Abstract
Bevacizumab (BEV, Avastin®) produces durable objective radiological responses of 20–26 %, median response durations of 16–18 weeks, and median overall survival (mOS) of 31–40 weeks. While the use of BEV is well-established, the lack of dose–response studies in glioblastoma (GBM) patients raises the question whether current dosing practice is optimal. As a result of differing approaches to BEV dosing that ranged from the FDA approved package insert dose of 10 mg/kg every 2 weeks to 7.5 mg/kg every 3–4 weeks, among physicians within Northern California Kaiser Permanente hospitals over 4+ years, we did an IRB-approved retrospective analysis of patients seen in Northern California Kaiser Permanente facilities and treated with BEV. Between September 1, 2008 and August 31, 2013, 181 patients received BEV for tumor progression/recurrence starting 2.6 weeks after completion of chemoradiation. The integrated BEV administered dose-week (AUCBEV) for all patients had a median AUCBEV of 3.6 mg·wk/kg). Maximum likelihood analysis found patients over 65 years did worse than younger patients (p = 0.004), women lived longer (p = 0.002), and patients treated below the AUCBEV did better than those treated above the median AUCBEV (p = 0.003). mOS for BEV starting 1 month after chemoradiation was 45 versus 68 weeks (p = 0.012) and BEV starting 3 months after chemoradiation was 40 versus 74 weeks (p = 0.0085). Dosing BEV at half the standard dose for progressive/recurrent GBM was at least equivalent to or, maybe better than standard dosing. Unexplained was the observation that females had longer OS with BEV than males.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bevacizumab (Avast®) was approved by the FDA for the treatment of recurrent glioblastoma (GBM) in May 2009 based on durable objective radiological responses. The two clinical trials leading to approval used IV bevacizumab (BEV) of 10 mg/kg every 2 weeks [1–4]. In one trial of 85 patients, partial responses (PR) were observed in 25.9 % (95 % CI: 17, 36); the median response duration was 4.2 months (95 % CI: 3, 6) [1]. In a second trial of 56 GBM patients, PR was 19.6 % (95 % CI: 11, 31) and the median response duration was 3.9 months (95 % CI: 2.4, 17.4) [4]. The overall survival (OS) reported for BEV from these studies ranged between 31 and 40 weeks [1, 4]. From these approval studies, adverse events of any grade were reported as infection, fatigue, headache, hypertension, epistaxis, and diarrhea. Adverse events of grade level 3–5 included bleeding/hemorrhage, central nervous system (CNS) hemorrhage, hypertension, venous and arterial thromboembolic events, wound-healing complications, proteinuria, gastrointestinal perforation, and reversible posterior leukoencephalopathy [2].
In addition to these studies and others reporting benefit for patients with recurrent GBM, it was also found that BEV stopped the progression of CNS radiation-induced brain necrosis [5–10]. One study also established class 1 evidence of benefit in a randomized placebo-controlled trial of symptomatic patients [6]. In that, and a prior study [5], the group at the University of Texas M.D. Anderson Cancer Center found that a dose of BEV of 7.5 mg/kg every 3 weeks was adequate to reduce radiographic effects of radiation necrosis. It has been a concern of ours, based on population pharmacokinetics of BEV that found a T1/2β of 19.9 days and accumulation of BEV on both a two-week and three-week schedule, [11] that a three-week dosing schedule would be satisfactory and more convenient for patients. Furthermore, since no dose–effect study had been done with BEV in GBM patients, it was problematic whether 10 mg/kg every 2 weeks, 5 mg/kg every 2 weeks, or 7.5 mg/kg every 3 weeks would provide any meaningful difference in the clinical efficacy of BEV in GBM patients. In addition, the oncology community endorsed BEV at 5 mg/kg every 2 weeks for colorectal, breast cancer, and NCSLC. A similar argument was made in an editorial by Wick and colleagues who questioned the scientific rationale for the 10 mg/kg every two-week dose of BEV in GBM patients since no dose–effect study had ever been done in GBM patients [12]. In 2010, one of us (VAL) started working as a neuro-oncologist for a Kaiser Permanente hospital and only treated patients with BEV at starting doses of 7.5 mg/kg or lower at intervals of 3 weeks. In 2010, Kaiser physicians and nurse practitioners used the FDA approved package insert dose of 10 mg/kg every 2 weeks. Over the next 4 years, most prescriptions for BEV in the treatment of progressive/recurrent GBM slowly fell in dose and increased in interval between treatments. As a result of changing practice patterns, we decided to do a retrospective analysis of patients seen in Northern California Kaiser Permanente facilities and treated with BEV for presumed progressive/recurrent glioblastoma. The goal of the study was to determine if there was a difference in patient OS and adverse events as a function of administered dose of BEV.
