Abstract
Background
Bevacizumab (BEV), at a standard dose of 10 mg/kg every 2 weeks is associated with prolonged progression-free survival (PFS) but no improvement in overall survival (OS) in recurrent glioblastoma (rGBM). Few studies have examined the potential dose-dependent efficacy of BEV. In Ontario, reimbursement for the costs of BEV varies, and as a result, our practice began to routinely use lower dose regimens. The main aim of this study was to ensure that there was no harm to patients who received the low dose protocol.
Methods
A single-center retrospective study of patients given BEV for rGBM between 2015 and 2020 was performed. Clinical and treatment data including BEV dose regimen [SD (10 mg/kg every 2 weeks) vs. LD (5 mg/kg every 2–3 weeks or 10 mg/kg every 3 weeks)] received at the time of rGBM diagnosis were captured. Overall survival (OS) and progression-free survival (PFS) on BEV were compared using the Kaplan–Meier product-limit method. Log-rank test was used to compare potential predictive factors. Cox regression model was performed for multivariable analysis of OS and PFS.
Results
A total of 96 patients were included with a median follow-up duration of 6.84 months (range 1.12–50.63 months) from the date of the first infusion. The LD group consisted of 55 of the 96 patients. By virtue of funding mechanisms for BEV, the median age in the LD group was significantly higher (62 vs. 54 years p = 0.009). There was no difference in MGMT status between the two groups (p = 0.60). The LD group had prolonged median PFS (5.89 months versus 3.22 months; p = 0.0112) and OS (10.23 months versus 6.28 months; p = 0.0010). Multivariable analysis including the dose of BEV, the extent of resection, gender, and age revealed that standard dose of BEV, subtotal resection, and female sex were associated with worse overall survival. Nine patients in the SD group vs. 18 patients in the LD group reported an adverse event related to BEV.
Conclusion
For patients with recurrent GBM, we found that a low dose regimen of BEV was associated with prolonged OS and PFS compared to the standard dose regimen. Lower dose schedules may be a better and more cost-effective option for patients with rGBM. Lower costs might provide more equitable access to this very important palliative drug.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prognosis for glioblastoma (GBM) remains poor despite advances in our understanding of glioma biology. Despite a large number of clinical trials testing experimental therapies, the standard of care remains surgery, radiation therapy, and temozolomide (TMZ) chemotherapy [1]. There are currently no proven survival-prolonging therapies for recurrent glioblastoma (rGBM) and the median survival after progression is 3–9 months. Bevacizumab is a recombinant humanized monoclonal IgG1 antibody against VEGF, which is linked to angiogenesis and tumor proliferation in GBM. In 2010, Health Canada approved the use of bevacizumab for rGBM based on promising but preliminary phase II data [2]. While bevacizumab can achieve impressive radiographic responses, and provide symptomatic and steroid-sparing benefits, improved survival in rGBM has not been demonstrated using bevacizumab alone or in combination with chemotherapy [3, 4]. Outcomes in Canadian rGBM patients treated with bevacizumab (alone or with chemotherapy) appear to be consistent with data reported from larger phase II or III clinical trials of bevacizumab in rGBM [5]. Bevacizumab has been increasingly used in Canada, in part because clinical experience suggests that selected patients benefit in terms of symptom palliation and maintenance of the quality of life. However, due to its prohibitive cost, many Canadian patients still do not have access to this agent through provincial healthcare systems, including in Ontario. The approved dose of bevacizumab is 10 mg/kg (per infusion every 2 weeks), based on the early trials of bevacizumab in GBM, however, there is a lack of dose-response studies in this population. Our institutional practice has been to administer 10 mg/kg every 2 weeks, but in self-paying patients who want treatment but are handicapped by the cost, we opted to reduce the regimen to 5 mg/kg every 2–3 weeks. Observational data from retrospective studies using 5–15 mg/kg every 2–3 weeks suggest no difference in progression-free survival (PFS) in rGBM patients [6, 7]. In addition, although larger trials are lacking, certain studies seem to suggest that a lower dose of bevacizumab may be related to improved survival [8, 9]. In addition to this potentially important finding, the use of lower doses of bevacizumab is compelling as lower costs could make this treatment more widely available for patients who currently lack better alternatives. There is also a potential reduction of side effects and adverse events related to a lower dose of bevacizumab. Finally, the administration of bevacizumab at 10 mg/kg in conjunction with chemotherapeutic agents has been of limited survival benefit, and salvage chemotherapy after bevacizumab has not been effective [10]. This is thought to be related to the modulation of the blood vessels at this particular dose of bevacizumab, which in turn decreases the tumor penetration of concomitant therapy [11]. It is unclear if lower doses of bevacizumab may allow for more optimized modulation of blood vessels to allow for more effective use of concomitant therapy, which may in turn improve survival.
