Abstract
Several molecular markers have been proposed as predictors of outcome in patients with high grade gliomas. We report a retrospective multicenter study of 97 consecutive adult patients with anaplastic astrocytoma (AA) treated with radiation therapy (RT) plus concomitant and adjuvant temozolomide (TMZ) between October 2004 and March 2012. Correlations between the isocitrate dehydrogenase 1 (IDH1) mutation and O-6-methylguanine-DNA methyltransferase (MGMT) promoter methylation with survival outcomes have been analyzed. At a median follow-up time of 46 months (range 12–89 months), median and 5-year overall survival rates were 50.5 months (95 % CI, 37.8–63.2) and 38 % (95 % CI, 25.7–50.7 %), and median and 5-year progression-free survival rates were 36 months (95 % CI, 28.5–44.0) and 22 % (95 % CI, 10–34 %), respectively. IDH1 mutation and MGMT promoter methylation were present in 54 and 60 % of evaluable patients, respectively. Multivariate Cox proportional hazards regression analysis showed that IDH1 mutation (P = 0.001), MGMT methylation (P = 0.01), age < 50 years (P = 0.02), and extent of resection (P = 0.04) were significantly associated with longer survival. Our study confirms the favorable prognostic value of IDH1 mutation and MGMT methylation in patients with AA treated with RT plus concomitant and adjuvant TMZ. The superiority of combined radiochemotherapy over other treatment modalities remains to be demonstrated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anaplastic astrocytoma (AA) accounts for approximately 8 % of all gliomas in adults, with an annual incidence rate of 0.44 per 100,000 persons [1]. Current treatments include surgery, radiation therapy (RT) and chemotherapy [2]; however, adjuvant RT remains the only category 1 recommended treatment for AA (www.nccn.org). Results from a meta-analysis including data from 12 randomized trials showed a significant prolongation of survival associated with chemotherapy in high grade gliomas with an absolute increase of a two-month in median survival time [3], although two randomized trials have failed to demonstrate a significant survival benefit of the addition of procarbazine, lomustine and vincristine (PCV) or adjuvant dibromodulcitol (DBD) and bichloroethylnitrosourea (BCNU) based chemotherapy to RT in patients with AA [4, 5]. More recently, the alkylating agent temozolomide (TMZ) has been employed as an alternative to PCV chemotherapy in patients with AA, anaplastic oligodendroglioma (AO) and oligoastrocytoma (AOA) [6–12]. In the German randomized phase III trial NOA-04 comparing the efficacy and safety of RT alone versus chemotherapy with either PCV or TMZ as initial therapy in patients with newly diagnosed grade III glioma, the reported median overall survival and progression-free survival rates were similar between groups, with no differences between patients treated with PCV and those treated with TMZ [8]. Few additional studies have retrospectively evaluated the combination of TMZ and RT in patients with AA reporting acceptable toxicity and survival benefit [11, 12]; however, its superiority over other treatment modalities as reported for glioblastoma remains to be proven.
The variable survival of 30–70 months reported after RT and/or chemotherapy in patients with AA has been associated with the presence of several prognostic factors. Age, extent of resection, and performance status have been well established as independent prognostic factors predictive of survival [13]. In addition, molecular markers such as chromosome 1p19q codeletion, O-6-methylguanine-DNA methyltransferase (MGMT) promoter methylation, and isocitrate dehydrogenase 1 (IDH1) mutational status have attracted great attention, and their use appears promising to predict prognosis, evaluate the response to treatment, and stratify patients for clinical trials. A strong correlation between MGMT promoter methylation and response to combined chemoradiotherapy with concomitant and adjuvant TMZ has been demonstrated in patients with glioblastoma [14–16]. In the European Organisation for Research and Treatment of Cancer (EORTC) and National Cancer Institute of Canada Clinical Trials Group (NCIC) randomized trial on the combination of RT plus TMZ in glioblastoma, the 2-year survival rates for methylated patients treated with standard RT and TMZ was 49 % compared with 15 % for unmethylated patients (P < 0.01) [15]. More recently, the favorable impact of chromosome 1p19q codeletion and IDH1 mutation on survival of anaplastic gliomas has been observed in few studies [17–20]. In the EORTC phase III randomized study on the association of RT with adjuvant PCV for anaplastic AO and AOA, the reduction in risk of death was 65 % for IDH1-mutated and 58 % for 1p19q codeleted tumors, respectively [20]. In contrast, the trial failed to demonstrate a correlation between the presence of MGMT promoter methylation and survival, and similar results have been observed in other few studies [21, 22].
