Abstract
Adding temozolomide (TMZ) to radiation for patients with newly-diagnosed anaplastic astrocytomas (AAs) is common clinical practice despite the lack of prospective studies demonstrating a survival advantage. Two retrospective studies, each with methodologic limitations, provide conflicting advice regarding treatment. This single-institution retrospective study was conducted to determine survival trends in patients with AA. All patients ≥18 years with newly-diagnosed AA treated at Johns Hopkins from 1995 to 2012 were included. As we incorporated TMZ into high-grade glioma treatment regimens in 2004, patients were divided into pre-2004 and post-2004 groups for analysis. Clinical, radiographic, and pathologic data were collected. Median overall survival (OS) was calculated using Kaplan–Meier estimates. A total of 196 patients were identified; 74 pre-2004 and 122 post-2004; mean age 47 ± 15 years; 57 % male; 87 % white, 69 % surgical debulking. Mean RT dose 5676 + 746 cGy; duration of concurrent chemoradiation 5.8 ± 0.8 weeks; and mean adjuvant chemotherapy 4.3 + 2.8 cycles. Baseline prognostic factors did not differ between groups. Chemotherapy was administered to 12 % of patients pre-2004 (TMZ = 1, procarbazine, lomustine and vincristine = 2, carmustine wafer = 6) and 94 % post-2004 (TMZ in all, p < 0.001). Median OS was 32 months (95 % CI 23–43). Survival was longer in the post-2004 cohort (37 mo, 24–64) than pre-2004 (27 mo, 19–40; HR 0.75, 0.53–1.06, p = 0.11). Multivariate analysis controlling for age, Karnofsky performance status, and extent of resection revealed a 36 % reduced risk of death (HR 0.64, 0.44–0.91, p = 0.015) in patients treated post-2004. This retrospective review found survival in newly diagnosed patients with AA improved with the addition of temozolomide to standard radiation. Until prospective randomized phase III data are available, these data support the practice of incorporating TMZ in the management of newly-diagnosed AA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malignant gliomas account for approximately 80 % of newly diagnosed malignant primary brain tumors and include both World Health Organization (WHO) grade III and grade IV astrocytomas [1]. In 2005, following the reporting of the randomized phase III EORTC 22981 study evaluating the impact of temozolomide (TMZ) on survival in glioblastoma (GBM), radiation therapy (RT) combined with TMZ became the standard of care for treating GBM [2]. Of the 573 patients enrolled in this study, however, only 16 (3 %) had WHO grade III anaplastic astrocytomas (AAs). Given the aggressive nature of the grade III neoplasms and the high risk of transformation to grade IV histology, some have extrapolated data from the EORTC 22981 study and include TMZ in the treatment of AAs [3, 4]; however, controversy remains.
Despite this trend toward incorporation of TMZ into treatment regimens for patients with newly diagnosed AA in some areas of the world, no prospective data exist to support its inclusion. While both the European Association for Neuro-Oncology (EANO) and the National Comprehensive Cancer Network (NCCN) Guidelines include radiation alone, chemotherapy alone, or combined modality therapy with TMZ as treatment options, controversy remains [5]. In the landmark RTOG 9402 and EORTC 26981 studies which first demonstrated the chemosensitivity of 1p19q codeleted anaplastic oligodendrogliomas to combination chemotherapy with procarbazine, lomustine (i.e. CCNU), and vincristine (PCV), similar chemosensitivity was not observed in those patients with non-codeleted anaplastic oligodendrogliomas [6, 7]. Survival was not different between the chemotherapy and radiation arms of the NOA-04 study which was designed to compare early radiation alone versus early chemotherapy (PCV or TMZ) followed by the alternative treatment at salvage [8]. However, this study did not include a combination chemoradiation arm to confirm the benefit of concurrent chemoradiation with TMZ. Two retrospective studies reporting on combination chemoradiation with TMZ in patients with newly diagnosed AA have cautioned its use even suggesting potential detriment [9, 10]. At our institution, following the reporting of data from the EORTC 22981 study, standard clinical practice shifted from recommending RT alone to recommending the incorporation of TMZ with RT for all patients with AA and GBM (i.e. high-grade astrocytomas, HGA). Herein, we review our experience prior to and following this shift in clinical practice and report on survival trends in patients with AAs.