Methods
Study design and participants
This is a single-center retrospective study performed at the Odette cancer center and encompassed a cohort of patients with a diagnosis of rGBM. Clinical charts from all patients with rGBM who received bevacizumab between 2015 and 2020 were reviewed using our institutional electronic medical records (SunnyCare, Connecting Ontario, PRO, and Sovera) and chart abstraction was done by trained clinicians/abstractors in accordance with institutional guidelines and ethics review board policies. Detailed patient demographics, IDH and MGMT promoter methylation status, treatment modalities (type and extent of resection, type, dose, and cycles of chemo-radiotherapy), adverse event, and outcome data were collected and stored using encrypted computers onto REDCAP, a widely used and secure data platform, and real-time data verification was performed periodically. Specifically, the dose of bevacizumab, number of infusions, and frequency of infusions were captured. A regimen of less than 10 mg/kg every 2 weeks was annotated as being a “low dose (LD)”, and a dose of 10 mg/kg every 2 weeks as the “standard dose (SD)”.
Definitions and outcomes
An integrated diagnosis adhering to the fifth edition of the WHO Classification of Tumors of the Central Nervous System (WHO CNS5) [12] was assigned in our study. In rare instances where the initial histopathological diagnosis showed an Astrocytoma, IDH-wildtype, WHO Grade 2 or 3 they were grouped with Glioblastoma, IDH-wildtype at the time of recurrence based on the radiological features rather than molecular features. Recurrence or progression definition was based on clinical and/or radiological criteria after the traditional surgical and/or chemo-radiation therapy period. Both focal and non-focal neurological symptoms/signs that can’t be attributed to the traditional therapy side effects or be better explained by an alternative diagnosis were used for the clinical recurrence. Radiologically, the Response Assessment in Neuro-Oncology (RANO) criteria was used to define recurrence and was assessed by an experienced neuro-radiologist [13].
Overall survival (OS) was measured in months from the diagnostic surgery date to the date of death and OS on bevacizumab was measured in months from the first infusion date to the date of death. Progression-free survival (PFS) on bevacizumab was measured in months from the first infusion date to the date of progression on bevacizumab or date of death whichever occurred first. Patients lost to follow-up were censored at their last appointment date.
Statistical analysis
Patient and radiological characteristics were compared across the two treatment groups (low dose vs. standard-dose bevacizumab) using Wilcoxon’t sum rank test for continuous measures and the chi-squared and/ or Fisher’s exact test, as appropriate, for categorical variables. OS, as well as PFS rates, were calculated using the Kaplan–Meier product-limit method. Log-rank test was used as a univariate analysis to compare levels of patient characteristics and other potential predictive factors. Cox regression model was performed for multivariable analysis for OS and PFS to assess the joint effect of those factors that were found potential in the univariate analysis. All P-values were 2-sided and for the statistical analyses, P < 0.05 was considered to indicate a statistically significant result. Statistical analysis was performed using version 9.4 of the SAS system for Windows, 2002–2012 SAS Institute, Inc., Cary, NC, USA.
