Abstract
There is no standard therapy for recurrent anaplastic astrocytoma (AA). Assess response and toxicity of lomustine (CCNU) in recurrent AA following prior surgery, radiotherapy and TMZ in a retrospective case series. Thirty-five adults (18 males; 17 females: median age 42.5 years) with TMZ refractory recurrent AA were treated with lomustine. Seven patients were treated at 1st recurrence and 28 patients were treated at 2nd recurrence. Prior salvage therapy included re-resection in 19, TMZ in 20 and radiotherapy in 7. A cycle of lomustine was defined as 110 mg/m2 on day 1 only administered once every 6–8 weeks. Success of treatment was defined as progression free survival at 6 months of 40 % or better. Grade 3 or 4 toxicities included anemia (14 patients), constipation (1), fatigue (4), lymphopenia (5), nausea/vomiting (2), neutropenia (8) and thrombocytopenia (10). No grade five toxicities were seen. The median number of cycles of therapy was 3 (range 1–6). Best radiographic response was progressive disease in 14 (40 %), stable disease in 19 (54 %) and partial response in 2 (5.7 %). Median progression free survival (PFS) was 4.5 months (range 1.5–12 months), 6-month PFS was 40 % and 12 month PFS was 11.4 %. Median survival after onset of CCNU was 9.5 months (range 2.5–15 months). Median overall survival was 2.7 years (range 1.7–4.3). In this small retrospective series of patients with recurrent AA refractory to TMZ, lomustine appears to have modest single agent with manageable toxicity. Confirmation in a larger series of similar patients is required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anaplastic gliomas (AG) are infrequently encountered gliomas (approximately 15 % of all gliomas) and comprise three histological variants; anaplastic astrocytoma (AA), anaplastic oligodendroglioma (AO) and anaplastic oligoastrocytoma (AOA) [1]. Molecular markers further characterize these tumors according to the presence or absence of the IDH1 mutation, 1p19q codeletion and the ATRX mutation [2–4]. Initial therapy in all gliomas including AG is maximum safe resection with a general consensus that greater extent of resection improves overall survival (OS) [5, 6]. Therapy following initial surgical resection is however less clear in AG with the exception of the 1p19q codeleted group of tumors in whom two recent prospective randomized phase 3 studies have indicated both a progression free (PFS) and OS advantage when treated with radiotherapy (RT) and PCV (procarbazine, lomustine [CCNU], and vincristine) chemotherapy as compared to RT only [7, 8]. Less certain is the best adjuvant therapy for uni- or nondeleted AG [5, 6, 9]. In the above mentioned randomized trials conducted in anaplastic oligodendroglial tumors, uni- or non- 1p19q deleted anaplastic oligodendroglial tumors (50–75 % of all tumors treated in these trials) demonstrated no PFS or OS advantage when treated with RT + PCV when compared to RT only [7, 8]. In the randomized phase III German NOA-04 trial of AG comparing RT only to chemotherapy only (either PCV or temozolomide [TMZ]) followed at first progression by crossover, there was no difference in time to second recurrence [9]. These data, the only currently available randomized studies in newly diagnosed AG, conclude that in uni- or nondeleted AG treatment following initial surgery may be either with RT only or chemotherapy (TMZ or PCV) only. This position regarding treatment has recently been articulated by the European Association of Neuro-Oncology (EANO) in a consensus guideline [6]. Nonetheless in clinical practice it remains commonplace for patients with AG to be treated in a similar manner to that of glioblastoma (GB) i.e. with RT and concurrent and adjuvant TMZ notwithstanding a lack of prospective evidence for this approach [10]. The currently accruing CATNON trial for newly diagnosed uni- or nondeleted AG will ultimately adjudicate the utility of TMZ when added to RT albeit unlike the NOA-04 trial there is no TMZ only with deferred RT arm.