Methods
A single-institution, retrospective cohort study was conducted of consecutive patients treated at the Sidney Kimmel Comprehensive Center between September 1995 and December 2012. After institutional review board approval was obtained, the Johns Hopkins Cancer Center Registry was queried for all adult patients (age ≥18) with a histopathologic diagnosis of primary WHO grade III anaplastic astrocytoma seen at the Johns Hopkins Hospital (JHH) during the pre-specified time period. Patients with a histopathologic diagnosis other than WHO grade III pure anaplastic astrocytoma (i.e. oligoastrocytoma), age <18, who did not receive adjuvant treatment and follow up at JHH, or whose grade III astrocytoma arose from a lower-grade neoplasm (i.e. secondary) were excluded. Given that this study was designed to assess the benefit of adding TMZ to RT, patients who did not undergo RT were excluded. Comprehensive medical record review was performed including clinic notes, operative notes, radiological images and results, and pathology reports. All images and pathology specimens procured at outside facilities were reviewed and confirmed by experienced neuroradiologists and neuropathologists at JHH.
Demographic characteristics including age, gender, ethnicity, and tumor histology were pooled from the cancer center registry and confirmed by medical record review. The date of diagnosis was defined as the first date of histopathologic confirmation of primary brain tumor. Extent of surgery was identified as gross total or subtotal resection or biopsy by operative and clinical notes and when available by post-operative imaging. Details of treatment including RT, total radiation dose, all chemotherapy types including bevacizumab, dosages, and durations were recorded. Karnofsky performance status (KPS) at or immediately before initiation of RT was dichotomized at 60 and recorded. Date of first recurrence was determined by histopathology when available or by clinicoradiographic data within the medical record. Dates of last contact were defined as the date of death or last follow up (if alive at data analysis) and were matched against the social security death index. Overall survival (OS) was defined as the time from the date of diagnosis to the date of death or last contact.
Based on institutional shift toward the incorporation of TMZ into treatment regimens for all patients with AA and GBM in the early spring of 2004, patients were divided into those receiving treatment prior to and following January 2004 (pre-2004 and post-2004, respectively). Patient characteristics (at disease diagnosis), oncologic treatments, and surgical procedures were summarized using descriptive statistics. Overall survival was calculated from the time of initial histological diagnosis to death from any cause. Survival time was censored if the subject was alive at the time of last follow-up. Survival probability was estimated using the Kaplan–Meier method [11]. Univariate analysis was used to assess for associations between known prognostic factors and overall survival. Important patient characteristics associated with survival were identified in the univariate analysis using a p value of <0.05. These characteristics were incorporated as covariates to construct the multivariate proportional-hazards regression model which was used to estimate the hazard ratio for death attributable to prognostic factors [12]. Given the exploratory nature of these analyses, no adjustment for multiple testing was performed and all observed outcomes should be considered descriptive. All p-values are reported as two-sided and analyses were performed using the SAS software (version 9.3, SAS institute, Cary, NC).
Results
A total of 445 patients were initially queried from the cancer center registry; 2 were excluded for incorrect pathology; 226 excluded for treatment performed at an outside facility, incomplete follow up, or radiation not administered; and 21 for age <18 (Supplemental Fig. 1). Of the 196 remaining in the active cohort, 74 were treated pre-2004 and 122 post-2004. Median age of the cohort was 46.6 ± 14.6 years; 57 % male; 87 % white, and 9 % black (Table 1). The majority of patients (n = 157, 80 %) had a KPS ≥ 60 at the initiation of RT. Gross total resection was performed in 32 %, subtotal resection 37 %, and biopsy in 31 %. Mean RT dose was 5676 ± 746 cGy; mean duration of concurrent chemoradiation 5.8 ± 0.8 weeks (n = 115), and mean number of adjuvant TMZ cycles 4.3 + 2.8 (n = 108), and PCV cycles 2.5 ± 2.1 (n = 2). Age (p = 0.75), gender (p = 0.46), ethnicity (p = 0.17), KPS (p = 1.00), and extent of resection (p = 0.39) did not differ between the groups.
Chemotherapy was administered to 12 % (n = 9) of patients pre-2004 and 94 % (n = 115) post-2004 (p < 0.001). Pre-2004, the majority of patients receiving any first-line chemotherapy (local or systemic) underwent Gliadel® wafer placement (n = 6). Only 3 patients received systemic chemotherapy (TMZ in 1, procarbazine-lomustine-vincristine, PCV, in 2). Post-2004 TMZ was the only first-line systemic chemotherapeutic agent administered. Gliadel® wafer was also placed in 1 patient.