Results
Between 2015 and 2020, 96 patients with a diagnosis of recurrent GBM were captured within this single-center cohort. Median follow-up was 20.3 months (range: 2.5–77.1 months) from the time of surgery. Of the 96 patients, 55 (57%) received the LD schedule and 41 (43%) were given the SD schedule. From the first day of BEV administration, the median follow-up was 6.84 months (range: 1.12–50.63 months). The median age of the entire cohort was 57 (range: 19–80 years) with a significantly older age seen in the LD group (median 62 years for LD, versus 54 years for SD, p = 0.009). Extent of resection was not significantly different between the two treatment groups (p = 0.97). There was no statistically significant difference in the number of cycles of TMZ administered in the adjuvant phase (p = 0.11). One patient in the low-dose cohort did not have an IDH status report but was presumed to be IDH-wildtype GBM based on age and radiographic appearance. MGMT status was not known for 58 patients, due to inconclusive results or lack of testing. Of the remaining patients, only 12 (31.58%) patients had a methylated MGMT promoter and 26 (68.42%) were unmethylated and MGMT status was not significantly different between the tow treatment groups (with the proportion of methylated being 34.78% versus 26.67%, p = 0.60) (Table 1).
Survival outcomes from diagnostic surgery
The median OS from the time of the surgery was 22.72 months (95% CI: 19.07–25.68 months) with a 2-year survival rate of 45.4%. Patients treated with adjuvant TMZ had a statistically significant survival benefit (p = 0.0012) with a median OS of 24.33 months (95% CI 21.37–26.70 months; 2-year OS 52.5%) compared to 16.04 months (95% CI 10.46–22.72 months; 2-year OS 8.8%) for those without adjuvant TMZ. On univariate analysis, the number of BEV infusions, dose regimen (low vs. standard), and adjuvant TMZ were significantly associated with prolonged survival while age, sex, extent of resection, and MGMT promoter methylation status were not.
Survival outcomes from the start of BEV infusions
From the first BEV infusion, the median PFS and OS were 4.14 months (95% CI 3.25–5.82 months) and 7.96 months (95% CI 6.58–9.9 months), respectively (Fig. 1). The 12-month PFS and OS survival rates were 18.7% and 26.3%, respectively. Stratified by the diagnostic surgery extent of resection, biopsy versus subtotal versus gross total, there was a statistically significant difference in the PFS (p = 0.0037) but not the OS (p = 0.1248). The median PFS was 2.88 months (95% CI 2.04–3.81 months) for a subtotal resection, 5.29 months (95% CI 3.26–8.25 months) for a gross total resection, and 7.4 months (95% CI 3.06–14.17 months) for a biopsy. There was no statistically significant difference in the overall survival when stratified by sex (p = 0.1247).
Survival outcomes for low versus standard dose from the start of BEV infusions
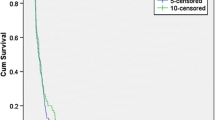
In the SD group, all patients received BEV 10 mg/kg every 2 weeks. In the LD group, 63.6% received 5 mg/kg every 3 weeks, 20% were 10 mg/kg every 3 weeks and 16.4% were 5 mg/kg every 2 weeks. No patients had a cross-over between the standard- and low-dose regimens (Table 2). The median number of BEV cycles was numerically higher in the SD (median of 4 cycles; range: 0–12) but not statistically different (p = 0.32) from the LD regimen (median of 2 cycles; range 0–12). At the time of the first infusion, the median dexamethasone dose (median of 4 mg per day; range: 0–16) and the proportion of patients with functional independence (65.30% in the LD versus 60% in the SD) were similar between both groups. Bevacizumab salvage therapy was started at an earlier point in the disease course in those receiving the SD (48.78% in SD versus 35.54% in LD at 1st recurrence) (Table 1). Compared to the SD group those in the LD group had a statistically significant improvement in PFS (p = 0.0112) and OS (p = 0.0010) (Fig. 2).
