Abstract
Epigenetic silencing of the O6-methylguanine-DNA methyltransferase (MGMT) gene is associated with improved survival in patients with high-grade gliomas (HGG), with varying estimates of magnitude. The objective of this meta-analysis is to determine the prognostic value of MGMT silencing, and assess its predictive value by treatment type. MEDLINE and EMBASE databases were searched for studies relating to gliomas and MGMT. Studies reporting overall survival (OS) by MGMT status in patients with HGG were considered potentially eligible. We excluded studies that did not control for potential confounding variables. A meta-analysis of studies was performed via random-effects modelling. Subgroup meta-analyses by treatment were performed according to a priori hypotheses. Twenty studies were ultimately eligible, including 2,018 patients. In the pooled analysis, MGMT silencing was associated with improved OS (HR = 0.436; 95% CI: 0.333–0.571; P < 0.001). The prognostic utility of MGMT status varies significantly by treatment type (P = 0.001): the HR for OS for MGMT silenced tumors is 0.190 (0.047–0.770), 0.403 (0.282–0.576), 0.743 (0.579–0.954), and 1.070 (0.722–1.585) for studies using surgery plus the addition of either: chemotherapy (CT), chemoradiotherapy (CRT), radiotherapy (RT), and nothing (surgery alone), respectively. Epigenetic silencing of MGMT is associated with markedly improved survival in patients with HGG who receive adjuvant therapy. MGMT silencing serves as a predictive marker, with the largest benefit seen in patients receiving CT as a component of adjuvant treatment, an intermediate benefit in patients receiving adjuvant RT, and no evidence to support benefit in those receiving surgery alone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 20,000 high-grade gliomas (HGG) are diagnosed each year in the US, and despite advances in chemotherapy (CT), radiotherapy (RT) and surgery, the prognosis of many of these patients is poor. Traditionally, prognostication for patients with HGGs has been based on factors such as age, performance status, cognitive function, tumor grade, and extent of resection [1, 2].
Silencing of the O6-methylguanine-DNA methyltransferase (MGMT) gene has been associated with longer overall survival (OS) in patients with HGG [3]. MGMT encodes a DNA-repair protein that counteracts the effect of treatment by removing alkyl groups from guanine, a target site for alkylating CT agents such as temozolomide (TMZ). MGMT has ubiquitous expression in human tissue with increased expression in some gliomas [4]. Silencing of the MGMT gene by epigenetic methylation of its promoter may disable this repair mechanism, thus increasing the cytotoxicity of CT, and potentially RT. Conversely, high MGMT activity may render cancer cells resistant to treatment. In addition, the presence of methyl groups on glioma DNA theoretically could increase radiosensitivity, irrespective of the level of MGMT expression [5].
The most reliable technique for evaluating MGMT status is controversial. The methylation-specific polymerase reaction (MSP), the most widely-used technique, is highly sensitive, but it is time-consuming and dependent on quality of tissue. Immunohistochemistry (IHC) is readily available in most diagnostic histopathology labs and is less expensive and time-intensive. A recent systematic review and meta-analysis evaluated the correlation between IHC and MSP in studies that compared MGMT promoter methylation by MSP with protein expression by IHC in the same patient cohorts; the study demonstrated that protein expression by IHC did not correlate with results obtained with MSP [6]. In light of these findings, MSP and IHC should not be used interchangeably. Therefore, in this meta-analysis, studies that measured protein expression by IHC alone were excluded.
Several clinical studies have demonstrated that methylation of the MGMT gene may have prognostic significance, though the magnitude of effect varies across studies, but is contradicted by others, and has not been demonstrated in all subgroups [3, 7, 8]. Precise estimates of the prognostic value of MGMT in all treatment groups could allow for refinement in clinical management, and would better inform clinicians and patients. The goal of this meta-analysis was to characterize the prognostic significance of MGMT silencing in patients with HGG, and to assess if MGMT silencing predicts survival differences that are specific to the form of therapy.