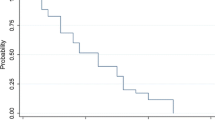
Median OS for the entire cohort was 32 months (95 % CI 22.8–43.1). Median OS was 27.4 months (95 % CI 19.1–39.8, 88 % deceased) pre-2004 compared to 36.7 months (95 % CI 24.4–63.6, 54 % deceased) post-2004 (Fig. 1, HR 0.75, 95 % CI 0.53–1.06, p = 0.11). In the univariate analysis, younger age, greater extent of resection, and KPS > 60 were significantly associated with improved survival (p < 0.007, Table 2). Median OS was 7.4 months (95 % CI 3.7–13.8) in those with KPS < 60 compared to 41.7 months (95 % CI 32–60.5) in those with KPS > 60 (HR 0.2, 95 % CI 0.1–0.3, p < 0.001, Fig. 2). In those with biopsy, median OS was 15.8 months (95 % CI 10.4–19.3), 38.4 (95 % CI 22.3–52.7) with subtotal resection, and 73.4 (95 % CI 47.3–122.8) with gross total resection (p < 0.0001). After controlling for each of these variables in the multivariate Cox proportional hazards regression model, a significant association between survival and era of treatment was observed (Table 2, HR 0.64, 0.44–0.91, p = 0.015). This accounted to a 36 % reduced risk of death post-2004.
Kaplan–Meier estimates of overall survival by treatment era. The unadjusted hazard ratio for death among patients in post-2004 group, as compared with those in pre-2004 group, was 0.75 (95 % CI 0.53–1.06; p = 0.1). The Hazard ratio was 0.64 (95 % CI 0.44–0.91, p = 0.0145) after adjusting for age, KPS and type of surgical procedure (a 40 % reduction of hazard of death)
Kaplan–Meier estimates of overall survival by performance status and extent of resection. Kaplan–Meier estimates of overall survival according to Karnosky performance status (a) and extent of resection (b). Median OS was 7.4 months (95 % CI 3.7–13.8) in those with KPS < 60 compared to 41.7 months (95 % CI 32–60.5) in those with KPS > 60 (HR 0.2, 95 % CI 0.1–0.3, p < 0.001, by log-rank test). Median OS was 15.8 months (95 % CI 10.4–19.3) in those with biopsy, 38.4 (95 % CI 22.3–52.7) with subtotal resection, and 73.4 (95 % CI 47.3–122.8) with gross total resection (p < 0.0001, by log-rank test). KPS Karnofsky performance status, STR subtotal resection, GTR gross total resection. Bev bevacizumab
Bevacizumab was administered at tumor recurrence in 21 patients (11.7 %) including 19 patients (15.5 %) post-2004 and 2 patients (2.7 %) pre-2004 (p = 0.004). Two additional patients received bevacizumab for steroid-sparing prior to recurrence. The median time to initiation of bevacizumab in this patient population was 45 months from diagnosis (range 10-111 months with a median of 90 months in the pre-2004 cohort and 39 months in the post-2004 cohort). Patients received a median of 6 cycles (range 1–29) of bevacizumab. To explore the possible association between bevacizumab use and survival, an analysis was performed on the post-2004 patient cohort who all received standard radiation and temozolomide. Although the median survival in this cohort was longer in the patients who received bevacizumab (58.9 months [95 % CI 26–73] versus 28.9 months [95 % CI 22–108]) this was not statistically different (p = 0.98, Supplemental Fig. 2). In addition, the univariate Cox proportional hazards regression analysis no differences in survival in patients receiving and not receiving bevacizumab (HR 1.01, 95 % CI 0.56–1.80, p = 0.98).
Discussion
In this study, unadjusted survival was not significantly different in the era after the standard incorporation of TMZ into first-line treatments regimens for patients with newly diagnosed AA compared to the era prior to its inclusion. After controlling for well-known prognostic factors, we report a significantly longer overall survival when TMZ was incorporated into standard first-line treatment. This adjusted survival impact amounted to a 36 % reduction in the adjusted risk of death for these patients with newly diagnosed AA. Age, extent of surgical resection, and KPS did not appear to drive this difference in survival, though data on molecular subtypes were not explored and could contribute to differences between these groups.
