vestibular schwannoma in the only hearing ear. Int J Otolaryngol 2012:157497
Chen CH, Shen CC, Sun MH, Ho WL, Huang CF, Kwan PC (2007) Histopathology of radiation necrosis with severe peritumoral edema after gamma knife radiosurgery for parasagittal meningioma. A report of two cases. Stereotact Funct Neurosurg 85:292–295
Chinn J, Miller J (1975) Animal model acoustic neuroma. Arch Otolaryngol 101:222–226
Chopra R, Kondziolka D, Niranjan A, Lunsford LD, Flickinger JC (2007) Long-term follow-up of acoustic schwannoma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol Biol Phys 68:845–851
Chuang CC, Chang CS, Tyan YS, Chuang KS, Tu HT, Huang CF (2012) Use of apparent diffusion coefficients in evaluating the response of vestibular schwannomas to Gamma Knife surgery. J Neurosurg 117(Suppl):63–68
Chung WY, Liu KD, Shiau CY, Wu HM, Wang LW, Guo WY, Ho DM, Pan DH (2005) Gamma knife surgery for vestibular schwannoma: 10-year experience of 195 cases. J Neurosurg 102(Suppl):87–96
Colletti L, Shannon R, Colletti V (2012) Auditory brainstem implants for neurofibromatosis type 2. Curr Opin Otolaryngol Head Neck Surg 20:353–357
Combs SE, Thilmann C, Debus J, Schulz-Ertner D (2006) Long-term outcome of stereotactic radiosurgery (SRS) in patients with acoustic neuromas. Int J Radiat Oncol Biol Phys 64:1341–1347
Combs SE, Welzel T, Kessel K, Habermehl D, Rieken S, Schramm O, Debus J (2013) Hearing preservation after radiotherapy for vestibular schwannomas is comparable to hearing deterioration in healthy adults and is accompanied by local tumor control and a highly preserved quality of life (QOL) as patients’ self-reported outcome. Radiother Oncol 106:175–180
Dandy WE (1925) An operation for the total removal of cerebellopontine (acoustic) tumors. Surg Gynecol Obstet 41:129–148
Eminowicz GK, Raman R, Conibear J, Plowman PN (2012) Bevacizumab treatment for vestibular schwannomas in neurofibromatosis type two: report of two cases, including responses after prior gamma knife and vascular endothelial growth factor inhibition therapy. J Laryngol Otol 126:79–82
Faber HT, de Wolf MJ, Cremers CW, Snik AF, Hol MK (2013) Benefit of Baha in the elderly with single-sided deafness. Eur Arch Otorhinolaryngol 270:1285–1291
Ferri GG, Modugno GC, Pirodda A, Fioravanti A, Calbucci F, Ceroni AR (2008) Conservative management of vestibular schwannomas: an effective strategy. Laryngoscope 118:951–957
Flickinger JC (2001) Cranial nerves. In: Shrieve DC (ed) Human radiation injury. Lippincott Williams & Wilkins, a Wolters Kluwer business, Philadelphia
Fong BM, Pezeshkian P, Nagasawa DT, De Salles A, Gopen Q, Yang I (2012) Hearing preservation after LINAC radiosurgery and LINAC radiotherapy for vestibular schwannoma. J Clin Neurosci 19:1065–1070
Fowler JF (1989) The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol 62:679–694
Gadre AK, Kwartler JA, Brackmann DE, House WF, Hitselberger WE (1990) Middle fossa decompression of the internal auditory canal in acoustic neuroma surgery: a therapeutic alternative. Laryngoscope 100:948–952
Gardner G, Robertson JH (1988) Hearing preservation in unilateral acoustic neuroma surgery. Ann Otol Rhinol Laryngol 97:55–66
Gerosa M, Mesiano N, Longhi M, De Simone A, Foroni R, Verlicchi A, Zanotti B, Nicolato A (2010) Gamma Knife surgery in vestibular schwannomas: impact on the anterior and posterior labyrinth. J Neurosurg 113(Suppl):128–135
Givre A, Olivecrona H (1949) Surgical experiences with acoustic tumors. J Neurosurg 6:396–407