Materials and methods
Objective
The primary objective of this meta-analysis was to answer the question: “What is the prognostic value of MGMT silencing for OS in patients with HGG?”. Specifically, we sought to determine the hazard ratio (HR) for OS of patients with MGMT-silenced tumors, compared to patients with MGMT-non-silenced tumors, to evaluate whether this HR changes based on the type of treatment (i.e. is it a predictive marker [9]), and to assess the robustness of our estimate using sensitivity analyses. The analysis methods and inclusion criteria were specified a priori and were conducted with reference to MOOSE and PRISMA guidelines [10, 11].
Search strategy and inclusion criteria
Combinations of terms were used to search the MEDLINE and EMBASE electronic databases from inception until July 2010, relating to the following two concepts: (1) gliomas (“glioma”, “glioblastoma”, “GBM”, etc.) and (2) MGMT (“MGMT”, “O6-methylguanine-methyltransferase”, etc.). Both text and exploded Medical Subject Heading (MeSH) terms were used. Only English-language articles studying human subjects were included. A total of 610 unique studies were identified (MEDLINE n = 370; EMBASE n = 551, with some duplicates).
Studies that assessed OS by MGMT status in patients with HGG, treated with surgery, potentially with the addition of either: CT, RT, or chemoradiotherapy (CRT) were included. Studies that did not report an adjusted HR for OS after controlling for potential confounding clinical variables in a multivariable analysis (e.g. Cox regression analysis including important clinical factors such age, grade, and/or performance status) were excluded, since, the accuracy of HRs estimated from Kaplan–Meier OS curves without a multivariable analysis are uncertain [12–14]. In addition, studies in which patients were assigned to different treatments based on MGMT status, studies which assessed MGMT status by protein expression, and case–control studies, were excluded.
Data abstraction
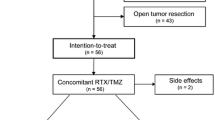
The data abstraction process is shown in Fig. 1. Title and abstract review of the selected reports were initially performed by one of two authors (RO or PB). Subsequently, the full text publication were independently screened by two of the authors (RO, DP, or PB), and their references checked for additional potential reports. Disagreements between reviewers were resolved by consensus.
We developed a data abstraction spreadsheet based on the Cochrane Consumers and Communication Review Group’s data extraction template. One review author (RO) extracted data from included studies and the second author (DP) checked the extracted data. Disagreements were resolved by discussion between the two authors. In instances where studies reported their outcomes more than once, the most recently published update was used for data extraction, and preference was given to outcomes that adjusted for previously demonstrated prognostic variables (e.g. age, performance status, gender, and treatment). Where a multivariable analysis was performed but HR for MGMT not reported, we contacted authors to obtain this information.
The following information was abstracted from included studies: (1) characteristics of study participants (including age, tumour grade, primary or recurrent tumors); (2) study inclusion and exclusion criteria; (3) type of treatments received; (4) method used to determine MGMT silencing (e.g. promoter methylation or protein expression). Unfortunately, extent of surgical resection was either mixed, or not available for the majority of studies, and therefore could not be reliably assessed. Studies were categorized as “primary only”, if study subjects MGMT status and OS were obtained from their initial brain tumor presentation (Table 1). Conversely, if all study subjects were presenting with recurrent disease, with MGMT status and OS was obtained from timing of their recurrence, they were categorized as “salvage only” (Table 1). Studies containing a mixture of both salvage and primary subjects were categorized as both (Table 1). The choice of study treatment category (Table 1) was based on the treatment being assessed at the time of the each study, since OS for each study was based on study treatment dates rather than previous or future salvage treatments, which this meta-analysis cannot account for. Ideally, progression-free survival (PFS) would have been assessed, since it is a valuable surrogate endpoint, which can provide definitive results with shorter follow-up than OS, and is therefore especially useful in studies with long natural histories; however, this was not analyzed for three major reasons: (1) PFS was not reported in most of the studies, which would result in elimination of several studies from the analysis, (2) progression is very difficult to define in the context of GBM, as pseudo-progression and radiation necrosis can result in misclassification, and (3) PFS depends on frequency of imaging follow-up, and studies in which imaging is done less frequently could over-estimate PFS. OS is a much more definitive endpoint that does not have these limitations. Therefore, our principle summary measure was the HR for OS comparing outcome by MGMT status (MGMT silenced versus MGMT not-silenced).