Methods
IRB approval was obtained to conduct a retrospective chart review of all patients treated with BEV for progressive/recurrent GBM in Northern California Kaiser Permanente facilities between September 1, 2008 and August 31, 2013. Our goal was to determine whether overall survival (OS) was affected by the BEV dose administered since it might have an impact on OS and adverse events. Patient records in Kaiser’s Epic Health Connect database were interrogated together with the intravenous drug dosing for BEV obtained from the Beacon database using the Beacon COPS/CAMMOLOT database to locate patients from Northern California that had received BEV for the treatment of progressive/recurrent GBM. We found 181 patients that fulfilled criteria for treatment of recurrent glioblastoma. Information that was sought for each patient include: age at first BEV dose, date of first BEV dose, BEV dose administered per course, number of courses of BEV, duration of all BEV treatments (first dose to last dose), total BEV administered, date and type of external beam radiation therapy (EBRT), date and type of most recent GBM surgery prior to first BEV dose, duration and number of cytotoxic chemotherapy treatment courses during BEV treatment, and overall survival (OS) from first BEV treatment or last contact date if the patient is still alive.
We determined OS from the time of first BEV dose for patients who received their first BEV dose at least 2.6 weeks after completion of EBRT. Since patients will receive a starting dose, but, over their remaining life, may have a change in weeks between treatments, lapses in BEV treatment for a variety of reasons, and reduction in treatment doses, we elected to use their integrated exposure dose as the primary measure of BEV dose. The approach we used to compute BEV an actual received dose per week value is similar to that used previously [13]. By this method,
In Eq. 2 the number 2 represents weeks from last treatment as an estimate of duration effect of the last dose of BEV.
Our initial statistical approach was to analyze the impact of various BEV dose parameters on OS. We used Kaplan–Meier representation [14] and compared the two curves using the log-rank test [15] and Gehan-modified Wilcoxon test [16]. We also used Cox proportional hazards regression model to provide point and interval estimates of the hazard ratios (HR) for overall survival in relation to age of initial Bevacizumab therapy, gender, full or reduced dose of Bevacizumab, and surgical status. Analysis was done with SAS v. 9.2 (SAS Institute Inc. Cary, NC). All statistical tests were two-sided.
Results
The demographics of the 181 patients found with sufficient information and at least two doses of BEV are shown in Table 1. The patient group is typical for GBM with a median age of 60 years, a male preponderance (1.8:1), surgical resection almost equally divided between subtotal resection (STR) and gross total resection (GTR) of gadolinium-contrast tumor based on MRI, and most patients receiving 60 Gy EBRT with less than 10 % receiving hypofractionated EBRT (39–42 Gy in 13–17 fractions). Using all 181 patients as a basis, the median AUCBEV was 3.6 mg·wk/kg. There were no statistical differences in baseline demographic and clinical characteristics between patients who received bevacizumab below the median dose and those who received equal to or above the median dose. To determine if the starting dose of BEV was reflected in the computed AUCBEV, we compared the starting dose of BEV to the AUCBEV for each patient. We found a low correlation coefficient of 0.44 and r2 of 0.19 indicating a poor correlation that further supported the AUCBEV analyses.