In this multicenter study we have evaluated the impact of clinical and molecular prognostic factors on clinical outcomes in patients with AA treated with RT combined with concomitant and adjuvant TMZ, with special attention to the role of IDH1 mutation and MGMT promoter methylation status.
Patients and methods
Patients
Adult patients with newly diagnosed histologically confirmed AA according to the WHO classification [23], with Karnofsky performance status (KPS) ≥ 60, who were treated with RT plus concomitant and adjuvant TMZ between October 2004 and March 2012 were evaluated in this multicentre retrospective study. All clinical relevant radiographic, surgical, and pathological information for patients who were treated at Sant’Andrea Hospital and Neuromed Institute were obtained from a multi-institutional prospective database on patients with brain tumors treated with RT. For patients treated at other centers, clinical data were collected from hospital charts and radiologic studies. Pathology was centrally reviewed (F.G.). Data regarding 129 patients with a diagnosis of AA treated during this period were examined. Patients with a prior diagnosis of low-grade glioma were included in the study provided that histologic confirmation of anaplastic transformation was obtained before treatment. A total of 97 patients were included in the final analysis after the exclusion of 32 patients because of insufficient pathology or clinical information. The Institutional Review Boards approved the study.
Treatment
Patients received conformal RT plus concomitant daily TMZ, followed by adjuvant TMZ. RT consisted of fractionated focal irradiation at the dose of 59.4–60 Gy delivered in 30–33 fractions of 1.8–2 Gy over a period of 6–6 1/2 weeks, started within 4–6 weeks from surgery. Magnetic resonance imaging (MRI) and/or computed tomography (CT) were used for treatment planning. The clinical target volume (CTV) was defined by the residual tumor and/or resection cavity (GTV) plus a margin of 1–2 cm. Concomitant chemotherapy consisted of TMZ at the dose of 75 mg/m2, given 7 days per week from the first day of RT. Adjuvant TMZ was started 4 weeks after the end of RT and delivered at dose 150–200 mg/m2 for 5 days every 28 days up to 6–12 cycles. Toxicity was evaluated according to the NCI Common Terminology Criteria for Adverse Events version 3.0 (CTCAE v3.0) (http://ctep.cancer.gov).
A clinical assessment of neurological status and tolerance to treatment was performed during RT and thereafter every month. MRI was repeated before RT, before adjuvant TMZ, every 2–3 months during adjuvant chemotherapy, and thereafter every 3–6 months, or as appropriate. According to RANO criteria [24], radiographic response was based on changes in tumor size of all measurable enhancing and nonenhancing lesions using contrast-enhanced T1-weighted and fluid-attenuated inversion recovery (FLAIR) MRI sequences. In brief, complete response (CR) was defined by complete disappearance of all enhancing measurable and nonmeasurable disease for at least 4 weeks apart, no new lesions, and stable or improved nonenhancing lesions. Partial response (PR) was defined as ≥50 % reduction in the size of all enhancing measurable lesions, no new lesions, and stable or improved nonenhancing lesions. Progressive disease (PD) was defined as >25 % increase in size of enhancing lesions, or as significant increase of nonenhancing tumor, or as any new lesion. Stable disease (SD) was defined as any other condition. Patients had to be on corticosteroid dose not greater than the dose at time of baseline scan, and stable or improved neurologic status. Responses were reviewed by the same neuroradiologist (A.B). Transient radiological abnormalities characterized by increased edema and contrast-enhancement on MRI, which occurred within 12 weeks from the end of RT, and stabilized or resolved on subsequent clinical and radiographic assessments without a change in therapy, were recorded as pseudoprogression [25].
Molecular biomarkers assessment
The methylation status of the MGMT promoter was determined by methylation-specific polymerase chain reaction (PCR) after sodium bisulfite DNA modification as described previously [16]. Immunohistochemistry for IDH1 was performed automatically with a Nexes instrument (Ventana) using a primary antibody anti IDH1-R-132H (Clone H09 Dianova). Antibody detection was performed by using a multilink streptavidin–biotin complex method, and antibodies were visualized by a diaminobenzidine chromagen method. Negative control samples were in the tumor-matched incubated with primary antibodies only. Thus, in the current study, the presence of IDH1 mutation refers to the presence of IDH1 R132H mutation.