Godefroy WP, Kaptein AA, Vogel JJ, van der Mey AG (2009) Conservative treatment of vestibular schwannoma: a follow-up study on clinical and quality-of-life outcome. Otol Neurotol 30:968–974
Grayeli AB, Kalamarides M, Ferrary E, Bouccara D, Elgharem H, Rey A, Sterkers O (2005) Conservative management versus surgery for small vestibular schwannomas. Acta Otolaryngol 125:1063–1068
Greiman MC, Lusk RP (1991) Pressure-induced modifications of the acoustic nerve. Part II: Auditory brain stem responses. Am J Otolaryngol 12:12–19
Hajioff D, Raut VV, Walsh RM, Bath AP, Bance ML, Guha A, Tator CH, Rutka JA (2008) Conservative management of vestibular schwannomas: third review of a 10-year prospective study. Clin Otolaryngol 33:255–259
Han JH, Kim DG, Chung HT, Paek SH, Kim YH, Kim CY, Kim JW, Kim YH, Jeong SS (2009) Long-term outcome of gamma knife radiosurgery for treatment of typical trigeminal neuralgia. Int J Radiat Oncol Biol Phys 75:822–827
Han JH, Kim DG, Chung HT, Paek SH, Park CK, Kim CY, Hwang SS, Park JH, Kim YH, Kim JW, Kim YH, Song SW, Kim IK, Jung HW (2012) The risk factors of symptomatic communicating hydrocephalus after stereotactic radiosurgery for unilateral vestibular schwannoma: the implication of brain atrophy. Int J Radiat Oncol Biol Phys 84:937–942
Han JH, Kim DG, Chung HT, Paek SH, Park CK, Kim CY, Kim YH, Kim JW, Kim YH, Song SW, Kim IK, Jung HW (2012) Hearing preservation in patients with unilateral vestibular schwannoma who undergo stereotactic radiosurgery: reinterpretation of the auditory brainstem response. Cancer 118:5441–5447
Handzel O, Ben-Ari O, Damian D, Priel MM, Cohen J, Himmelfarb M (2013) Smartphone-based hearing test as an aid in the initial evaluation of unilateral sudden sensorineural hearing loss. Audiol Neurootol 18:201–207
Hasegawa T, Kida Y, Kato T, Iizuka H, Kuramitsu S, Yamamoto T (2013) Long-term safety and efficacy of stereotactic radiosurgery for vestibular schwannomas: evaluation of 440 patients more than 10 years after treatment with Gamma Knife surgery. J Neurosurg 118:557–565
Hasegawa T, Kida Y, Kato T, Iizuka H, Yamamoto T (2011) Factors associated with hearing preservation after Gamma Knife surgery for vestibular schwannomas in patients who retain serviceable hearing. J Neurosurg 115:1078–1086
Hasegawa T, Kida Y, Kobayashi T, Yoshimoto M, Mori Y, Yoshida J (2005) Long-term outcomes in patients with vestibular schwannomas treated using gamma knife surgery: 10-year follow up. J Neurosurg 102:10–16
Hasegawa T, Kida Y, Yoshimoto M, Koike J, Goto K (2006) Evaluation of tumor expansion after stereotactic radiosurgery in patients harboring vestibular schwannomas. Neurosurgery 58:1119–1128; discussion 1119–1128
Hietanen A, Era P, Sorri M, Heikkinen E (2004) Changes in hearing in 80-year-old people: a 10-year follow-up study. Int J Audiol 43:126–135
Huang E, Strother DR, Davis QG, Chiu JK, Lu HH, Carpenter LS, Mai WY, Chintagumpala MM, South M, Grant WH, Butler EB, Woo SY (2002) Intensity-modulated radiation therapy for pediatric medulloblastoma: early report on the reduction of ototoxicity. Int J Radiat Oncol Biol Phys 52:599–605
Iwai Y, Yamanaka K, Kubo T, Aiba T (2008) Gamma knife radiosurgery for intracanalicular acoustic neuromas. J Clin Neurosci 15:993–997
Iwai Y, Yamanaka K, Shiotani M, Uyama T (2003) Radiosurgery for acoustic neuromas: results of low-dose treatment. Neurosurgery 53:282–287; discussion 287–288
Iwai Y, Yamanaka K, Yamagata K, Yasui T (2007) Surgery after radiosurgery for acoustic neuromas: surgical strategy and histological findings. Neurosurgery 60:ONS75–ONS82; discussion ONS82
