Abstract
Background Cerebral radiation necrosis is a serious complication of radiation treatment for brain tumors. Therapeutic options include corticosteroids, anticoagulation and hyperbaric oxygen with limited efficacy. Bevacizumab, an antibody against VEGF had been reported to reduce edema in patients with suspected radiation necrosis. We retrospectively reviewed 6 patients with biopsy proven cerebral radiation necrosis treated with bevacizumab between 2006 and 2008. Results Interval MRI follow-up demonstrated radiographic response in all patients with an average reduction of 79% for the post gadolinium studies and 49% for the FLAIR images. The initial partial radiographic response was noted for up to a mean follow-up time of 5.9 months (6 weeks to 18 months). Conclusion Bevacizumab appears to produce radiographic response and clinical benefits in the treatment of patients with cerebral radionecrosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
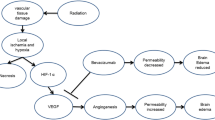
Cerebral radiation necrosis is a recognized potential treatment complication that usually develops in brain tumor patients one to three years after radiation [1]. The clinical manifestations of localized brain necrosis depend on where the necrosis is located and can result in focal neurological deficits or more generalized signs and symptoms of increased intracranial pressure [1, 2]. Radiation necrosis is associated with vascular endothelial damage resulting in irreversible fibrinoid necrosis of small arterial vessels [1, 3]. It is a continuous process leading to tissue hypoxia, necrosis, edema, demyelination and concomitant release of vascular endothelial growth factor (VEGF) [1, 3–5]. Surgical decompression of any radiation-necrosis-related mass effect can provide helpful palliative effect but does not reverse the process in the majority of patients [6]. Corticosteroids usually produce prompt symptomatic improvement [1, 3]. Therapeutic anticoagulation and hyperbaric oxygen therapy have also been reported to provide benefit, but their efficacy is far from established [1, 4, 7–9]. Bevacizumab is a humanized monoclonal antibody against vascular endothelial growth factor (i.e., VEGF, also known as vascular permeability factor) [3]. Since preventing VEGF from reaching its capillary targets is a logical treatment strategy for radiation necrosis, bevacizumab might be an effective treatment option [3].
Materials and methods
Six patients with gliomas previously treated with radiation and chemotherapies were biopsied for imaging progression. In addition to magnetic resonance imaging (MRI), perfusion computed tomography (CT) scans were performed as part of the pre-operative studies. Biopsy sites were correlated with areas of low blood volume and permeability on CT perfusion. All patients were confirmed to have radiation necrosis on histopathology. The patients were then treated with bevacizumab alone (10 mg/kg) or combined with Irinotecan (CPT 11) both given via infusion every 2 weeks. Two of six (2/6) patients were treated with bevacizumab plus irinotecan. One patient was reported to have majority radiation necrosis with the presence of isolated tumor cells in the resection specimen, so it was felt that it was indicated to treat for tumor as well as radiation effect (Table 1). The treating oncologist for the other patient elected to treat with bevacizumab and irinotecan because of concern of possible tumor missed by biopsy.
MRI studies were then obtained at 6-week intervals to determine response to treatment. Bidimensional measurements (the product of the longest diameter and its longest perpendicular diameter per MacDonald criteria) of the largest radiation necrosis lesions were recorded for both gadolinium-enhanced and FLAIR sequences [10]. The difference in the measurements was expressed as a percentage change from the pre-treatment MRI images. Data were collected pertaining to dexamethasone dose, complications, duration of response and clinical outcome.
Results
We treated six patients with an age range of 41–65. Of the three patients diagnosed with glioblastoma multiforme, there was one anaplastic astrocytoma, one anaplastic ependymoma, and one astrocytoma (Table 1). Biopsy results showed pure cerebral radiation necrosis in 5/6 patients with one patient reported to have radiation necrosis and tumor (Table 1). Of the six patients in the study, two have received prior stereotactic radiosurgery and two patients were previously treated with hyperbaric oxygen (Table 1). The average time from fractionated external beam treatment to bevacizumab was 19.1 months. One patient was treated with stereotactic radiosurgery (16 Gy) and another patient was given fractionated stereotactic radiotherapy (36 Gy) with an average interval of 6.5 months prior to receiving bevacizumab. Four patients were treated with bevacizumab alone and two patients received irinotecan and bevacizumab. These patients were given an average of 6.8 infusions. Compared to baseline pre-bevacizumab contrast and FLAIR studies, the average reduction in the post-gadolinium studies was 79% and 49% for the FLAIR images (Table 2, Figs. 1, 2, 3, 4). The duration of response ranged from 6 weeks to 18 months (average of 5.9 months). One patient’s treatment was discontinued due to an infection that developed at the incision site, and another patient’s treatment was discontinued due to profound fatigue.