If studies presented adjusted HR for patient subgroups by treatment, these data were extracted in addition to the overall study result. Conversely, if subgroup analyses were unadjusted (i.e. crude) [3, 15] then this data was not extracted. If the 95% CI interval was not reported, it was calculated from the HR and associated P value [16]. For one study the authors sent us their raw data, and granted us permission to generate the HR and 95% CI [17].
Assessment of potential publication bias
For each trial we plotted the log HR by the standard error, allowing for visual inspection for publication bias via a ‘funnel plot’; the spread expands at the bottom of the graph, as a result of decreasing accuracy among smaller studies. The graph should resemble a symmetrical inverted funnel, in the absence of bias; in the presence of bias, the plot should appear skewed and asymmetrical. Because graphical evaluation can be subjective, we also estimated the number of unpublished studies that would bring the P value above 0.05 significance level, using the ‘Classic fail-safe N’ method.
Assessment of heterogeneity
The Breslow–Day test was used to assess for heterogeneity. Furthermore, we present the I 2 statistic as a measure of inconsistency of the prognostic value of MGMT status, since it represents the percentage of total variation across studies due to heterogeneity, and does not inherently depend on the number of studies [18]. Meta-analysis was performed using random-effects modelling. Pre-defined subgroup analyses by grade, treatment type, type of CT, and treatment intent (newly diagnosed versus salvage) were specified. In two of the studies, adjusted subgroup analysis by treatments were reported, and therefore used when assessing if the prognostic value of MGMT status is associated with the treatment received (Table 1; Fig. 2) [7, 19, 20]. Analysis of variance (ANOVA) was used to test whether there was a relationship between the HR and the subgroup category explored. In other words, ANOVA was used to assess if there was a statistically significant difference in HR for OS among different subgroups of studies.
Hazard ratio of death with MGMT silenced versus MGMT non-silenced. a Surgery and CT only studies, b surgery and CRT studies, c surgery and RT studies, d surgery only studies, and e mixed treatment studies. The centre of each square is the HR for individual trials and the corresponding horizontal line is the 95% CI. The area of the square is proportional to the number of deaths in each trial. The black diamond is the pooled HR and the horizontal tip of the diamond is the 95% CI
Sensitivity analyses
Through sensitivity analyses, we examined if our pooled estimate of the prognostic value of MGMT status was largely influenced by studies including grade III tumors, non-CRT treatment, or salvage treatment. Data analysis was done using Comprehensive Meta-Analysis software (version 2.2.05, Englewood, NJ, USA).
Results
Search results
Twenty studies met the inclusion criteria for this meta-analysis, including a total of 2,018 patients (Table 1) [7, 17, 20–37]. Two of these studies also reported subgroup analyses. All studies included patients treated for HGG with surgery, since this is necessary in order to obtain tissue for MGMT analysis. Most studies reported on patients who received either RT, CRT, or CT after surgery.
Meta-analysis
In the pooled analysis MGMT silencing was associated with improved OS (HR 0.436; 95% CI 0.333–0.571; P < 0.001). However, we detected significant heterogeneity [I 2 = 75.2%; Q = 76.7; degrees of freedom (df) = 19; P < 0.001], and therefore do not present the pooled analysis via a forest plot. We pre-specified examination of the heterogeneity by grade and treatment. We continued to detect significant heterogeneity in studies restricted to (1) grade IV gliomas (I 2 = 74.8; Q = 67.5; df = 17; P < 0.001), (2) primary treatment only (not salvage) (I 2 = 78.8; Q = 70.9; df = 15; P < 0.001), and (3) surgery and CRT only (I 2 = 70.3; Q = 40.4; df = 12; P < 0.001). In contrast, there was no significant heterogeneity within the other treatment groups, which supports our a priori hypothesis to explore whether the HR was dependent on treatment used.