Kano H, Kondziolka D, Khan A, Flickinger JC, Lunsford LD (2009) Predictors of hearing preservation after stereotactic radiosurgery for acoustic neuroma. J Neurosurg 111:863–873
Kapoor S, Batra S, Carson K, Shuck J, Kharkar S, Gandhi R, Jackson J, Wemmer J, Terezakis S, Shokek O, Kleinberg L, Rigamonti D (2011) Long-term outcomes of vestibular schwannomas treated with fractionated stereotactic radiotherapy: an institutional experience. Int J Radiat Oncol Biol Phys 81:647–653
Kari E, Friedman RA (2012) Hearing preservation: microsurgery. Curr Opin Otolaryngol Head Neck Surg 20:358–366
Kim CH, Chung KW, Kong DS, Nam DH, Park K, Kim JH, Hong SH, Cho YS, Chung WH, Lee JI (2010) Prognostic factors of hearing preservation after gamma knife radiosurgery for vestibular schwannoma. J Clin Neurosci 17:214–218
Kim JW, Kim DG, Paek SH, Chung HT, Kim YH, Han JH, Park CK, Jung HW (2011) Efficacy of corticosteroids in hearing preservation after radiosurgery for vestibular schwannoma: a prospective study. Stereotact Funct Neurosurg 89:25–33
Kim KM, Park CK, Chung HT, Paek SH, Jung HW, Kim DG (2007) Long-term outcomes of gamma knife stereotactic radiosurgery of vestibular schwannomas. J Korean Neurosurg Soc 42:286–292
Kim YH, Kim DG, Han JH, Chung HT, Kim IK, Song SW, Park JH, Kim JW, Kim YH, Park CK, Kim CY, Paek SH, Jung HW (2012) Radiosurgery for para-IAC meningiomas: the effect of radiation dose to the cochlea on hearing outcome. Int J Radiat Oncol Biol Phys 84:675–680
Kim YH, Kim DG, Han JH, Chung HT, Kim IK, Song SW, Park JH, Kim JW, Kim YH, Park CK, Kim CY, Paek SH, Jung HW (2013) Hearing outcomes after stereotactic radiosurgery for unilateral intracanalicular vestibular schwannomas: implication of transient volume expansion. Int J Radiat Oncol Biol Phys 85:61–67
Landi A, Marina R, DeGrandi C, Crespi A, Montanari G, Sganzerla EP, Gaini SM (2001) Accuracy of stereotactic localisation with magnetic resonance compared to CT scan: experimental findings. Acta Neurochir (Wien) 143:593–601
Lapsiwala SB, Pyle GM, Kaemmerle AW, Sasse FJ, Badie B (2002) Correlation between auditory function and internal auditory canal pressure in patients with vestibular schwannomas. J Neurosurg 96:872–876
Lee SR, Yang KA, Kim SK, Kim SH (2012) Radiation-induced intratumoral necrosis and peritumoral edema after gamma knife radiosurgery for intracranial meningiomas. J Korean Neurosurg Soc 52:98–102
Leksell L (1951) The stereotaxic method and radiosurgery of the brain. Acta Chir Scand 102:316–319
Leksell L (1971) A note on the treatment of acoustic tumours. Acta Chir Scand 137:763–765
Lin LM, Bowditch S, Anderson MJ, May B, Cox KM, Niparko JK (2006) Amplification in the rehabilitation of unilateral deafness: speech in noise and directional hearing effects with bone-anchored hearing and contralateral routing of signal amplification. Otol Neurotol 27:172–182
Linskey ME (2000) Stereotactic radiosurgery versus stereotactic radiotherapy for patients with vestibular schwannoma: a Leksell Gamma Knife Society 2000 debate. J Neurosurg 93(Suppl 3):90–95
Linskey ME (2008) Hearing preservation in vestibular schwannoma stereotactic radiosurgery: what really matters? J Neurosurg 109(Suppl):129–136
Linthicum FH Jr, Brackmann DE (1980) Bilateral acoustic tumors. A diagnostic and surgical challenge. Arch Otolaryngol 106:729–733
Litre F, Rousseaux P, Jovenin N, Bazin A, Peruzzi P, Wdowczyk D, Colin P (2013) Fractionated stereotactic radiotherapy for acoustic neuromas: a prospective monocenter study of about 158 cases. Radiother Oncol 106:169–174