The approach in recurrent AG is not dissimilar to that in GB wherein patients deemed to benefit from re-resection, surgery is performed with or with implantation of carmustine (BCNU) wafers (Gliadel) [5, 6, 11]. Additionally patients may be treated with re-irradiation in instances of small well- circumscribed recurrent tumors though currently there is limited retrospective data in support of this strategy [12, 13]. Most commonly patients with recurrent AA are offered systemic therapy either chemotherapy or bevacizumab [14–35]. Chemotherapy in most instances utilizes an alkylator and usually re-challenge with TMZ or a nitrosourea-based regimen such as PCV. When available a clinical trial may be proffered. Notwithstanding recent trials in recurrent GB that demonstrated the utility of single agent CCNU (demonstrated in the enzastaurin and REGAL trials), there is limited data regarding this treatment strategy in recurrent TMZ refractory AA [36, 37]. It is unclear from the literature whether PCV chemotherapy is advantageous with respect to anti-glioma efficacy compared to single agent CCNU notwithstanding increased cost and toxicity of PCV chemotherapy. The current retrospective study in part addresses this deficiency in a case series of 35 patients with recurrent AA treated with differing up-front strategies all however having progressed on RT and TMZ and treated with salvage single agent CCNU. The primary objective of this retrospective study was to observe whether CCNU given once every 6–8 weeks could delay progression in patients with radiographically recurrent AA as determined by 6-month progression free survival (PFS-6).
Patients and methods
This was a retrospective unsponsored study that searched a database for patients with recurrent AA treated with single agent CCNU following prior treatment with surgery, RT and TMZ between the years 2000 and 2013. All patients consented verbally to treatment after being apprised of alternative therapies and receiving disclosure of potential risks and benefits of CCNU. None of the 35 patients had been treated on an investigational trial prior to treatment with CCNU. The retrospective study was approved by the university institutional review board.
Objectives and end points
The primary objective was to determine the efficacy of CCNU in the treatment of patients with TMZ refractory recurrent AA. The primary end point was determining progression free survival at 6-months (PFS-6). Secondary end points included toxicity and OS. Toxicity was evaluated in all patients.
Selection of patients
All patients had a histologically proven supratentorial AA that progressed following RT and TMZ. Patients had no or one salvage chemotherapy excluding bevacizumab and nitrosoureas. All patients had recovered from prior chemotherapy with normal hematologic, renal and hepatic function. All patients had radiographically measurable intracranial disease wherein recurrent tumor was bi-dimensionally measurable (at least 10 mm in one dimension) by cranial contrast-enhanced magnetic resonance imaging (MRI) [38, 39]. Histological confirmation of tumor recurrence was not required.
Drug schedule
Lomustine (CCNU) was administered to all patients at a dose of 110 mg/m2 on day 1 only every 6–8 weeks. Concurrent dexamethasone was permitted for control of neurological symptoms and signs. CCNU was administered orally with antiemetic premedication (ondansetron) and without pre- or post-chemotherapy hydration. In patients having undergone re-resection, CCNU commenced 2–4 weeks following surgery and after establishment of normal craniotomy wound healing.
A cycle of therapy was operationally defined as 42 days. Treatment with CCNU was continued every 42 days from day 1 provided that all hematologic toxicity from the previous cycle had resolved to grade 2 or less (except for lymphopenia in which grade did not affect treatment), and all non-hematologic toxicity had recovered to grade 1 or less. If recovery had not occurred by day 42, the subsequent cycle of CCNU was delayed until these criteria were met. All toxicities including hematologic due to CCNU therapy were graded retrospectively according to the NCI Common Toxicity Criteria (version 4.0).
No CCNU dose escalations were used. Dose reduction for toxicity was by 25 % in patients with grade 3 toxicity except in instances of lymphopenia.
Method of evaluation
Laboratory tests (complete blood counts and metabolic panel) were obtained once every 42 days at treatment commencement. Complete blood counts were also obtained at day 21 of each cycle, neurologic examination and contrast-enhanced cranial MR was performed after the each cycle of CCNU. A maximum of 6 cycles of CCNU was administered. No pulmonary function studies were used unless clinically indicated.