Kaplan–Meier curves stratified by bevacizumab regimen from the date of the first infusion for recurrent GBM. The median progression-free survival (a) was 5.89 months (95% CI 3.72–7.5 months) in the LD group compared to 3.22 months (95% CI 2.27–4.70 months) in the SD group. The median overall survival (b) was 10.23 months (95% CI 7.79–11.87 months) in the LD group compared to 6.28 months (95% CI 3.98–7.79 months) in the SD group
In the low dose regimen, the median PFS was 5.89 months (95% CI 3.72–7.5 months; 6-month PFS 49.8%) compared to a median of 3.22 months (95% CI 2.27–4.70 months; 6-month PFS 23%) in the standard dose regimen from the time of the first infusion (Fig. 2a). Gross total resection at the time of the diagnostic surgery and low dose of BEV were factors found to have a positive impact on PFS with those receiving a lower dose found to be 2.67-fold less likely to progress during our follow up time (HR = 0.375, 95% CI 0.23–0.61; p < 0.001).
In contrast to the median OS of 6.28 months (95% CI 3.98–7.79 months; 6-month OS 51.7%) seen with the SD regimen, the LD group had a median OS of 10.23 months (95% CI 7.79–11.87 months; 6-month OS 77.3%) resulting in an approximate survival gain of 4 months (Fig. 2b). Multivariable analysis revealed that low dose of BEV, gross total resection at the time of diagnosis, and male sex were associated with better survival. Of note, those receiving a low dose had 2.56-fold better chances of survival (HR = 0.39, 95% CI 0.24–0.64; p = 0.0002). This gain was more pronounced when the OS was measured from the time of the diagnostic surgery with a median survival of 24.26 months (95% CI 20.71–31.99 months; 2-year OS 51.8%) versus 18.87 months (95% CI 14.37–25.64 months; 2-year OS 37.2%) (Fig. 3).
Adverse events
Table 3 summarizes the safety profile of BEV in our patients. Overall, the infusions were well tolerated in both the LD and SD groups. The most common adverse effect was fatigue followed by arthralgia and hypertension, more commonly encountered with the lower dose of bevacizumab. Serious adverse events necessitating discontinuation of treatment were seen in three patients receiving the LD. These included venous thromboembolism and severe fatigue/arthralgia while on the 5 mg/kg every 3 weeks regimen and nephrotic syndrome while on the 10 mg/kg every 3 weeks regimen. One patient on SD had asymptomatic thrombocytopenia (nadir at 51,000), however, the reason for treatment discontinuation was lack of bevacizumab efficacy. No Grade 4 or 5 toxicities were encountered.
Discussion
Bevacizumab has been incorporated into the care of IDH-wt GBM patients, particularly in the recurrent setting where salvage therapies are limited. Bevacizumab has never been compared to best supportive care alone but evidence from large phase 2/3 studies [2, 4, 14, 15] has shown an improvement in the median PFS ranging from 3 to 5.6 months with BEV-based regimens. In contrast to this improvement in PFS [16, 17], no overall survival benefit has been shown [18]. Our SD cohort had a median PFS of 3.22 months (95% CI 2.27–4.70 months; 6-month PFS 23%) and a median OS of 6.28 months (95% CI 3.98–7.79 months; 6-month OS 51.7%) which is consistent with historical outcomes. Two phase II trials led to an accelerated approval by the U.S. Food and Drug Administration on May 5, 2009, for the use of BEV as a monotherapy in rGBM. Shortly afterward, in 2010, approval was granted by Health Canada [2, 15, 19]. Both trials assessed the efficacy of BEV standard dose of 10 mg/kg in patients with rGBM who were previously treated with traditional radiotherapy and TMZ chemotherapy. While none of the later studies in rGBM showed an improvement in OS, the 10 mg/kg regimen has since become the standard regimen.