Dexamethasone dose ranged from 0 to 4 mg per day (ave = 1 mg) before starting treatment (Table 2). The 2 patients who had progressed despite hyperbaric oxygen treatment had been on high-dose dexamethasone previously and had plateaued therefore dexamethasone taper was started. Furthermore, tapering was felt to be justified owing to significant steroid side effects, and evidence from the follow-up MRI post HBO2 did not show increased mass effect associated with the increased enhancement. The remaining 4 patients’ pre-treatment MRIs did not demonstrate significant mass effect and to minimize steroid toxicity, the dexamethasone total daily dose was kept at the lowest possible dose to maintain clinical status. All patients were successfully tapered off dexamethasone.
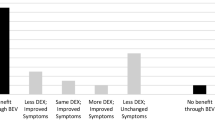
Of the six patients treated, three are still alive and clinically improved although one seemed to have significant fatigue with bevacizumab infusion (Table 1). The remaining three patients died, two due to tumor progression and one from pneumonia; however, these patients did not report worsening symptoms while on bevacizumab treatment.
Discussion
Cerebral radiation necrosis is a recognized potential complication with debilitating sequelae among patients receiving therapeutic radiation of the central nervous system [1, 7]. This typically develops one to three years after radiation with a risk of 2 to 19% depending on the dose and fractionation [1, 2, 7, 11]. Levin et al. noted over 50% incidence of radiation necrosis in one study of patients with malignant glioma treated with carboplatin [12]. It has also been reported that chemotherapy can have an additive effect on the development of cerebral radiation necrosis in the setting of radiotherapy [13]. The reported incidence of radionecrosis ranged from 2.5 to 24% with a mean time interval from end of radiotherapy until necrosis of 11.6 months [1, 14–17]. Furthermore, the incidence of radiation necrosis varies over time and as a function of glioma histology, with radiation necrosis occurring earlier in glioblastoma (median close to 10 months) patients than anaplastic glioma (median close to 18 months) [18].
The primary mechanism of the delayed injury in radiation associated with necrosis is secondary to vascular endothelial injury resulting in endothelial thickening, lymphocytic and macrophagic infiltrates, presence of cytokines, hyalinization, fibrinoid deposition, thrombosis, and occlusion [19]. Furthermore, with prolonged radiation exposure, cytokines such as tumor necrosis factor, interleukin-1, and tissue growth factor became overexpressed resulting in a cascade of inflammatory events and vascular injury [1, 19]. Demyelination, oligodendrocyte dropout, axonal swelling, reactive gliosis, and disruption of the blood-brain barrier also can be observed [1, 19]. The disruption of the blood-brain barrier (as characterized by gadolinium) may be mediated partly by VEGF that is released in response to hypoxia [3]. The damage is usually irreversible and in some cases may be relentlessly progressive with a tendency to recur even after surgical resection [1]. The prognosis is usually guarded in these patients [1].
Surgical decompression of any radionecrosis-related mass effect can provide helpful palliative benefit but is associated with high risks of complications and may be considered for symptomatic patients who had failed medical therapy [5]. Medical options are aimed at controlling vasogenic edema and/or controlling vessel thrombosis. Corticosteroids usually produce prompt symptomatic improvement of cerebral edema but patients can become symptomatic after discontinuance thereby requiring long-term treatment with the associated medical complications of chronic steroid use [1]. The use of anticoagulant has been reported because radiation necrosis pathophysiology involves vessel thrombosis and subsequent occlusion. Glantz et al. reported clinical improvement in 5/8 patients with biopsy-confirmed cerebral radiation necrosis with the use of heparin followed by warfarin anticoagulation for 3–6 months [9]; however, symptoms reemerged after anticoagulation was discontinued. Hyperbaric oxygen promotes perfusion and angiogenesis. Oxygen is delivered at 20–24 atm for 20–30 sessions. Each session lasts approximately 90–120 min. Chuba et al. published their experience on the use of hyperbaric oxygen therapy in 10 patients (8 had biopsy-proven radiation necrosis) [7]. He reported clinical improvement in 6/10 patients with 3 having documented radiographic response. However, many of these patients also were receiving concomitant high-dose steroid therapy.