Modified neuroradiographic response criteria as defined by MacDonald were used [38, 39]. In patients with SD, PR or CR, one additional cycle of CCNU was to be administered, following which patients were assessed again as described. Patients were continued on CCNU therapy until documentation of PD at which time patients were removed from CCNU and were either monitored or offered alternative therapy.
Progression free survival (PFS) and OS were defined as the time from the first day of treatment with CCNU until progression (PFS) or death (OS).
Analysis
The primary objective of the retrospective study was to observe whether CCNU could delay progression in adult patients with recurrent AA. Historical values were obtained from analysis of a database of 350 patients with recurrent high-grade glioma (150 AG) treated on consecutive prospective phase II trials, in which 6-month progression free survival (PFS-6) was 31 % for AG [40, 41]. The median survival, time to progression and the associated 95 % confidence intervals were computed. Kaplan–Meier plots were constructed to display the estimated probabilities of OS and progression free survival. An outcome was considered potentially relevant if the PFS-6 was ≥40 %.
Results
Study population
Thirty-five patients (18 males; 17 females) median age 42.5 years (range 24–63) with recurrent TMZ refractory AA were treated with CCNU between the years 2000–2013 (Table 1). Recurrent AA was defined by objective neuroradiographic progression (>25 % increase in tumor size) as compared with prior baseline neuroradiographic images using modified criteria as reported by MacDonald [35]. All patients underwent cranial MRI demonstrating progressive disease within two weeks of CCNU administration. Nineteen patients (54 %) underwent a re-operation (complete resection in 7; partial in 12) in which repeat tumor histology was consistent with AA.
Patient performance status using the Karnofsky scale ranged from 70 to 100 (median 80) at the time of documented tumor recurrence and initiation of CCNU therapy. Tumor locations, including multilobar tumors were as follows: frontal lobe (n = 16), temporal lobe (n = 8), parietal lobe (n = 4), occipital lobe (n = 2), insula (n = 2), thalamus (n = 2) and basal ganglia (1). Thirty-three patients had lobar tumors; two patients had multilobar tumors. Pathology (institutionally reviewed) showed all tumors to be AA by WHO criteria.
All patients underwent initial surgery in which a complete resection was accomplished in 15, partial in 10 and biopsy only in 10 (Table 1). Nineteen patients (54 %) underwent a second surgery prior to CCNU administration.
All patients had previous treatment with limited-field RT (Table 1) and in all, conventional fractionated RT was used in which 1.8–2.0 Gy was administered daily, with a median tumor dose of 60 Gy (range 59–60 Gy). No patient was treated with stereotactic radiotherapy.
Initial treatment varied and included RT only followed at 1st recurrence with TMZ (20 patients; 57 %), RT and concurrent and post-RT TMZ (11 patients; 31 %) and TMZ only followed by RT at 1st recurrence (4 patients; 11 %). TMZ was administered in the standard fashion (concurrent RT and TMZ 75 mg/m2/day for 42 days; post-RT 150–200 mg/m2/day for 5-days every 4 weeks) (Table 1). Patients received a median of 6 TMZ cycles of therapy (range 3–12 cycles). Median time to initiation of CCNU following initial surgery was 15 months with a range of 6–28 months. A total of 115 cycles of CCNU were administered. A minimum of 1 cycle of CCNU was administered to each patient with a median of 3 cycles (range 1–6). CCNU was dose reduced for myelotoxicity in 15 (42.9 %) patients. No other anti-glioma agents aside from dexamethasone were utilized during CCNU treatment.
Molecular markers
All patients (100 %) underwent 1p19q deletion evaluation by FISH and none were codeleted. 14 (40 %) were uni-deleted for 1p or 19q. Thirty (86 %) patients had IDH1 mutation determined by immunohistochemistry of which 20 (67 %) manifested a mutated genotype. Only 10 (28.5 %) patients had ATRX mutation status determined by immunohistochemistry of which 6 (60 %) were found to be mutated. Only 6 patients (17 %) had MGMT promoter methylation status determined by methylation specific PCR.