To date, no prospective phase III data exists to inform the decision to include TMZ in the first-line management of AA. While the EORTC 26053 CATNON study (Concurrent and Adjuvant Temozolomide chemotherapy in NON-1p/19q deleted anaplastic glioma, NCT00626990) has completed patient accrual and will address this question and the utility of concurrent and adjuvant TMZ in the management of non-codeleted AAs results will not be available for several years. Prior retrospective studies evaluating the utility of TMZ in patients with AA have not demonstrated longer survival in patients receiving TMZ [9, 10]. However, important methodological concerns limit the clinical application of these results. In one study, important prognostic differences such as KPS and radiation dose were observed in patients receiving concurrent chemotherapy and may reflect selection biases favoring incorporation of chemotherapy in patients with better functional status [9]. In the other study, progression free survival (PFS) was reported to be significantly shorter in patients treated with concurrent chemoradiation with TMZ compared to those who were treated with RT alone or RT followed by adjuvant TMZ [10]. However, this finding may reflect a higher proportion of patients who develop radiographic evidence of pseudoprogression and not reflect the underlying biology of the cancer. Patients with rapid progression to GBM were also excluded in this study and outcome analysis included only certain patient subgroups [10]. In both studies retrospective analysis was performed of cohorts who were treated over a single time interval and patients were stratified by treatment type within this period potentially introducing important patient and treatment selection biases.
Several important methodological differences exist between these prior reports and our current study which may explain the differences in our results. In the current investigation, standard institutional practice shifted from treating with RT alone to the inclusion of concurrent and adjuvant TMZ with RT at a clearly defined time coinciding with the circulation of results from the EORTC 29981 trial. This created a logical time point for dividing patients by time (i.e. era of treatment) rather than by treatment selection over a single timeframe. All patients treated with at least RT prior to and following this time were included. Thus analysis was performed by time and not by treatment delivered which may limit important potential confounders and selection biases. The lack of observed difference between the pre-2004 and post-2004 groups in clinical, tumor, and treatment characteristics with only chemotherapy administration being significantly different between groups supports this method of patient selection by era. Data on molecular characteristics of the tumors (i.e. O6-methylguanine-DNA-methyltransferase, MGMT, promoter methylation or isocitrate dehydrogenase 1, IDH1, mutation) were not available to explore differences in these important prognostic factors by treatment era. The use of bevacizumab at recurrence did differ between the two groups (two patients before vs. 19 after 2004). However, in the post-2004 cohort only 17 % of patients received bevacizumab and this was used late in the patient’s illness as it was started a median of 39 months from diagnosis. In addition, overall survival was not different in the patients receiving bevacizumab. This is consistent with results from several large randomized phase III studies which demonstrated no survival advantage when bevacizumab was added to standard radiation and temozolomide in patients with high grade gliomas [13, 14].
In general, data on the utility of chemotherapy in treating anaplastic gliomas has been mixed [15, 16]. Neoadjuvant, concurrent, and adjuvant therapies have been investigated. In the late 1990s, the addition of adjuvant PCV or carmustine and dibromodulcitol (DBD) to RT was not shown to improve survival in newly diagnosed anaplastic gliomas based on two prospective, randomized phase III studies [17, 18]. However, in one study initial pathologic interpretation of WHO grade III glioma was ultimately re-interpreted to GBM in 25 % and low-grade glioma in 23 % of patients by central pathologic review [17]. Objective responses were reported following neoadjuvant TMZ in patients with high-grade gliomas for which anaplastic lesions accounted for a substantial number [19, 20]. In the setting of recurrent anaplastic glioma, a pivotal single-arm phase II study of 162 patients treated with TMZ monotherapy demonstrated a 46 % 6-month PFS and 24 % 12-month PFS [21]. Based on these results, TMZ was approved in the United States for the treatment of recurrent AA. Several large phase III studies have evaluated the role of chemotherapy in patients with newly diagnosed anaplastic oligodendrogliomas and have included analysis of the non-codeleted AOs which share clinical, molecular, and biologic features with anaplastic astrocytomas [6–8]. While sequential radiation with PCV did not appear to improve survival in non-codeleted patients in the RTOG 9402 and EORTC 26951 studies [6, 7], chemotherapy with either PCV or TMZ alone was similar to radiation alone in the NOA-04 study [8]. Ultimately, results of the phase III CATNON study will be required to confirm the role of TMZ in the management of non-codeleted anaplastic gliomas. The continued controversy regarding these therapeutic options is reflected in the NCCN Guidelines, which includes radiation alone, chemotherapy alone (PCV or TMZ), and concurrent chemoradiation with TMZ.