On subgroup meta-analysis by treatment (Fig. 2), only treatment type was significantly associated with the prognostic utility of MGMT status (P = 0.001; Table 2). In other words, there is a highly significant difference in HR for OS by the treatment received. Furthermore, in studies which received mixed treatments, the HR for OS was within the average value (HR = 0.419; Fig. 2e). Since the surgery and CT or surgery studies were limited in number, an exploratory analyses comparing surgery and CRT versus surgery and RT was performed, suggesting that MGMT status was significantly more prognostic in the former group (P = 0.006; Table 2). As a measure of the robustness of the OS benefit associated with MGMT silencing, it is evident in all subgroups by the 95% confidence intervals (CI) in Table 2, with the exception of the surgery alone studies subgroup. In addition, sensitivity analyses confirm that our estimate of the overall HR of OS by MGMT status is robust across various scenarios (Table 3).
Assessment for publication bias
Publication bias was initially assessed visually through plotting the log HR by the standard error (Fig. 3); a lack of complete symmetry in this figure suggests that a small number of missing studies with a log HR of 0 (i.e. HR = 1) may not have been published. It is estimated that 610 such null studies would have to be unpublished, in order to bring the P value for a pooled HR estimate to be larger than 0.05, using the Classic fail-safe N method.
Discussion
This meta-analysis of 20 studies, involving 2,018 patients, shows that MGMT gene silencing is associated with a survival advantage in patients undergoing treatment for HGG, and this survival advantage is of a large magnitude (HR of death of 0.436; 95% CI: 0.333–0.571), and similar to the subgroup of studies where all patients received surgery followed by concurrent CRT (HR 0.403; 95% CI 0.282–0.576). If the 2-year survival in patients with MGMT-non-silenced tumors is assumed to be 10%, a HR of 0.436 would correspond to a 2-year survival of 37% in patients with MGMT-silenced tumors, assuming an exponential survival function. This HR estimate has been generated using studies which have all adjusted for other major baseline prognostic factors through multivariable analysis, such as age, gender, and performance status. A benefit of MGMT silencing is evident across all groups of patients who received adjuvant CT, CRT or RT, and for patients treated initially or at recurrence.
This study also demonstrates a significant difference in the estimated effect of MGMT based on treatment type, indicating that MGMT is a predictive marker, and that it is preferable to use the HR specific to the treatment received, rather than a pooled estimate [9]. However, caution should be used interpreting these results given the small number of studies that were not in the CRT category. The HR of death associated with MGMT silencing was lowest in the surgery and CT studies, intermediate in the studies including CRT, and highest in patients treated with surgery alone (Table 2). This finding is consistent with the biological role of MGMT. Alkylating chemotherapies such as nitrosoureas or temozolomide induce cell death by forming cross-links between DNA strands through alkylation of the O6 position of guanine, and MGMT is a DNA repair enzyme that removes these alkyl groups and confers resistance to these agents [38]. Thus, it follows that the effect of MGMT silencing should be most pronounced where alkylating agents are the primary treatment modality.
Like alkylating CT, DNA damage from RT can be mediated through alkylation of DNA at the O6 position of guanine. In addition, MGMT activity has been demonstrated to correlate with tumor resistance in glioma cell lines and xenografts treated with ionizing radiation [39]. However, alkylation of DNA at the O6 position of guanine is just one, minor pathway of RT mechanism of action [5]; therefore, one would expect MGMT activity to have a lesser impact on survival in patients treated with radiation, than in those treated with alkylating CT. This indeed was the case in our meta-analysis (Table 2). Furthermore, MGMT status was not prognostic in patients treated with surgery alone, suggesting that MGMT status is only prognostic when the treatment used is targeted at the pathway MGMT repairs; however, this conclusion is based on data from only a subgroup of a single study, and therefore further studies assessing the prognostic utility of MGMT status in patient receiving only surgery are clearly warranted.