Statistical analysis
Overall survival (OS) and progression-free survival (PFS) were estimated using the Kaplan–Meier method calculated from the time of histological diagnosis. For patients with a history of a low grade glioma OS and PFS were calculated from the time of histological confirmation of anaplastic transformation. For univariate analysis, the log-rank test was used for categorical variables, and the Cox proportional hazards model was used for continuous variables. Significant prognostic factors (P < 0.05) were included in a multivariate analysis performed using a Cox proportional hazards regression model. Statistical evaluation was performed using a commercial statistical software package (StatView, version 5.0, SAS Institute, Cary, NC).
Results
Ninety-seven consecutive patients (55 males and 42 females) with AA who underwent RT plus TMZ between October 2004 and March 2012 were analyzed. Pretreatment characteristics for eligible patients are listed in Table 1. The median age was 43 years (range 20–71 years), and median KPS was 90 (range, 60–100). Twenty-six patients were considered to have macroscopically complete resection, 22 patients subtotal resection (>90 %), and 42 patients incomplete resection on the basis of an intraoperative or immediate postoperative MRI. Seven patients underwent stereotactic biopsy. Twenty-eight patients had an initially grade II astrocytoma that have undergone histologically proven “malignant transformation”. Adjuvant chemotherapy consisted of 6 cycles of TMZ in 33 patients and 12 cycles in 64 patients, respectively. Fifty-two patients had died at the time of analysis (April 2013).
PFS and OS analysis
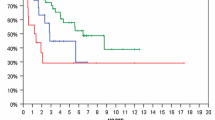
At a median follow-up time of 46 months (range, 12–89 months), median OS and 5-year survival rates were 50.5 months (95 % CI, 37.8–63.2) and 38 % (95 % CI, 25.7–50.7 %), respectively. Median and 5-year PFS rates were 36 months (95 % CI, 28.5–44.0) and 22 % (95 % CI, 10–34 %), respectively (Fig. 1). Treatment response was achieved in 43 patients, including 16 complete responses and 27 partial responses. SD occurred in 40 and disease progression in 14 patients. In patients who progressed, salvage treatment was chemotherapy alone (n = 17) or in combination with surgery (n = 12) and stereotactic irradiation (n = 9). Chemotherapy was fotemustine in 8, dose-dense TMZ in 14, and bevacizumab in 16 patients, respectively.
Prognostic factors
IDH1 mutation was detected in 51 (54 %) of 94 evaluable patients, and MGMT promoter was methylated in 51 (61 %) of 84 evaluable patients. Eighty-two patients had results for both chromosome IDH1 mutation and MGMT promoter methylation status. Logistic regression analysis showed a significant correlation between IDH1 and MGMT promoter methylation status (OR 2.9, 95 %CI 1.6–8.5; P = 0.02); nine tumors with the IDH1 mutation showed an unmethylated MGMT. In patients without IDH1 mutation, MGMT promoter was methylated in 24 and unmethylated in 16 cases, respectively.
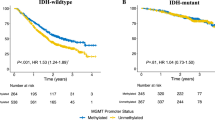
Presence of IDH1 mutation and MGMT promoter methylation were associated with better outcomes. Median OS was 34 months in patients with IDH1 nonmutated tumors and 67 months in those with IDH1 mutated tumors (P < 0.0001), with respective PFS of 18 and 60 months (P < 0.0001) (Fig. 2). The median OS and PFS rates were 60 and 45 months in methylated tumors and 34 and 18 months in unmethylated tumors (P < 0.0001), respectively. Co-evaluation of IDH1 and MGMT status revealed that patients with IDH1 mutation and methylated MGMT promoter had the longest OS and PFS, followed by patients with IDH1 mutation and unmethylated MGMT promoter, patients without IDH1 mutation and MGMT promoter methylation, and patients with neither IDH1 mutation nor MGMT promoter methylation (P < 0.0001) (Fig. 3).
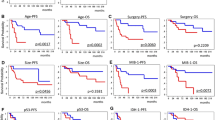
Extent of resection (subtotal/total resection vs. partial resection/biopsy) (P = 0.01), KPS (KPS ≤ 70 vs. KPS > 70; P = 0.02), and age (≥50 vs. <50, P = 0.01) were found to be significantly correlated with OS. Similarly, KPS (P = 0.03), and age (P = 0.01) had an effect on PFS (P = 0.02). In contrast, site of tumor, previous low grade glioma, and number of chemotherapy cycles (6 vs. 12 cycles) did not affect either OS or PFS.