Although definitive data supporting the inclusion of TMZ into treatment regimens for patients with AA is lacking, its use appears to be common in clinical practice in the USA [3, 4]. TMZ does have important safety, toxicity, and cost considerations which must also be contemplated when considering the role of this agent. While myelosuppression is less common with TMZ than other cytotoxic chemotherapies, rate of hospitalization during the first course of therapy has been estimated between 11 and 19 % with deaths having been observed [22, 23]. Female and older patients appear to have a higher incidence of grade 3 or 4 myelotoxicity, a risk which may in part be due to underlying genetic polymorphisms [22]. According to a British study which modeled the average cost per patient treated with surgery, RT and TMZ for a high-grade glioma, TMZ was estimated to add an additional cost of around £7800 to existing costs [24]. In addition, data on the impact of TMZ on future malignant potential at relapse suggest that TMZ can induce new driver mutations and higher-grade malignancy at recurrence which supports caution when considering utilization of this agent without evidence of benefit [25].
Despite the efforts to design this study to reduce selection biases and confounders of survival, the study is limited by its retrospective design. Data on MGMT promoter methylation, IDH1 mutation and expression, and other molecular features of these tumors were not available to determine if differences in survival were related to these important prognostic factors [26]. Histopathologic diagnosis of grade III gliomas can be challenging with variations have been reported between pathologists and over time [27]. While efforts were made for all pathology to be reviewed by experienced neuropathologists at our institution, prospective collection with formal central review is optimal. Differences in survival have also been shown to exist over time as supportive treatment improves [28].
Conclusion
In conclusion, since the reporting of results from the EORTC 22981 study by Stupp and colleagues in 2005, TMZ has become standard of care for the management of GBM and its use has been widely extrapolated to AA. Data supporting the incorporation of TMZ into treatment regimens do not exist and pre-existing methodologically limited retrospective studies have suggested potential detrimental effects on PFS. The improved adjusted survival observed in this retrospective study supports the use of TMZ in the treatment of newly diagnosed AA. Results of the phase III CATNON study (Concurrent and Adjuvant Temozolomide chemotherapy in NON-1p/19q deleted anaplastic glioma, NCT00626990) will provide important data to inform this treatment decision.
References
Dolecek TA, Propp JM, NE Stroup, Kruchko C (2012) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol 14:v1–v49
Stupp R, Mason W, van den Bent M et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. NEJM 352:987–996
Chang SM, Parney IF, Huang W, Anderson FA, Asher AL, Bernstein M, Lillehei KO, Brem H, Berger MS, Laws ER (2005) Patterns of care for adults with newly diagnosed malignant glioma. JAMA 293:557–564
Ray S, Bonafede MM, Mohile NA (2014) Treatment patterns, survival, and healthcare costs of patients with malignant gliomas in a large US commercially insured population. Am Health Drug Benefits 7(3):140–149
Weller M, van den Bent M, Hopkins K et al (2014) EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol 15:e395–e403
Cairncross G, Wang M, Shaw E et al (2013) Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol 31:337–343
Van Den Bent MJ, Brandes AA, Taphoorn MJB et al (2013) Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol 31:344–350
Wick W, Hartmann C, Engel C et al (2009) NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol 27:5874–5880
Scoccianti S, Magrini SM, Ricardi U et al (2012) Radiotherapy and temozolomide in anaplastic astrocytoma: a retrospective multicenter study by the Central Nervous System Study Group of AIRO (Italian Association of Radiation Oncology). Neuro Oncol 14:798–807
Shonka NA, Theeler B, Cahill D, Yung A, Smith L, Lei X, Gilbert MR (2013) Outcomes for patients with anaplastic astrocytoma treated with chemoradiation, radiation therapy alone or radiation therapy followed by chemotherapy: a retrospective review within the era of temozolomide. J Neurooncol 113:305–311