Toxicity
Toxicity was recorded for all grades for all patients by type using the NCI common toxicity criteria (version 4.0). Table 2 lists all grade 3–4 toxicity observed with each figure representing the sum of the highest grade of toxicity attained, per toxicity, per cycle for all patients. A total of 115 treatment cycles were administered of which there were 27 (23.5 %) grade 3 adverse events (AEs) and 5 (4.3 %) grade 4 AEs. No grade 5 toxicity was observed. The most common grade 3–4 AEs was thrombocytopenia (8.6 %), neutropenia (6.9 %), lymphopenia (4.3 %), thrombocytopenia (2.8), and fatigue (3.4 %). Four patients required transfusion, 1 with packed red blood cells and 3 with platelets. Two patients developed febrile neutropenia however body fluid cultures were negative. No treatment-related deaths occurred.
Response
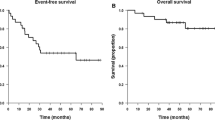
All patients were assessable for response. Following one cycle of CCCNU, 14 patients (40 %) demonstrated progressive disease, 19 (54 %) showed stable disease and 2 (5.7 %) demonstrated a partial response. At the conclusion of CCNU, Karnofsky performance status ranged from 40 to 70 with a median of 60 in the entire study group. Patients who failed to respond to CCNU were offered alternative or supportive therapy. Median PFS was 4.5 months (range 1.5–12 months) [95 % CI = 3.0–6.0] (Fig. 1). Six-month PFS was 40 % (95 % CI = 24–55.5 %) and 12-month PFS was 11.4 % (95 % CI = 4–14 %). Survival in the entire cohort ranged from 2.5 to 15 months after onset of CCNU with a median of 9.5 months (95 % CI = 7.0–10.5 months; Fig. 2). Nine patients (25.7 %) were treated with bevacizumab after disease progression on CCNU. All patients have died, and all deaths were directly attributable to the effects of progressive intracranial tumor. Median OS from initial surgery and diagnosis was 2.7 years (range 1.7–4.3) [95 % CI = 2.2–3.1 years]. Regarding the primary observational endpoint of the study (6-month PFS), the results appeared similar to a historical control (AA: expected ≥40 %; observed 40 %). Analysis by molecular phenotype indicated a trend for improved survival in patients with IDH1 mutation that appeared enhanced by the presence of the ATRX mutation however the overall numbers of evaluable tumors (including MGMT status) were too small for formal analysis.
Discussion
At present, newly diagnosed AA are treated with one of 3 up-front strategies: RT only (used in 20 patients in the current study) as articulated in the NCCN and EANO brain tumor guidelines and based upon the German NOA-04 trial results; chemotherapy only (administered to four patients in the current study) and based upon the NOA-04 trial results; and the use of RT and concurrent and adjuvant TMZ (used in 11 patients in the current study) simulating the treatment of GB as demonstrated in the EORTC/NCIC seminal study [5, 6, 9, 10]. Nonetheless both the EORTC and RTOG prospective and randomized studies of anaplastic oligodendroglial tumors demonstrated no OS benefit in the uni- or nondeleted large cohort of patients with the addition of PCV to RT as compared to RT only [7, 8]. These two studies consequently question the current common practice of treating newly diagnosed AA (irrespective of IDH1 mutational status) with RT + TMZ, a question likely to be answered by the open and accruing CATNON trial comparing RT only to RT + TMZ given in three different TMZ schedules. Notwithstanding a potential benefit of RT + PCV with respect to PFS in the EORTC 26951 trial in non-codeleted AG (not seen however in the RTOG 9402 trial), it is likely this benefit is realized only in a subpopulation of molecularly defined tumors containing for example both IDH1 and ATX mutations. In addition, NOA-04 trial showed apparent equivalence of TMZ and PCV when given as a single modality therapy in newly diagnosed AA and similarly the British Medical Research Council trial demonstrated equivalency of TMZ and PCV when given at first recurrence following initial surgery and RT of AG [9, 16]. The current retrospective study using three differing treatment strategies reflects the ambiguity regarding the up-front treatment of AA and likely is reflective of many neuro-oncology centers.