Multivariate analysis showed that extent of resection (P = 0.04), age < 50 years (P = 0.02), MGMT promoter methylation (P = 0.01), and IDH1 mutation (P = 0.001) remained significant prognostic factors associated with longer OS (Table 2). IDH1 mutation (P = 0.001), MGMT methylation (P = 0.03), and age < 50 years (P = 0.03) were of favourable prognostic significance for PFS, with IDH-1 being the strongest independent factor.
Toxicity
All patients were evaluated for toxicity during RT with concomitant TMZ and the adjuvant therapy period. All patients completed the planned programme of RT. During adjuvant TMZ, 13 % of patients had grade 3/4 thrombocytopenia, 8 % grade 3/4 neutropenia, 3 % grade 3/4 anemia, and 17 % grade 3/4 lymphocytopenia. Chemotherapy was stopped in 7 patients, and delayed or reduced in 16 patients. A moderate-to-severe fatigue occurred in 22 patients, and grade 2 nausea in 4 patients. MRI imaging abnormalities suggestive of pseudoprogression were observed in 21 patients, occurring in 3 unmethylated patients and 18 methylated patients (P = 0.01). Pseudoprogression was associated with grade 2/3 neurological deterioration in 11 patients and required high-dose dexamethasone for more than 4 months in 8 patients.
Discussion
In this multicenter study we have evaluated the impact of clinical and molecular prognostic factors in a series of patients with AA treated with RT plus concomitant and adjuvant TMZ. IDH1 mutation was the most important factor associated with improved survival in our population, followed by MGMT methylation status, age and extent of surgery.
Significance of IDH1 mutation as strong favorable prognostic factor for OS and PFS in grade III gliomas has been analyzed in few series [8, 17, 19, 20, 26]. In the NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic gliomas with PCV or TMZ, the presence of IDH1 was the most prominent single prognostic factor for PFS in patients with AA treated with RT or CHT, regardless of the type of chemotherapy [8]. Hartmann et al. [17] found that IDH1 mutation, which was present in 60 % of 145 AA evaluated in the study, was associated with significant longer survival, being approximately 67 and 13 months in patients with or without IDH1 mutation, respectively. MGMT promoter methylation is a favorable prognostic factor for OS and a predictive biomarker for benefit from alkylating agent chemotherapy in patients with glioblastoma [14–16, 27] and AA [28, 29]. In our series the MGMT promoter methylation, which was present in 61 % of patients, was significantly associated with longer PFS and OS, conferring a 64 % reduction in risk for death. According to our findings, we suggest the importance of combined IDH1 and MGMT analysis to predict survival in patients with AA treated with combined radiochemotherapy: AA with IDH1 mutation and MGMT methylation had the more favorable outcome, followed by AA with IDH1 mutation and unmethylated MGMT promoter, AA without IDH1 mutation and methylated MGMT promoter, and AA without IDH1 mutation and unmethylated MGMT promoter. While our study indicates that either IDH1 mutation or MGMT methylation identify subgroups of AA with improved survival, their role as predictive factor for chemotherapy and RT response needs to be clarified in future studies.
The optimal treatment for AA remains matter of debate. In the present series OS was 50.5 months and PFS was 36 months, which are in the best range of studies reporting on the use of RT and CHT alone or in combination in AA [6, 8, 11, 12]. Combs et al. [6] found no survival benefit in patients treated with RT plus TMZ over RT alone. Median OS and PFS were 15 and 6 months in patients treated with radiochemotherapy and 13 and 7 months in those treated with RT alone, respectively. In a recent retrospective review of 163 patients with AA, a comparable median survival of 5.7 years was observed in patients treated with RT alone, concurrent RT and TMZ, or RT followed by TMZ [12]. PFS was longer in patients receiving RT alone over those treated with RT plus concurrent or adjuvant chemotherapy, although difference in PFS between groups may have been confounded, at least in part, by the possibility of pseudoprogression. Finally, in the NOA-4 trial PFS was 10.8 months in patients treated with RT and 18.2 months in those treated with either PCV or TMZ, leading the authors to recommend chemotherapy alone as first-line treatment for patients with AA [8]. While RT plus CHT has emerged as the most effective treatment for patients with AO and AOA [20, 30], the superiority of regimens incorporating chemotherapy in AA remains to be proven. The CATNON (Concurrent and Adjuvant Temozolomide Chemotherapy in NON 1p/19q Deleted Anaplastic Glioma) Intergroup trial, which is currently testing the use of RT with or without concurrent and/or adjuvant TMZ in patients with non-1p/19q deleted anaplastic glioma will be essential to define the optimal treatment of these tumors.