In this retrospective study, we demonstrated that the LD schedule is associated with a statistically significant improvement in both progression-free and overall survival as compared to the SD schedule of 10 mg/kg given every 2 weeks. Our study demonstrates that there appears to be dose and schedule-dependent efficacy of BEV for recurrent glioblastoma. This finding is compelling for several reasons. While many studies have looked into the role of bevacizumab in rGBM (Table 3), dose-finding studies have generally not been pursued in the rGBM population. In colorectal cancer, alternative schedules of 5 mg/kg every 2 weeks or 7.5 mg/kg every 3 weeks are widely used. Initial reports of responses using dosing schedules as low as 5 mg/kg every 2 weeks encouraged the development of BEV for this indication [20, 21]. A single-arm phase 2 study confirmed the antitumor activity of 7.5 mg/kg every 3 weeks rGBM [6]. However, a meta-analysis of 548 patients with rGBM found no difference in the BEV dose-response benefit between 5 mg/kg and 10–15 mg/kg. Intriguingly, a single-center retrospective study of 181 patients compared the use of 10 mg/kg every 2 weeks and 7.5 mg/kg every 3 weeks and found a trend towards better survival in the lower dose group [9]. Indeed, it was this study by Levin et al. that helped to inform our practice of using lower-dose schedules for self-paying patients with financial hardship. Our main purpose of this study was to see if there was any evidence of worse survival outcomes with the lower dose schedule. Not only did we find no evidence of harm, but our findings also suggest a survival benefit in the lower dose group.
There may be other advantages to an LD schedule beyond survival alone. Although the adverse event rates in our study were low, we wondered if there would be less toxicity with the LD schedule. Our study doesn’t have the power to detect differences in these subgroups. On the surface, there appear to be more AEs reported in the LD group; however, there was a very large difference in median age between our study cohorts. Older patients tend not to have third-party prescription insurance and financial toxicity is a concern with expensive anti-cancer agents. In our study, the age bias may have led to the appearance of more adverse effects in the LD group. Age of course is a very powerful predictor of worse survival in GBM, making it all the more remarkable that we were able to detect better survival in the much older low-dose group.
Drug costs are a barrier to equity and access in all areas of oncology. Lower dose, less frequently administered drug regimens that are more cost-effective may improve access to these expensive therapeutics.
Our study has several limitations including its retrospective nature, potential bias in patient selection, and relatively small sample size. MGMT methylation status was unavailable for most patients. As 60% of patients are unmethylated and progress sooner, an imbalance in the distribution of methylation status could bias results, although BEV does not have an MGMT-dependent mechanism of action. The majority of our patients in the LD group received 5 mg/kg q 3 weekly, a cost reduction of 66% compared to standard dosing. We were unable to compare the different LD schedules due to the small number of patients in each group. A prospective clinical trial comparing different lower dose regimens with a well-captured clinician and patient-reported outcomes would be needed to better evaluate the potential survival and quality of life benefits of a specific lower dose regimen in rGBM.
References
Wen PY, Weller M, Lee EQ, Alexander BM, Barnholtz-Sloan JS, Barthel FP, Batchelor TT, Bindra RS, Chang SM, Chiocca EA, Cloughesy TF, DeGroot JF, Galanis E, Gilbert MR, Hegi ME, Horbinski C, Huang RY, Lassman AB, Le Rhun E, van den Bent MJ (2020) Glioblastoma in adults: a society for neuro-oncology (SNO) and European society of neuro-oncology (EANO) consensus review on current management and future directions. Neuro Oncol 22(8):1073–1113. https://doi.org/10.1093/neuonc/noaa106
Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27(28):4733–4740. https://doi.org/10.1200/JCO.2008.19.8721
Wick W, Gorlia T, Bendszus M, Taphoorn M, Sahm F, Harting I, Brandes AA, Taal W, Domont J, Idbaih A, Campone M, Clement PM, Stupp R, Fabbro M, Le Rhun E, Dubois F, Weller M, von Deimling A, Golfinopoulos V, van den Bent MJ (2017) Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med 377(20):1954–1963. https://doi.org/10.1056/NEJMoa1707358
Taal W, Oosterkamp HM, Walenkamp AM, Dubbink HJ, Beerepoot LV, Hanse MC, Buter J, Honkoop AH, Boerman D, de Vos FY, Dinjens WN, Enting RH, Taphoorn MJ, van den Berkmortel FW, Jansen RL, Brandsma D, Bromberg JE, van Heuvel I, Vernhout RM, van den Bent MJ (2014) Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol 15(9):943–953. https://doi.org/10.1016/S1470-2045(14)70314-6
Lim Fat MJ, Maurice C, Maganti M, Mason WP (2018) Bevacizumab in recurrent high-grade gliomas: a canadian retrospective study. Can J Neurol Sci 45(1):56–61. https://doi.org/10.1017/cjn.2017.248
Raizer JJ, Grimm S, Chamberlain MC, Nicholas MK, Chandler JP, Muro K, Dubner S, Rademaker AW, Renfrow J, Bredel M (2010) A phase 2 trial of single-agent bevacizumab given in an every-3-week schedule for patients with recurrent high-grade gliomas. Cancer 116(22):5297–5305. https://doi.org/10.1002/cncr.25462
Wong ET, Gautam S, Malchow C, Lun M, Pan E, Brem S (2011) Bevacizumab for recurrent glioblastoma multiforme: a meta-analysis. J Natl Compr Canc Netw 9(4):403–407. https://doi.org/10.6004/jnccn.2011.0037
Ajlan A, Thomas P, Albakr A, Nagpal S, Recht L (2017) Optimizing bevacizumab dosing in glioblastoma: less is more. J Neurooncol 135(1):99–105. https://doi.org/10.1007/s11060-017-2553-2
Levin VA, Mendelssohn ND, Chan J, Stovall MC, Peak SJ, Yee JL, Hui RL, Chen DM (2015) Impact of bevacizumab administered dose on overall survival of patients with progressive glioblastoma. J Neurooncol 122(1):145–150. https://doi.org/10.1007/s11060-014-1693-x
Quant EC, Norden AD, Drappatz J, Muzikansky A, Doherty L, Lafrankie D, Ciampa A, Kesari S, Wen PY (2009) Role of a second chemotherapy in recurrent malignant glioma patients who progress on bevacizumab. Neuro Oncol 11(5):550–555. https://doi.org/10.1215/15228517-2009-006
Gerstner ER, Emblem KE, Chang K, Vakulenko-Lagun B, Yen YF, Beers AL, Dietrich J, Plotkin SR, Catana C, Hooker JM, Duda DG, Rosen B, Kalpathy-Cramer J, Jain RK, Batchelor T (2020) Bevacizumab reduces permeability and concurrent temozolomide delivery in a subset of patients with recurrent glioblastoma. Clin Cancer Res 26(1):206–212. https://doi.org/10.1158/1078-0432.CCR-19-1739
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 23(8):1231–1251. https://doi.org/10.1093/neuonc/noab106
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28(11):1963–1972. https://doi.org/10.1200/JCO.2009.26.3541
Wick W, Brandes AA, Gorlia T, Bendszus M, Sahm F, Taal W, Taphoorn MJB, Domont J, Idbaih A, Campone M, Clement PM, Stupp R, Fabbro M, Rhun EL, Dubois F, Klein M, Platten M, Weller M, Golfinopoulos V, Bent MJVD (2016) EORTC 26101 phase III trial exploring the combination of bevacizumab and lomustine in patients with first progression of a glioblastoma. J Clin Oncol 34(15suppl):2001–2001. https://doi.org/10.1200/JCO.2016.34.15_suppl.2001
Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, Park J, Albert PS, Fine HA (2009) Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27(5):740–745. https://doi.org/10.1200/JCO.2008.16.3055
Schritz A, Aouali N, Fischer A, Dessenne C, Adams R, Berchem G, Huiart L, Schmitz S (2021) Systematic review and network meta-analysis of the efficacy of existing treatments for patients with recurrent glioblastoma. Neurooncol Adv 3(1):vdab052. https://doi.org/10.1093/noajnl/vdab052