There were no significant differences observed in the HR of death associated with MGMT silencing across the other subgroups tested: type of CT, intent of treatment (primary vs. salvage), or grade (Table 2). Although there appeared to be a trend to a lower HR for studies of grade III only patients compared to studies with only grade IV studies, there was only one study with only grade III patients, which limits the interpretation (Tables 1, 2). The trend for studies with salvage only patients to have a lower HR for OS by MGMT status, is likely due to confounding, in that most patients treated for salvage were also treated with CT only (Tables 1, 2). Sensitivity analysis revealed that our pooled estimate of effect was robust and did not change appreciably in the various scenarios tested (Table 3).
In the era of molecularly targeted therapy, these results could have important therapeutic implications. If these differences in survival associated with MGMT silencing are indeed causal in nature (which cannot be proven here), then MGMT provides an attractive therapeutic target. If the enzyme could be silenced pharmacologically, then theoretically this large survival benefit associated with MGMT silencing could be conferred to patients with MGMT activity [40]. Currently, inhibitors of the MGMT enzyme are being evaluated in clinical trials of recurrent malignant gliomas [41]. Although the value of these drugs may not be known for several years, in the meantime, the results of this meta-analysis will have important implications for patients and physicians. Accurate estimates of survival are important to assist in decisions regarding aggressiveness of treatment. Patients and physicians must balance the potential benefits of treatment against the negative effects of such treatments on quality of life, especially when life expectancy is short. Better estimates of prognosis and life expectancy will assist in such decisions.
The results of this study should be considered in the context of its strengths and limitations. As a meta-analysis, it combines the power of numerous studies and large numbers of patients to provide more precise estimates of effects and to allow for better-powered subgroup analyses. However, as a tradeoff for improved precision, there are several inherent limitations, specifically regarding the risks of selection bias, and from combining data from studies that may be heterogeneous. Unpublished studies and studies not reporting adjusted HRs for OS do not contribute to the estimates of the effect of MGMT calculated herein. It is conceivable that negative studies were less likely to be published. Nonetheless, we calculated that over six hundred null studies would be needed in order to statistically negate the benefit of MGMT silencing that is reported here. Secondly, as a result of the aggressive nature of HGGs, most patients in these studies received treatment other than the study treatment, either previously (e.g. initial CRT in salvage CT studies [32]) or in the future (e.g. salvage CT in upfront studies [34]), which could influence their prognosis. Since individual patient data on OS from the other non-study treatments is not available, these heterogeneous treatments could not be accounted for. Thirdly, extent of surgery could not be assessed, since this data was often not available, or was mixed within study patients. Fourthly, not all studies adjusted the prognostic value of MGMT for the same baseline factors, and some factors (such as p53, Ki-67, epidermal growth factor receptor, cyclin-dependent kinases, allelic losses on chromosome arms 1p and 19q, isocitrate dehydrogenase 1 mutations, or other gene expression parameters) were rarely used to adjust the estimate of MGMT effect [37]. Ideally, all available baseline factors would be used to adjust the estimate of effect, but this is only possible in the setting of an individual patient data meta-analysis that contains the same variables. Studies also varied in methods of classifying tumors as MGMT-silenced. These important differences likely contributed to the amount of heterogeneity detected between studies.
We have several recommendations for future studies reporting on the prognostic value of MGMT. Investigators should report HRs for all variables included in their multivariable models, not only variables that are statistically significant. Clear reporting of MGMT detection techniques and a priori specification of cutoff thresholds is essential. Currently, the best method of MGMT detection is unknown. Comparison of different detection techniques with receiver-operator characteristic curves would allow for standardized detection techniques, and thereafter uniform detection procedures across studies. Finally, we support several authors’ recommendation that new RPA prognostic models should be constructed, accounting for MGMT status [15].