Lunsford LD, Kondziolka D, Maitz A, Flickinger JC (1998) Black holes, white dwarfs and supernovas: imaging after radiosurgery. Stereotact Funct Neurosurg 70(Suppl 1):2–10
Lustig LR, Yeagle J, Driscoll CL, Blevins N, Francis H, Niparko JK (2006) Cochlear implantation in patients with neurofibromatosis type 2 and bilateral vestibular schwannoma. Otol Neurotol 27:512–518
Ma L, Sahgal A, Descovich M, Cho YB, Chuang C, Huang K, Laperriere NJ, Shrieve DC, Larson DA (2010) Equivalence in dose fall-off for isocentric and nonisocentric intracranial treatment modalities and its impact on dose fractionation schemes. Int J Radiat Oncol Biol Phys 76:943–948
Malhotra PS, Sharma P, Fishman MA, Grumbine FL, Tholey R, Dam VQ, Dasgupta A, Pequignot E, Willcox TO (2009) Clinical, radiographic, and audiometric predictors in conservative management of vestibular schwannoma. Otol Neurotol 30:507–514
Mandl ES, Meijer OW, Slotman BJ, Vandertop WP, Peerdeman SM (2010) Stereotactic radiation therapy for large vestibular schwannomas. Radiother Oncol 95:94–98
Maniakas A, Saliba I (2012) Conservative management versus stereotactic radiation for vestibular schwannomas: a meta-analysis of patients with more than 5 years’ follow-up. Otol Neurotol 33:230–238
Maniakas A, Saliba I (2012) Microsurgery versus stereotactic radiation for small vestibular schwannomas: a meta-analysis of patients with more than 5 years’ follow-up. Otol Neurotol 33:1611–1620
Massager N, Nissim O, Delbrouck C, Delpierre I, Devriendt D, Desmedt F, Wikler D, Brotchi J, Levivier M (2007) Irradiation of cochlear structures during vestibular schwannoma radiosurgery and associated hearing outcome. J Neurosurg 107:733–739
Massager N, Nissim O, Delbrouck C, Devriendt D, David P, Desmedt F, Wikler D, Hassid S, Brotchi J, Levivier M (2006) Role of intracanalicular volumetric and dosimetric parameters on hearing preservation after vestibular schwannoma radiosurgery. Int J Radiat Oncol Biol Phys 64:1331–1340
Merkus P, Lella FD, Trapani GD, Pasanisi E, Beltrame MA, Zanetti D, Negri M, Sanna M (2014) Indications and contraindications of auditory brainstem implants: systematic review and illustrative cases. Eur Arch Otorhinolaryngol 271:3–13
Nagano O, Higuchi Y, Serizawa T, Ono J, Matsuda S, Yamakami I, Saeki N (2008) Transient expansion of vestibular schwannoma following stereotactic radiosurgery. J Neurosurg 109:811–816
Niranjan A, Mathieu D, Flickinger JC, Kondziolka D, Lunsford LD (2008) Hearing preservation after intracanalicular vestibular schwannoma radiosurgery. Neurosurgery 63:1054–1062; discussion 1062–1053
Nonaka Y, Fukushima T, Watanabe K, Friedman AH, Sampson JH, McElveen JT Jr, Cunningham CD 3rd, Zomorodi AR (2013) Contemporary surgical management of vestibular schwannomas: analysis of complications and lessons learned over the past decade. Neurosurgery 72:ons103–ons115; discussion ons115
Northfield DW (1970) Surgical treatment of acoustic neurinoma. The conventional approach. Proc R Soc Med 63:769–775
Noudel R, Gomis P, Duntze J, Marnet D, Bazin A, Roche PH (2009) Hearing preservation and facial nerve function after microsurgery for intracanalicular vestibular schwannomas: comparison of middle fossa and retrosigmoid approaches. Acta Neurochir (Wien) 151:935–944; discussion 944–935
Odat HA, Piccirillo E, Sequino G, Taibah A, Sanna M (2011) Management strategy of vestibular schwannoma in neurofibromatosis type 2. Otol Neurotol 32:1163–1170
Paek SH, Chung HT, Jeong SS, Park CK, Kim CY, Kim JE, Kim DG, Jung HW (2005) Hearing preservation after gamma knife stereotactic radiosurgery of vestibular schwannoma. Cancer 104:580–590