Zhang T, Xin Q, Kang JM (2021) Bevacizumab for recurrent glioblastoma: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci 25(21):6480–6491. https://doi.org/10.26355/eurrev_202111_27092
Johnson DR, Omuro AMP, Ravelo A, Sommer N, Guerin A, Ionescu-Ittu R, Shi S, Macalalad A, Uhm JH (2018) Overall survival in patients with glioblastoma before and after bevacizumab approval. Curr Med Res Opin 34(5):813–820. https://doi.org/10.1080/03007995.2017.1392294
Cohen MH, Shen YL, Keegan P, Pazdur R (2009) FDA drug approval summary: bevacizumab (avastin) as treatment of recurrent glioblastoma multiforme. Oncologist 14(11):1131–1138. https://doi.org/10.1634/theoncologist.2009-0121
Wick W, Weller M, van den Bent M, Stupp R (2010) Bevacizumab and recurrent malignant gliomas: a European perspective. J Clin Oncol 28(12):e188-189. https://doi.org/10.1200/JCO.2009.26.9027
Stark-Vance V (2005) Bevacizumab and CPT-11 in the treatment of relapsed malignant glioma [Abstract 342]. Neuro Oncol 7(3):369. https://doi.org/10.1215/S1152851705200388
Ananthnarayan S, Bahng J, Roring J, Nghiemphu P, Lai A, Cloughesy T, Pope WB (2008) Time course of imaging changes of GBM during extended bevacizumab treatment. J Neurooncol 88(3):339–347. https://doi.org/10.1007/s11060-008-9573-x
Nghiemphu PL, Liu W, Lee Y, Than T, Graham C, Lai A, Green RM, Pope WB, Liau LM, Mischel PS, Nelson SF, Elashoff R, Cloughesy TF (2009) Bevacizumab and chemotherapy for recurrent glioblastoma: a single-institution experience. Neurology 72(14):1217–1222. https://doi.org/10.1212/01.wnl.0000345668.03039.90
Kaloshi G, Brace G, Rroji A, Bushati T, Roci E, Hoxha M, Fejzo G, Petrela M (2013) Bevacizumab alone at 5 mg/kg in an every-3-week schedule for patients with recurrent glioblastomas: a single center experience. Tumori 99(5):601–603. https://doi.org/10.1700/1377.15309
Weathers SP, Han X, Liu DD, Conrad CA, Gilbert MR, Loghin ME, O’Brien BJ, Penas-Prado M, Puduvalli VK, Tremont-Lukats I, Colen RR, Yung WKA, de Groot JF (2016) A randomized phase II trial of standard dose bevacizumab versus low dose bevacizumab plus lomustine (CCNU) in adults with recurrent glioblastoma. J Neurooncol 129(3):487–494. https://doi.org/10.1007/s11060-016-2195-9
Heiland DH, Masalha W, Franco P, Machein MR, Weyerbrock A (2016) Progression-free and overall survival in patients with recurrent glioblastoma multiforme treated with last-line bevacizumab versus bevacizumab/lomustine. J Neurooncol 126(3):567–575. https://doi.org/10.1007/s11060-015-2002-z
Blumenthal DT, Mendel L, Bokstein F (2016) The optimal regimen of bevacizumab for recurrent glioblastoma: does dose matter? J Neurooncol 127(3):493–502. https://doi.org/10.1007/s11060-015-2025-5
Gleeson JP, Keane F, Keegan NM, Mammadov E, Harrold E, Alhusaini A, Harte J, Eakin-Love A, O’Halloran PJ, MacNally S, Hennessy BT, Breathnach OS, Grogan L, Morris PG (2020) Similar overall survival with reduced vs. standard dose bevacizumab monotherapy in progressive glioblastoma. Cancer Med 9(2):469–475. https://doi.org/10.1002/cam4.2616
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
JP and ML contributed to the study’s conception and design. Data collection was performed by IG, EC, and AT. Data analysis was performed by EA. The first draft of the manuscript was written by JM and ML and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was approved by our local REB (SUN-3574) and a waiver for patient consent was obtained due to the retrospective nature of this study .
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Melhem, J.M., Tahir, A., Calabrese, E. et al. Dose-dependent efficacy of bevacizumab in recurrent glioblastoma. J Neurooncol 161, 633–641 (2023). https://doi.org/10.1007/s11060-023-04248-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04248-z