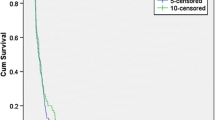
Since patients and their MRI can show subacute radiation effect (also called pseudo-progression) after completion of EBRT and up to about week 12 [17–23], we elected to present three groups of patients that had MRI progression of tumor starting 2.6 weeks after completion of EBRT, at 1 month (4.3 weeks) after EBRT, and at 3 months (13 weeks) after EBRT. We analyzed these three patient groups using the median AUCBEV of 3.6 as the breakpoint. These three groups are shown in Figs. 1, 2 and 3. For the entire group of 181 patients who progressed after 2.6 weeks (Fig. 1), the mOS is 60 weeks for those treated at <3.6 mg·wk/kg and mOS is 45 weeks for those treated ≥3.6 mg·wk/kg with a log-rank p = 0.029. For those after 1 month (4.3 weeks, Fig. 2) the mOS is 68 weeks for those treated at <3.6 mg·wk/kg and mOS is 45 weeks for those treated ≥3.6 mg·wk/kg with a log-rank p = 0.012. For those progressing after 3 months (13 weeks, Fig. 3) the mOS is 74 weeks for those treated at <3.6 mg·wk/kg and mOS is 40 weeks for those treated ≥3.6 mg·wk/kg with a log-rank p = 0.0085.
Kaplan–Meier representation of all 181 patients treated with IV BEV starting 2.6 weeks after completion of EBRT. For patients treated below the median AUCBEV exposure dose of 3.6 mg·wk/kg, the mOS was 60 weeks (E/N = 72/106); for patients equal to or above that median, mOS was 45 weeks (E/N = 60/75). The differences between the two curves by the log-rank test was significant at p = 0.029
Kaplan–Meier representation of 155 patients treated with IV BEV starting 4.3 weeks (1 month) after completion of EBRT. For patients treated below the median AUCBEV exposure dose of 3.6 mg·wk/kg, the mOS was 68 weeks (E/N = 53/81); for patients equal to or above that median, mOS was 45 weeks (E/N = 59/74). The differences between the two curves by the log-rank test was significant at p = 0.012
Kaplan–Meier representation of 97 patients treated with IV BEV starting 13 weeks (3 month) after completion of EBRT. For patients treated below the median AUCBEV exposure dose of 3.6 mg·wk/kg, the mOS was 74 weeks (E/N = 30/50); for patients equal to or above that median, mOS was 40 weeks (E/N = 39/47). The differences between the two curves by the log-rank test was significant at p = 0.0085
Analysis of covariates (Table 2) that impact OS for the 181 patient group finds that patients over 65 years do worse than those younger patients (p = 0.0035), female gender lived longer (p = 0.0024), and patients treated at AUCBEV <3.6 mg·wk/kg do better than those treated at higher AUCBEV (p = 0.0027). Surprising was the finding that female gender led to longer OS. In an effort to better understand this observation, we evaluated gender for differences in Karnofsky Performance Score or extent of surgery. By Chi square test there was no difference in initial Karnofsky Performance Score, equal to or above 90, and gender. Likewise, there was no difference in surgical status (biopsy, subtotal resection, or gross total resection) and gender.
There were no differences in AE for patients treated above and below the median AUCBEV (Table 3). The determination of hypertension (HTN) due to or exacerbated by differing doses of BEV was investigated through medical and antihypertensive drug histories. Eighty (44 %) patients had HTN before starting BEV; there were 12/91(13 %) in the group equal to or above the median AUCBEV and 17/90(19 %) in the group below the median AUCBEV. Worsening in HTN or new HTN was seen in 26/91(29 %) in the above median AUCBEV and 32/90(36 %) in the below median AUCBEV group (p = 0.34). Thus, it appears that worsening or new HTN was similar in patients regardless of BEV dosing.
Discussion
BEV is used for palliative therapy in patients with progressive/recurrent GBM based on studies that showed PR in 20–26 %, a median response duration of ~4 months, and OS of 7–9 months [1, 4].
Newer studies have also confirmed similar results with the BELOB trial in the Netherlands showing median PFS of 3 months and mOS of 8 months [24]. In another study, BEV treatment was evaluated based on whether BEV was given for early or late progression/recurrence [25]. In that study, median PFS was 4 months and mOS was nearly 10 months and similar for all three recurrence groups.