Less clear is the preferred treatment for recurrent AA as present therapies duplicate strategies used for recurrent GB. These include salvage therapy with TMZ if not otherwise treated with TMZ (utilized in 20 patients [57 %] treated initially with surgery and RT only in the current study) and RT if not previously irradiated (administered to 4 patients [11 %] treated initially with TMZ only). In patients with prior combined modality treatment (11 patients [31 %] so treated in the current study), treatment strategies might include enrollment in a clinical trial, re-challenge with TMZ as demonstrated by the NCIC RESCUE trial, use of nitrosourea-based chemotherapy such as PCV or CNNU and single agent bevacizumab [Table 3]. Unlike prospective clinical trials in recurrent GB (REGAL and the enzastaurin study), there are no similar trials that inform as to the utility of CCNU in recurrent AA having failed (first- or second-line) TMZ [36, 37]. The objective of the current retrospective study was to identify the utility of CCNU as an effective salvage therapy following prior treatment with TMZ.
In the current cohort of patients with recurrent AA, 24 patients (68.5 %) received CCNU as a second salvage therapy and 16 patients (31.5 %) received CCNU as first salvage therapy. All patients had an initial diagnosis of AA following first surgery and 19 (54 %) underwent a second reoperation with repeat confirmation of AA pathology recognizing the limitations of the current WHO grading system in patients with previously treated gliomas. Apart from reoperation and reexamination of tumor, it is uncertain if the remaining 16 patients with recurrent AA had dedifferentiated and transformed to a secondary GB. Consequently the current study may represent an admixture of both recurrent AA and secondary GB. As illustrated in Table 3, studies of recurrent AA are comparatively scarce but aside from single agent bevacizumab these trials are similar with respect to outcome as measured by objective radiographic response (median 13 %: range 5–35 %), median PFS (median 4.1: range 2–11.5 months) and PFS-6 (median 36 %: range 12–70 %) [11, 15, 17–23, 25, 27–32, 35, 42]. The current retrospective study using single agent CCNU had comparable outcomes (response 5.7 %, median PFS 4.5 months and PFS-6 40 %). Five studies in Table 3 utilized TMZ before an investigational regimen excluding bevacizumab and therefore are most comparable to the prior study [19, 20, 23, 25, 28, 31]. Similar to CCNU salvage therapy used in the current study, median radiographic response was 22.5 %, median PFS was 4.1 months and PFS-6 was 40 %. It therefore seems reasonable based on efficacy to consider single agent CCNU as a modestly effective salvage regimen after failure of TMZ in recurrent AA. Three trials all utilizing 6-thioguanine and CCNU combination regimens that biochemically potentiate alkylator-based cytotoxicity have reported better outcomes however these multiday multidrug regimens are infrequently utilized likely due to unfamiliarity as well as regimen complexity [21, 22, 25]. Median OS in non-bevacizumab containing regimens (Table 3) is 11 months similar to the current study (9.5 months; range 6.9–18.5 months) however these regimens vary with respect to the incidence of prior chemotherapy administration, number of prior salvage treatments as well as prior TMZ treatment. Bevacizumab, used either alone or in combination (Table 3) may as in GB represent another salvage strategy following disease progression after alkylator-based chemotherapy in recurrent AA recognizing that currently bevacizumab is not currently approved for recurrent AA [32, 35, 42–47]. In the current study, 9 patients received bevacizumab following progression on CCNU (Table 1) that modestly impacted OS (median PFS 4 months, median OS 5 months [range 3–9]) but had no impact on the primary study endpoint, PFS while on single agent CCNU.
Based on the results of this small retrospective study, salvage therapy with CCNU, after progression on TMZ in patients with recurrent AA, appears to be a modestly effective treatment strategy with manageable toxicity. Recognizing the limitations of a small retrospective trial such as this, a prospective trial with CCNU administered as a single agent would appear warranted and might serve as a control comparator in a randomized study of novel treatments in patients with recurrent AA.