Kalbfleisch J, Prentice R (2002) The statistical analysis of failure time data. Wiley, Hoboken
Cox DR, Society S, Methodological SB (2007) regression models and life-tables. J R Stat Soc Ser B 34:187–220
Gilbert MR, Dignam JJ, Armstrong TS et al (2014) A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 370:699–708
Chinot OL, Wick W, Mason W et al (2014) Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 370:709–722
Prados BMD, Scott C, Curran WJ, Nelson DF, Leibel S, Kramer S (2007) anaplastic astrocytoma: a retrospective review of radiation carmustine or PCV adjuvant chemotherapy. Society 17:3389–3395
Brandes AA, Nicolardi L, Tosoni A, Gardiman M, Iuzzolino P, Ghimenton C, Reni M, Rotilio A, Sotti G, Ermani M (2006) Survival following adjuvant PCV or temozolomide for anaplastic astrocytoma. Neuro Oncol 8:253–260
Hildebrand J, Gorlia T, Kros JM, Afra D, Frenay M, Omuro A, Stupp R, Lacombe D, Allgeier A, van den Bent MJ (2008) Adjuvant dibromodulcitol and BCNU chemotherapy in anaplastic astrocytoma: results of a randomised European Organisation for Research and Treatment of Cancer phase III study (EORTC study 26882). Eur J Cancer 44:1210–1216
Hildebrand J, Sahmoud T, Mignolet F, Brucher J, Afra D (1994) Adjuvant therapy with dibromodulcitol and BCNU increases survival of adults with malignant gliomas. EORTC Brain Tumor Group. Neurology 44:1479–1483
Brada M, Ashley S, Dowe A et al (2005) Neoadjuvant phase II multicentre study of new agents in patients with malignant glioma after minimal surgery. Report of a cohort of 187 patients treated with temozolomide. Ann Oncol 16:942–949
Gilbert MR, Friedman HS, Kuttesch JF, Prados MD, Olson JJ, Reaman GH, Zaknoen SL (2002) A phase II study of temozolomide in patients with newly diagnosed supratentorial malignant glioma before radiation therapy. Neuro Oncol 4:261–267
Yung BWKA, Prados MD, Yaya-tur R et al (2015) With anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. J Clin Oncol 17:2762–2771
Armstrong TS, Cao Y, Scheurer ME, Vera-Bolaños E, Manning R, Okcu MF, Bondy M, Zhou R, Gilbert MR (2009) Risk analysis of severe myelotoxicity with temozolomide: the effects of clinical and genetic factors. Neuro Oncol 11:825–832
Gerber DE, Grossman S, Zeltzman M, Parisi M, Kleinberg L (2007) The impact of thrombocytopenia from temozolomide and radiation in newly diagnosed adults with high-grade gliomas. Neuro Oncol 9:47–52
Rogers G, Garside R, Mealing S, Pitt M, Anderson R, Dyer M, Stein K, Somerville M (2008) Carmustine implants for the treatment of newly diagnosed high-grade gliomas. Pharmacoeconomics 26:33–44
Johnson BE, Mazor T, Hong C et al (2014) Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science 343:189–193
Kizilbash SH, Giannini C, Voss JS, Decker PA, Jenkins RB, Hardie J, Laack NN, Parney IF, Uhm JH, Buckner JC (2014) The impact of concurrent temozolomide with adjuvant radiation and IDH mutation status among patients with anaplastic astrocytoma. J Neurooncol 120:85–93
Van Den Bent MJ (2010) Interobserver variation of the histopathological diagnosis in clinical trials on glioma: a clinician’s perspective. Acta Neuropathol 120:297–304
Grossman SA, Ye X, Piantadosi S, Desideri S, Nabors LB, Rosenfeld M, Fisher J (2010) Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res 16:2443–2449
Funding
This study received no internal or external funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
Dr. Strowd reports no disclosures or conflicts of interest. Dr. Abuali reports no disclosures or conflicts of interest. Ms. Lu reports no disclosures or conflicts of interest. Dr. Ye reports no disclosures or conflicts of interest. Dr. Grossman reports no disclosures or conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Strowd, R.E., Abuali, I., Ye, X. et al. The role of temozolomide in the management of patients with newly diagnosed anaplastic astrocytoma: a comparison of survival in the era prior to and following the availability of temozolomide. J Neurooncol 127, 165–171 (2016). https://doi.org/10.1007/s11060-015-2028-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-015-2028-2