Bevacizumab is a humanized murine monoclonal antibody directed against VEGF. VEGF is released in the presence of hypoxia and necrosis leading to increased permeability of the blood-brain barrier with increased edema [20, 21]. Therefore, reversal of edema and imaging abnormalities may be achieved by preventing VEGF from reaching its capillary targets [3]. Gonzales et al. [3] reported their experience in 8 patients with radiation necrosis treated with bevacizumab. There were six patients exhibiting radiographic characteristics of radiation necrosis. Of the eight patients, only two patients had biopsy proven radiation necrosis. All patients showed radiographic improvement with an average reduction of 44% in the contrast studies and 59.75% in the FLAIR studies. The average dexamethasone dose before treatment with bevacizumab was 10.5 mg per day with reduction to an average of 3 mg per day on follow-up.
Our experience confirmed the 100% radiographic response noted by Gonzales et al. [3] with an average reduction in post contrast studies of 79% and Flair to 49% (Table 2). Furthermore, all patients in our study had biopsy proven cerebral radiation necrosis. The patients were also on a much lower dose of steroids (ave of 1 mg/day) before initiating treatment with bevacizumab and were tapered subsequently therefore the radiographic response cannot be attributed to dexamethasone therapy. We were also able to follow up on these patients with a mean period of 5.9 months (6 weeks to 18 months). The index lesions have remained stable in two patients that showed distal tumor progression. No patient showed clinical decline during the treatment with two patients reported to have improved symptoms.
The impressive imaging changes observed in the follow-up studies of these six patients with biopsy-confirmed radiation necrosis are likely related to the relative normalization of the blood-brain barrier secondary to decreased levels of VEGF brought about by bevacizumab. The optimal duration of bevacizumab treatment for radiation necrosis is unknown. We suggest discontinuing treatment after 6 months followed by surveillance imaging every 3 months. This study is limited because of the small number of patients and hence no firm conclusions can be drawn; however, the impressive results and overall safety profile of these patients is encouraging.
Further studies should be undertaken to fully establish the role of bevacizumab in the treatment of cerebral radiation necrosis.
References
Giglio P, Gilbert MR (2003) Cerebral radiation necrosis. Neurology 9(4):180–188. doi:10.1097/01.nrl.0000080951.78533.c4
Cross N, Glantz M (2003) Neurologic complications of radiation therapy. Neurol Clin N Am 21(1):249–277
Gonzales J, Kumar AJ, Conrad CA, Levin VA (2007) Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys 67(2):323–326. doi:10.1016/j.ijrobp.2006.10.010
Brandes A, Tosoni A, Spagnolli F, Frezza G, Leonardi M, Calbucci F, Franceschi E (2008) Disease progression after concomitant radiochemotherapy treatment: pitfalls in neurooncology. Neuro-oncol 10(3):361–367. doi:10.1215/15228517-2008-008
Ricci P, Karis J, Heiserman J, Fram EK, Bice AN, Drayer BP (1998) Differentiating recurrent tumor from radiation necrosis: time for re-evaluation of positron emission tomography? AJNR Am J Neuroradiol 19(3):407–413
McPherson C, Warnick R (2004) Results of contemporary surgical management of radiation necrosis using frameless stereotaxis and intraoperative magnetic resonance imaging. J Neurooncol 68(1):41–47. doi:10.1023/B:NEON.0000024744.16031.e9