References
Prados MD, Gutin PH, Phillips TL et al (1992) Anaplastic astrocytoma: a review of 357 patients treated between 1977 and 1989. Int J Radiat Oncol Biol Phys 23:3–8
Wiestler B, Capper D, Holland-Letz T et al (2013) ATRX loss refines the classification of anaplastic gliomas and identifies a subgroup of IDH mutant astrocytic tumors with better prognosis. Acta Neuropathol 126:443–451
Hartmann C, Hentschel B, Wick W et al (2010) Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol 120:707–718
Cairncross JG, Wang M, Jenkins RB et al (2014) Benefit From Procarbazine, Lomustine, and Vincristine in Oligodendroglial Tumors Is Associated With Mutation of IDH. J Clin Oncol 32:783–790
Nabors LB, Ammirati M, Bierman PJ et al (2013) Central nervous system cancers. J Natl Compr Cancer Netw 11:1114–1151
Weller M, van den Bent M, Hopkins K et al (2014) EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol 15:e395–e403
Cairncross G, Wang M, Shaw E et al (2013) Phase III Trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol 31:337–343
van den Bent MJ, Brandes AA, Taphoorn MJ et al (2013) Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol 31:344–350
Wick W, Hartmann C, Engel C et al (2009) NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine and vincristine or temozolomide. J Clin Oncol 27:5874–5880
Stupp R, Mason WP, van den Bent M et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New Engl J Med 352:987–996
Brem H, Piantadosi S, Burger PC et al (1995) Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. Lancet 345:1008–1012
Combs SE, Thilmann C, Edler L et al (2005) Efficacy of fractionated stereotactic reirradiation in recurrent gliomas: long-term results in 172 patients treated in a single institution. J Clin Oncol 23:8863–8869
Fogh SE, Andrews DW, Glass J et al (2010) Hypofractionated stereotactic radiation therapy: an effective therapy for recurrent high-grade gliomas. J Clin Oncol 28:3048–3053
Perry JR, Belanger K, Mason WP et al (2010) Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol 28:2051–2057
Yung WK, Prados MD, Yaya-Tur R et al (1999) Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal brain tumor group. J Clin Oncol 17:2762–2771
Brada M, Stenning S, Gabe R et al (2010) Temozolomide versus procarbazine, lomustine and vincristine in recurrent high-grade glioma. J Clin Oncol 28:4601–4608
Yung WKA, Kyritsis AP, Gleason MJ, Levin VA (1992) Treatment of recurrent malignant gliomas with high-dose 13-cis-retinoic acid. Clin Cancer Res 2:1931–1935
Chamberlain MC, Kormanik P (1999) Salvage chemotherapy with taxol for recurrent anaplastic astrocytomas. J Clin Oncol 43:71–78
Chamberlain M, Tsao-Wei D, Blumenthal D, Glantz MJ (2008) Salvage chemotherapy with CPT-11 for recurrent temozolomide-refractory anaplastic astrocytoma. Cancer 112:2038–2045
Chamberlain MC, Tsao-Wei D, Groshen S (2006) Salvage chemotherapy with cyclophosphamide for temozolomide refractory anaplastic astrocytoma. Cancer 106:172–179
Levin VA, Prados MD (1992) Treatment of recurrent gliomas and metastatic brain tumors with a polydrug protocol designed to combat nitrosoureas resistance. J Clin Oncol 10:766–771
Levin VA, Prados MD, Yung WKA, Gleason MJ, Ictech S, Malec M (1992) Treatment of recurrent gliomas with enflornithine. J Natl Cancer Inst 84:1432–1437
Jaeckle KA, Hess KR, Yung WK et al (2003) Phase II evaluation of temozolomide and 13-cis-retinoic acid for the treatment of recurrent and progressive malignant glioma: a North American brain tumor consortium study. J Clin Oncol 1:2305–2311
Levin VA, Ictech S, Hess KR (2007) Impact of phase II trials with progression-free survival as end-points on survival-based phase III studies in patients with anaplastic gliomas. BMJ Cancer 7:106–113