The use of IV BEV for the treatment of recurrent GBM appears to be effective whether using the FDA suggested dose of 10 mg/kg every 2 weeks or doses lower such as 7.5 mg/kg every 3 weeks. The analysis of the Northern California Kaiser Permanente set of 181 patients shows that there is persistent difference in OS with median AUCBEV that favors lower dosing compared to the FDA approved Roche/Genentech dosing recommendation. In addition, our mOS exceeds that quoted in the literature for progressive/recurrent GBM treated with BEV [1, 2, 4, 24, 25]. Using a reduced dose of BEV of 7.5 mg/kg every 3 weeks. Raizer and colleagues found a median PFS of 2.7 months and mOS of 6.4 months [26]. Wong and colleagues did a meta-analysis of 548 patients from the literature that were treated at 5, 10, and 15 mg/kg for progressive/recurrent GBM but found no difference PFS at 6 months or OS at 6 months [27].
To better understand the basis for this, we divided our patients into groups of varying time after EBRT as we assumed that subacute radiation effect (pseudoprogression) might be a confounding issue and affect response to BEV treatment. By 3 months after EBRT, we expected that most patients who progressed would do so because of tumor growth or, possibly, a mixture of radiation effect and tumor growth. Nonetheless, in all three groups studied, whether progressive by MRI at 2.6 weeks, 1 or 3 months after EBRT, patients treated at lower doses (AUCBEV <3.6 mg·wk/kg) than the FDA-approved dose showed longer mOS.
We also interrogated the patients surviving more than 1 year on BEV and found that approximately 45 % of patients survived 52 weeks or longer from the first date of treatment with BEV. We found that a significantly greater percent of patients were in the reduced-dose group, 53 % versus 37 % (p = 0.03 by Chi square test). After adjusting for gender, surgical status, and whether they initiated treatment at an age greater than 65 years, we found that those who received reduced dose of BEV were more than twice as likely (logistic regression odds ratio 2.16, 95 % CI, 1.14, 4.03) to survive at least 52 weeks.
Patients treated above the median 3.6 mg·wk/kg in this study have comparable mOS to the 31- to 40-week mOS reported in the literature for full dose BEV [1, 4]. Other than receiving a lower dose of BEV, we have been unable to determine an independent causal link between those patients receiving an AUCBEV below the median to those above the median. We could find no differences in covariates of age, gender, extent of surgery, and Karnofsky Performance Score between those treated below or above median AUCBEV. What remains unexplained as well is why female gender led to better outcome.
Lastly, we looked at the time from last BEV dose to death. The median was 12.2 weeks (range 1–73 weeks) in 132 patients with an event. Of this group, 4.5 % had last BEV dose to death ≤2 weeks and 10 % ≤3 weeks. This in keeping with a recent report of Rodriguez and colleagues who reported on 6089 patients with solid tumors treated at the University of Texas M.D. Anderson Cancer Center [28].
In conclusion, treatment for recurrent GBM with BEV appears to improve survival at a dose lower than that in the FDA drug insert. In addition, since the interval between doses moves from 2 to 3 weeks, patients find it more convenient and less costly per month of treatment. Given the potential benefit of lower dose BEV to our patients, it would be helpful to have prospective randomized dose-finding study for progressive/recurrent GBM patients to confirm the validity of our observation.