Pai I, Dhar V, Kelleher C, Nunn T, Connor S, Jiang D, O’Connor AF (2013) Cochlear implantation in patients with vestibular schwannoma: a single United Kingdom center experience. Laryngoscope 123:2019–2023
Pennybacker JB, Cairns H (1950) Results in 130 cases of acoustic neurinoma. J Neurol Neurosurg Psychiatry 13:272–277
Phi JH, Paek SH, Chung HT, Jeong SS, Park CK, Jung HW, Kim DG (2007) Gamma Knife surgery and trigeminal schwannoma: is it possible to preserve cranial nerve function? J Neurosurg 107:727–732
Plotkin SR, Stemmer-Rachamimov AO, Barker FG 2nd, Halpin C, Padera TP, Tyrrell A, Sorensen AG, Jain RK, di Tomaso E (2009) Hearing improvement after bevacizumab in patients with neurofibromatosis type 2. N Engl J Med 361:358–367
Pollock BE (2006) Management of vestibular schwannomas that enlarge after stereotactic radiosurgery: treatment recommendations based on a 15 year experience. Neurosurgery 58:241–248; discussion 241–248
Pollock BE, Link MJ, Foote RL (2009) Failure rate of contemporary low-dose radiosurgical technique for vestibular schwannoma. J Neurosurg 111:840–844
Ramsden RT (1995) The bloody angle: 100 years of acoustic neuroma surgery. J R Soc Med 88:464–468
Raut VV, Walsh RM, Bath AP, Bance ML, Guha A, Tator CH, Rutka JA (2004) Conservative management of vestibular schwannomas – second review of a prospective longitudinal study. Clin Otolaryngol Allied Sci 29:505–514
Rosenberg SI (2000) Natural history of acoustic neuromas. Laryngoscope 110:497–508
Sakamoto T, Shirato H, Takeichi N, Aoyama H, Kagei K, Nishioka T, Fukuda S (2001) Medication for hearing loss after fractionated stereotactic radiotherapy (SRT) for vestibular schwannoma. Int J Radiat Oncol Biol Phys 50:1295–1298
Samii M (1995) Hearing preservation in bilateral acoustic neurinomas. Br J Neurosurg 9:413–424
Samii M, Gerganov V, Samii A (2008) Hearing preservation after complete microsurgical removal in vestibular schwannomas. Prog Neurol Surg 21:136–141
Samii M, Matthies C (1997) Management of 1000 vestibular schwannomas (acoustic neuromas): hearing function in 1000 tumor resections. Neurosurgery 40:248–260; discussion 260–242
Samii M, Matthies C, Tatagiba M (1997) Management of vestibular schwannomas (acoustic neuromas): auditory and facial nerve function after resection of 120 vestibular schwannomas in patients with neurofibromatosis 2. Neurosurgery 40:696–705; discussion 705–696
Sanna M, Di Lella F, Guida M, Merkus P (2012) Auditory brainstem implants in NF2 patients: results and review of the literature. Otol Neurotol 33:154–164
Slattery WH, Hoa M, Bonne N, Friedman RA, Schwartz MS, Fisher LM, Brackmann DE (2011) Middle fossa decompression for hearing preservation: a review of institutional results and indications. Otol Neurotol 32:1017–1024
Stangerup SE, Caye-Thomasen P (2012) Epidemiology and natural history of vestibular schwannomas. Otolaryngol Clin North Am 45(257–268):vii
Stangerup SE, Caye-Thomasen P, Tos M, Thomsen J (2008) Change in hearing during ‘wait and scan’ management of patients with vestibular schwannoma. J Laryngol Otol 122:673–681
Sughrue ME, Kaur R, Kane AJ, Rutkowski MJ, Yang I, Pitts LH, Tihan T, Parsa AT (2011) Intratumoral hemorrhage and fibrosis in vestibular schwannoma: a possible mechanism for hearing loss. J Neurosurg 114:386–393
Sughrue ME, Yang I, Aranda D, Lobo K, Pitts LH, Cheung SW, Parsa AT (2010) The natural history of untreated sporadic vestibular schwannomas: a comprehensive review of hearing outcomes. J Neurosurg 112:163–167