Walbert T, Gilbert MR, Groves MD et al (2011) Combination of 6-thioguanine, capecitabine, and celecoxib with temozolomide or lomustine for recurrent high-grade glioma. J Neurooncol 102:273–280
Kyritsis AP, Levin VA (2011) An algorithm for chemotherapy treatment of recurrent glioma patients after temozolomide failure in the general oncology setting. Cancer Chemother Pharmacol 67:971–983
Hess KR, Wong ET, Jaeckle KA, Kyritsis AP, Levin VA, Prados MD, Yung WK (1999) Response and progression in recurrent malignant glioma. Neuro Oncol 1:282–288
Desjardins A, Quinn JA, Vredenburgh JJ et al (2007) Phase II study of imatinib mesylate and hydroxyurea for recurrent grade III malignant gliomas. J Neurooncol 83:53–60
Kyritsis AP, Yung WK, Jaeckle KA et al (1999) Combination of 6-thioguanine, procarbazine, lomustine, and hydroxyurea for patients with recurrent malignant gliomas. Neurosurg 39:921–926
Kunschner LJ, Yung WKA, Levin VA, Jaeckle KA (1999) Carboplatin and 13-cis-retinoic acid for recurrent glioblastoma multiforme. Neuro-Oncol 1:320–325
Franceschi E, Bartolotti M, Dall’Occa P et al (2012) Anaplastic glioma at first recurrence: outcome analysis. J Clin Oncol 30(suppl.):2061 (abstract)
Norden AD, Young GS, Setayesh K et al (2008) Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology 70:779–787
Friedman HS, Prados MD, Wen PY et al (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27:4733–4740
Kreisl TN, Kim L, Moore K et al (2009) Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27:740–745
Desjardins A, Reardon DA, Herndon JE 2nd et al (2008) Bevacizumab plus irinotecan in recurrent WHO grade 3 malignant gliomas. Clin Cancer Res 14:7068–7073
Wick W, Puduvalli VK, Chamberlain MC et al (2010) Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol 28:1168–1174
Batchelor T, Mulholland P, Neyns B et al (2013) Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol 31:3212–3218
MacDonald DR, Cascino TL, Schold SC et al (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972
Wong ET, Hess KR, Gleason MJ et al (1999) Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol 17:2572–2578
Lamborn KR, Yung AWK, Chang SM et al (2008) Progression free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. J Neurooncol 10:162–170
Chamberlain MC, Johnston S (2009) Salvage chemotherapy with bevacizumab for recurrent alkylator-refractory anaplastic astrocytoma. J Neuro-Oncol 91:359–367
Kreisl TN, Zhang W, Odia Y et al (2011) A phase II trial of single-agent bevacizumab in patients with recurrent anaplastic glioma. Neuro-Oncol 13:1143–1150
Reardon DA, Desjardins A, Vredenburgh JJ et al (2009) Metronomic chemotherapy with daily, oral etoposide plus bevacizumab for recurrent malignant glioma: a phase II study. Br J Cancer 101:1986–1994
Sathornsumetee S, Desjardins A, Vredenburgh JJ et al (2010) Phase II trial of bevacizumab and erlotinib in patients with recurrent malignant glioma. Neuro-Oncol 12:1300–1310
Seystahl KL, Wiestler B, Hundsberger T, Happold C, Wick W, Weller M, Wick A (2013) Bevacizumab alone or in combination with irinotecan in recurrent WHO grade II and grade III gliomas. Eur Neurol 69:95–101
Delios A. Brebnnan CW, Huse JT, Colevas K, Omuro AMP (2012) Bevacizumab for recurrent WHO grade III anaplastic glioma (AG). J Clin Oncol 30(suppl.):2028 (abstract)
Acknowledgments
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chamberlain, M.C. Salvage therapy with lomustine for temozolomide refractory recurrent anaplastic astrocytoma: a retrospective study. J Neurooncol 122, 329–338 (2015). https://doi.org/10.1007/s11060-014-1714-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-014-1714-9