Chuba P, Aronin P, Bhambhani K, Eichenhorn M, Zamarano L, Cianci P, Mulbauer M, Porter AT, Fontanesi J (1997) Hyperbaric oxygen therapy for radiation induced brain injury in children. Cancer 80(10):2005–2012. doi:10.1002/(SICI)1097-0142(19971115)80:10<2005::AID-CNCR19>3.0.CO;2-0
Leber KA, Eder HG, Kovac H, Anegg U, Pendl G (1998) Treatment of cerebral radionecrosis by hyperbaric oxygen therapy. Stereotact Funct Neurosurg 70(suppl 1):229–236. doi:10.1159/000056426
Glantz MJ, Burger PC, Friedman AH, Radtke RA, Massey EW, Schold SC Jr (1994) Treatment of radiation-induced nervous system injury with heparin and warfarin. Neurology 44(11):2020–2027
Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Corn BW, Yousem DM, Scott CB, Rotman M, Asbell SO, Nelson DF, Martin L, Curran WJ Jr (1994) White matter changes are correlated significantly with radiation dose Observations from a randomized dose-escalation trial for malignant glioma (Radiation Therapy Oncology Group 83-02). Cancer 74(10):2828–2835. doi:10.1002/1097-0142(19941115)74:10<2828::AID-CNCR2820741014>3.0.CO;2-K
Levin VA, Yung WK, Bruner J, Kyritsis A, Leeds N, Gleason MJ, Hess KR, Meyers CA, Ictech SA, Chang E, Maor MH (2002) Phase II study of accelerated fractionation radiation therapy with carboplatin followed by PCV chemotherapy for the treatment of anaplastic gliomas. Int J Radiat Oncol Biol Phys 53(1):58–66. doi:10.1016/S0360-3016(01)02819-X
Jain R, Scarpace L, Ellika S, Schultz LR, Rock JP, Rosenblum ML, Patel SC, Lee TY, Mikkelsen T (2007) First-pass perfusion computed tomography: initial experience in differentiating recurrent brain tumors from radiation effects and radiation necrosis. Neurosurgery 61(4):778–787
Ruben JD, Dally M, Bailey M, Smith R, McLean CA, Fedele P (2006) Cerebral radiation necrosis: incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int J Radiat Oncol Biol Phys 65(2):499–508. doi:10.1016/j.ijrobp.2005.12.002
Peterson K, Clark HB, Hall WA, Truwit CL (1995) Multifocal enhancing magnetic resonance imaging lesions following cranial irradiation. Ann Neurol 38(2):237–244. doi:10.1002/ana.410380217
Glantz MJ, Choy H, Kearns CM, Cole BF, Mills P, Zuhowski EG, Saris S, Rhodes CH, Stopa E, Egorin MJ (1996) Phase I study of weekly outpatient paclitaxel and concurrent cranial irradiation in adults with astrocytomas. J Clin Oncol 14(2):600–609
Chamberlain MC, Glantz MJ, Chalmers L, Van Horn A, Sloan AE (2007) Early necrosis following concurrent Temodar and radiotherapy in patients with glioblastoma. J Neurooncol 82:81–83. doi:10.1007/s11060-006-9241-y
Kumar AJ, Leeds NE, Fuller GN, Van Tassel P, Maor MH, Sawaya RE, Levin VA (2000) Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology 217(2):377–384
Cheng KM, Chan CM, Fu YT, Ho LC, Cheung FC, Law CK (2001) Acute hemorrhage in late radiation necrosis of the temporal lobe: report of five cases and review of the literature. J Neurooncol 51(2):143–150. doi:10.1023/A:1010631112015
Kim JH, Chung YG, Kim CY, Kim HK, Lee HK (2004) Upregulation of VEGF and FGF2 in normal rat brain after experimental intraoperative radiation therapy. J Korean Med Sci 19(6):879–886
Li Y-Q, Ballinger JR, Nordal RA, Su ZF, Wong CS (2001) Hypoxia in radiation-induced blood-spinal cord barrier breakdown. Cancer Res 61:3348–3354
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Torcuator, R., Zuniga, R., Mohan, Y.S. et al. Initial experience with bevacizumab treatment for biopsy confirmed cerebral radiation necrosis. J Neurooncol 94, 63–68 (2009). https://doi.org/10.1007/s11060-009-9801-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-009-9801-z