In conclusion, our study confirms the prognostic value of IDH1 mutation and MGMT promoter methylation for longer survival in patients with AA treated with RT plus TMZ. Survival outcomes are consistent with those reported with the use of RT or CHT alone, and the superiority of combined radiochemotherapy in patients with AA over other treatment modalities remains to be demonstrated. Stratification of patients according to histological and molecular characteristics should be considered in future studies assessing the role of RT and CHT in patients with AA.
References
Dolecek TA, Propp JM, Stroup NE, Kruchko C (2012) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro Oncol 14(Suppl 5):v1–v49
Sathornsumetee S, Rich JN, Reardon DA (2007) Diagnosis and treatment of high-grade astrocytoma. Neurol Clin 25:1111–1139
Glioma Meta-Analysis Trialists (GMT) Group (2002) Chemotherapy for high-grade glioma. Cochrane Database Syst Rev 4:CD003913
Medical Research Council Brain Tumor Working Party (2001) Randomized trial of procarbazine, lomustine, and vincristine in the adjuvant treatment of high-grade astrocytoma: a Medical Research Council trial. J Clin Oncol 19:509–518
Hildebrand J, Gorlia T, Kros JM, Afra D, Frenay M, Omuro A, Stupp R, Lacombe D, Allgeier A, van den Bent MJ, EORTC Brain Tumour Group investigators (2008) Adjuvant dibromodulcitol and BCNU chemotherapy in anaplastic astrocytoma: results of a randomised European Organisation for Research and Treatment of Cancer phase III study (EORTC study 26882). Eur J Cancer 44:1210–1216
Combs SE, Nagy M, Edler L, Rausch R, Bischof M, Welzel T, Debus J, Schulz-Ertner D (2008) Comparative evaluation of radiochemotherapy with temozolomide versus standard-of-care postoperative radiation alone in patients with WHO grade III astrocytic tumors. Radiother Oncol 88:177–182
Mikkelsen T, Doyle T, Anderson J, Margolis J, Paleologos N, Gutierrez J, Croteau D, Hasselbach L, Avedissian R, Schultz L (2009) Temozolomide single-agent chemotherapy for newly diagnosed anaplastic oligodendroglioma. J Neurooncol 92:57–63
Wick W, Hartmann C, Engel C, Stoffels M, Felsberg J, Stockhammer F, Sabel MC, Koeppen S, Ketter R, Meyermann R, Rapp M, Meisner C, Kortmann RD, Pietsch T, Wiestler OD, Ernemann U, Bamberg M, Reifenberger G, von Deimling A, Weller M (2009) NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol 27:5874–5880
Gan HK, Rosenthal MA, Dowling A, Kalnins R, Algar E, Wong N, Benson A, Woods AM, Cher L (2010) A phase II trial of primary temozolomide in patients with grade III oligodendroglial brain tumors. Neuro Oncol 12:500–507
Ducray F, del Rio MS, Carpentier C, Psimaras D, Idbaih A, Dehais C, Kaloshi G, Mokhtari K, Taillibert S, Laigle-Donadey F, Omuro A, Sanson M, Delattre JY, Hoang-Xuan K (2011) Up-front temozolomide in elderly patients with anaplastic oligodendroglioma and oligoastrocytoma. J Neurooncol 101:457–462
Kim YH, Park CK, Cho WH, Kim IA, Moon S, Choe G, Park SH, Kim IH, Kim DG, Jung HW, Lee MM, Bae SH, Cha SH, Kim CY (2011) Temozolomide during and after radiation therapy for WHO grade III gliomas: preliminary report of a prospective multicenter study. J Neurooncol 103:503–512
Shonka NA, Theeler B, Cahill D, Yung A, Smith L, Lei X, Gilbert MR (2013) Outcomes for patients with anaplastic astrocytoma treated with chemoradiation, radiation therapy alone or radiation therapy followed by chemotherapy: a retrospective review within the era of temozolomide. J Neurooncol 113:305–311
Curran WJ Jr, Scott CB, Horton J, Nelson JS, Weinstein AS, Fischbach AJ, Chang CH, Rotman M, Asbell SO, Krisch RE et al (1993) Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst 85:704–710
Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO, European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups, National Cancer Institute of Canada Clinical Trials Group (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466