References
Friedman HS, Prados MD, Wen PY et al (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27(28):4733–4740
Cohen MH, Shen YL, Keegan P, Pazdur R (2009) FDA drug approval summary: Bevacizumab (Avastin®) as treatment of recurrent Glioblastoma Multiforme. Oncologist 14(11):1131–1138
Desjardins A, Reardon DA, Herndon JE II et al (2008) Bevacizumab plus irinotecan in recurrent WHO grade 3 malignant gliomas. Clin Cancer Res 14(21):7068–7073
Kreisl TN, Kim L, Moore K et al (2009) Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27(5):740–745
Gonzalez J, Kumar AJ, Conrad CA, Levin VA (2007) Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys 67(2):323–326
Levin VA, Bidaut L, Hou P et al (2011) Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys 79(5):1487–1495
Wong ET, Huberman M, Lu XQ, Mahadevan A (2008) Bevacizumab Reverses Cerebral Radiation Necrosis. J Clin Oncol 26:5649–5650
Liu AK, Macy ME, Foreman NK (2009) Bevacizumab as therapy for radiation necrosis in four children with pontine gliomas. Int J Radiat Oncol Biol Phys 75(4):1148–1154
Torcuator R, Zuniga R, Mohan YS et al (2009) Initial experience with bevacizumab treatment for biopsy confirmed cerebral radiation necrosis. J Neurooncol 94(1):63–68
Lubelski D, Abdullah K, Weil R, Marko N (2013) Bevacizumab for radiation necrosis following treatment of high grade glioma: a systematic review of the literature. J Neurooncol 115(3):317–322
Lu JF, Bruno R, Eppler S, Novotny W, Lum B, Gaudreault J (2008) Clinical pharmacokinetics of bevacizumab in patients with solid tumors. Cancer Chemother Pharmacol 62(5):779–786
Wick W, Weller M, van den Bent M, Stupp R (2010) Bevacizumab and recurrent malignant gliomas: a European perspective. J Clin Oncol. 28(12):e188–e189 (author reply e190-182)
Levin VA, Edwards MS, Wright DC et al (1980) Modified procarbazine, CCNU, and vincristine (PCV 3) combination chemotherapy in the treatment of malignant brain tumors. Cancer Treat Rep 64(2–3):237–244
Kaplan EL, Meier P (1958) Nonparametric estimates from incomplete observations. J Am Statist Assoc 53:457–481
Mantel N (1966) Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 50(3):163–170
Gehan EA (1965) A generalized Wilcoxon test for comparing arbitrarily singly-censored samples. Biometrika 52:203–223
Hoffman WF, Levin VA, Wilson CB (1979) Evaluation of malignant glioma patients during the postirradiation period. J Neurosurg 50(5):624–628
Brandes AA, Tosoni A, Spagnolli F et al (2008) Disease progression or pseudoprogression after concomitant radiochemotherapy treatment: pitfalls in neurooncology. Neuro Oncol. 10(3):361–367
Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ (2008) Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol 9(5):453–461
Chamberlain MC (2008) Pseudoprogression in glioblastoma. J Clin Oncol 26(26):4359 author reply 4359–4360
Brandsma D, van den Bent MJ (2009) Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol 22(6):633–638
Chaskis C, Neyns B, Michotte A, de Ridder M, Everaert H (2009) Pseudoprogression after radiotherapy with concurrent temozolomide for high-grade glioma: clinical observations and working recommendations. Surg Neurol 72(4):423–428
Clarke JL, Chang S (2009) Pseudoprogression and pseudoresponse: challenges in brain tumor imaging. Curr Neurol Neurosci Rep 9(3):241–246
Taal W, Oosterkamp HM, Walenkamp AM et al (2014) Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol 15(9):943–953
Piccioni DE, Selfridge J, Mody RR et al (2014) Deferred use of bevacizumab for recurrent glioblastoma is not associated with diminished efficacy. Neuro Oncol 16(6):815–822
Raizer JJ, Grimm S, Chamberlain MC et al (2010) A phase 2 trial of single-agent bevacizumab given in an every-3-week schedule for patients with recurrent high-grade gliomas. Cancer 116(22):5297–5305
Wong ET, Gautam S, Malchow C, Lun M, Pan E, Brem S (2011) Bevacizumab for recurrent glioblastoma multiforme: a meta-analysis. J Natl Compr Canc Netw 9(4):403–407
Rodriguez MA, DeJesus AY, Cheng L (2014) Use of chemotherapy within the last 14 days of life in patients treated at a comprehensive cancer center. JAMA Intern Med 174(6):989–991
Funding
Internal Kaiser Permanente funding.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Levin, V.A., Mendelssohn, N.D., Chan, J. et al. Impact of bevacizumab administered dose on overall survival of patients with progressive glioblastoma. J Neurooncol 122, 145–150 (2015). https://doi.org/10.1007/s11060-014-1693-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-014-1693-x