Szeifert GT, Figarella-Branger D, Roche PH, Regis J (2004) Histopathological observations on vestibular schwannomas after Gamma Knife radiosurgery: the Marseille experience. Neurochirurgie 50:327–337
Thomas C, Di Maio S, Ma R, Vollans E, Chu C, Math M, Clark B, Lee R, McKenzie M, Martin M, Toyota B (2007) Hearing preservation following fractionated stereotactic radiotherapy for vestibular schwannomas: prognostic implications of cochlear dose. J Neurosurg 107:917–926
Timmer FC, Hanssens PE, van Haren AE, Mulder JJ, Cremers CW, Beynon AJ, van Overbeeke JJ, Graamans K (2009) Gamma knife radiosurgery for vestibular schwannomas: results of hearing preservation in relation to the cochlear radiation dose. Laryngoscope 119:1076–1081
Trotter MI, Briggs RJ (2010) Cochlear implantation in neurofibromatosis type 2 after radiation therapy. Otol Neurotol 31:216–219
van de Langenberg R, Dohmen AJ, de Bondt BJ, Nelemans PJ, Baumert BG, Stokroos RJ (2012) Volume changes after stereotactic LINAC radiotherapy in vestibular schwannoma: control rate and growth patterns. Int J Radiat Oncol Biol Phys 84:343–349
van Eck AT, Horstmann GA (2005) Increased preservation of functional hearing after gamma knife surgery for vestibular schwannoma. J Neurosurg 102(Suppl):204–206
Varughese JK, Wentzel-Larsen T, Vassbotn F, Moen G, Lund-Johansen M (2010) Analysis of vestibular schwannoma size in multiple dimensions: a comparative cohort study of different measurement techniques. Clin Otolaryngol 35:97–103
Vokurka EA, Herwadkar A, Thacker NA, Ramsden RT, Jackson A (2002) Using Bayesian tissue classification to improve the accuracy of vestibular schwannoma volume and growth measurement. AJNR Am J Neuroradiol 23:459–467
Wanibuchi M, Fukushima T, Friedman AH, Watanabe K, Akiyama Y, Mikami T, Iihoshi S, Murakami T, Sugino T, Mikuni N (2014) Hearing preservation surgery for vestibular schwannomas via the retrosigmoid transmeatal approach: surgical tips. Neurosurg Rev 37:431–444
Wazen JJ, Spitzer JB, Ghossaini SN, Fayad JN, Niparko JK, Cox K, Brackmann DE, Soli SD (2003) Transcranial contralateral cochlear stimulation in unilateral deafness. Otolaryngol Head Neck Surg 129:248–254
Wei BP, Stathopoulos D, O’Leary S (2013) Steroids for idiopathic sudden sensorineural hearing loss. Cochrane Database Syst Rev 7:CD003998
Whitehouse K, Foroughi M, Shone G, Hatfield R (2010) Vestibular schwannomas – when should conservative management be reconsidered? Br J Neurosurg 24:185–190
Wong HK, Lahdenranta J, Kamoun WS, Chan AW, McClatchey AI, Plotkin SR, Jain RK, di Tomaso E (2010) Anti-vascular endothelial growth factor therapies as a novel therapeutic approach to treating neurofibromatosis-related tumors. Cancer Res 70:3483–3493
Wowra B, Muacevic A, Jess-Hempen A, Hempel JM, Muller-Schunk S, Tonn JC (2005) Outpatient gamma knife surgery for vestibular schwannoma: definition of the therapeutic profile based on a 10-year experience. J Neurosurg 102(Suppl):114–118
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Han, J.H., Kim, D.G., Chung, HT., Paek, S.H., Jung, HW. (2016). Hearing Outcomes After Stereotactic Radiosurgery for Vestibular Schwannomas. In: Schramm, J. (eds) Advances and Technical Standards in Neurosurgery. Advances and Technical Standards in Neurosurgery, vol 43. Springer, Cham. https://doi.org/10.1007/978-3-319-21359-0_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-21359-0_1
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-21358-3
Online ISBN: 978-3-319-21359-0
eBook Packages: MedicineMedicine (R0)