Minniti G, Salvati M, Arcella A, Buttarelli F, D’Elia A, Lanzetta G, Esposito V, Scarpino S, Maurizi Enrici R, Giangaspero F (2011) Correlation between O6-methylguanine-DNA methyltransferase and survival in elderly patients with glioblastoma treated with radiotherapy plus concomitant and adjuvant temozolomide. J Neurooncol 102:311–316
Hartmann C, Hentschel B, Wick W, Capper D, Felsberg J, Simon M, Westphal M, Schackert G, Meyermann R, Pietsch T, Reifenberger G, Weller M, Loeffler M, von Deimling A (2010) Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol 120:707–718
Frenel JS, Leux C, Loussouarn D, Le Loupp AG, Leclair F, Aumont M, Mervoyer A, Martin S, Denis MG, Campone M (2013) Combining two biomarkers, IDH1/2 mutations and 1p/19q codeletion, to stratify anaplastic oligodendroglioma in three groups: a single-center experience. J Neurooncol 114:85–91
Jiang H, Ren X, Cui X, Wang J, Jia W, Zhou Z, Lin S (2013) 1p/19q codeletion and IDH1/2 mutation identified a subtype of anaplastic oligoastrocytomas with prognosis as favorable as anaplastic oligodendrogliomas. Neuro Oncol 15:775–782
Van den Bent MJ, Brandes AA, Taphoorn MJ, Kros JM, Kouwenhoven MC, Delattre JY, Bernsen HJ, Frenay M, Tijssen CC, Grisold W, Sipos L, Enting RH, French PJ, Dinjens WN, Vecht CJ, Allgeier A, Lacombe D, Gorlia T, Hoang-Xuan K (2013) Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol 31:344–350
Brandes AA, Tosoni A, Vastola F, Pasetto LM, Coria B, Danieli D, Iuzzolino P, Gardiman M, Talacchi A, Ermani M (2004) Efficacy and feasibility of standard procarbazine, lomustine, and vincristine chemotherapy in anaplastic oligodendroglioma and oligoastrocytoma recurrent after radiotherapy. A phase II study. Cancer 101:2079–2085
Minniti G, Arcella A, Scaringi C, Lanzetta G, Di Stefano D, Scarpino S, Pace A, Giangaspero F, Osti MF, Enrici RM (2014) Chemoradiation for anaplastic oligodendrogliomas: clinical outcomes and prognostic value of molecular markers. J Neurooncol 116:275–282
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (2007) World Health Organization classification of tumours. Pathology and genetics of tumours of the nervous system. IARC, Lyon
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972
Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ (2008) Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol 9:453–461
Shibahara I, Sonoda Y, Kanamori M, Saito R, Yamashita Y, Kumabe T, Watanabe M, Suzuki H, Kato S, Ishioka C, Tominaga T (2012) IDH1/2 gene status defines the prognosis and molecular profiles in patients with grade III gliomas. Int J Clin Oncol 17:161–551
Olson RA, Brastianos PK, Palma DA (2011) Prognostic and predictive value of epigenetic silencing of MGMT in patients with high grade gliomas: a systematic review and meta-analysis. J Neurooncol 105:325–335
Kamiryo T, Tada K, Shiraishi S, Shinojima N, Kochi M, Ushio Y (2004) Correlation between promoter hypermethylation of the O6-methylguanine-deoxyribonucleic acid methyltransferase gene and prognosis in patients with high-grade astrocytic tumors treated with surgery, radiotherapy, and 1-(4-amino-2-methyl-5-pyrimidinyl)methyl-3-(2-chloroethyl)-3 nitrosourea-based chemotherapy. Neurosurgery 54:349–357
Wick W, Meisner C, Hentschel B, Platten M, Schilling A, Wiestler B, Sabel MC, Koeppen S, Ketter R, Weiler M, Tabatabai G, von Deimling A, Gramatzki D, Westphal M, Schackert G, Loeffler M, Simon M, Reifenberger G, Weller M (2013) Prognostic or predictive value of MGMT promoter methylation in gliomas depends on IDH1 mutation. Neurology 81:1515–1522
Cairncross G, Wang M, Shaw E, Jenkins R, Brachman D, Buckner J, Fink K, Souhami L, Laperriere N, Curran W, Mehta M (2013) Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol 31:337–343
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Minniti, G., Scaringi, C., Arcella, A. et al. IDH1 mutation and MGMT methylation status predict survival in patients with anaplastic astrocytoma treated with temozolomide-based chemoradiotherapy. J Neurooncol 118, 377–383 (2014). https://doi.org/10.1007/s11060-014-1443-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-014-1443-0