Conclusion
Epigenetic silencing of MGMT is associated with markedly improved survival in patients with HGG. The largest benefit is apparent in patients receiving CT as a component of adjuvant treatment, an intermediate benefit in those receiving adjuvant RT, and no evidence to support benefit in those receiving surgery alone. There was no difference in the effect of MGMT silencing based on type of CT, primary versus salvage treatment, or tumor grade.
References
Curran WJ Jr, Scott CB, Horton J, Nelson JS, Weinstein AS, Fischbach AJ, Chang CH, Rotman M, Asbell SO, Krisch RE, Nelson DF (1993) Recursive partitioning analysis of prognostic factors in three radiation therapy oncology group malignant glioma trials. J Natl Cancer Inst 85:704–710. doi:10.1093/jnci/85.9.704
Olson R, Tyldesley S, Carolan H, Parkinson M, Chhanabhai T, McKenzie M (2010) Prospective comparison of the prognostic utility of the Mini Mental State Examination and the Montreal Cognitive Assessment in patients with brain metastases. Support Care Cancer. doi:10.1007/s00520-010-1028-1
Hegi ME, Diserens A, Gorlia T, Hamou M, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JEC, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003. doi:10.1056/NEJMoa043331
Esteller M, Hamilton SR, Burger PC, Baylin SB, Herman JG (1999) Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Res 59:793–797
Hall EJ, Giaccia AJ (2006) Radiobiology for the radiologist. Lippincott Williams & Wilkins, Philadelphia
Brell M, Ibanez J, Tortosa A (2011) O6-methylguanine-DNA methyltransferase protein expression by immunohistochemistry in brain and non-brain systemic tumours: systematic review and meta-analysis of correlation with methylation-specific polymerase chain reaction. BMC Cancer 11:35. doi:10.1186/1471-2407-11-35
Zawlik I, Vaccarella S, Kita D, Mittelbronn M, Franceschi S, Ohgaki H (2009) Promoter methylation and polymorphisms of the MGMT gene in glioblastomas: a population-based study. Neuroepidemiology 32:21–29. doi:10.1159/000170088
Mineura K, Yanagisawa T, Watanabe K, Kowada M, Yasui N (1996) Human brain tumor O6-methylguanine-DNA methyltransferase mRNA and its significance as an indicator of selective chloroethylnitrosourea. Chemotherapy 69:420–425. doi:10.1002/(SICI)1097-0215(19961021)69:5<420:AID-IJC12>3.0.CO;2-6
Esteller M, Herman JG (2004) Generating mutations but providing chemosensitivity: the role of O6-methylguanine DNA methyltransferase in human cancer. Oncogene 23:1–8. doi:10.1038/sj.onc.1207316
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Annals of Internal Medicine 151:W-65, W-94. doi:10.1059/0003-4819-151-4-200908180-00136
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB, for the Meta-Analysis of Observational Studies in Epidemiology Group (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 283:2008–2012. doi:10.1001/jama.283.15.2008
Duchateau L, Collette L, Sylvester R, Pignon JP (2000) Estimating number of events from the Kaplan–Meier curve for incorporation in a literature-based meta-analysis: what you don’t see you can’t get!. Biometrics 56:886–892
Michiels S, Piedbois P, Burdett S, Syz N, Stewart L, Pignon JP (2005) Meta-analysis when only the median survival times are known: a comparison with individual patient data results. Int J Technol Assess Health Care 21:119–125
Hirooka T, Hamada C, Yoshimura I (2009) A note on estimating treatment effect for time-to-event data in a literature-based meta-analysis. Methods Inf Med 48:104–112. doi:10.3414/ME0535
Gorlia T, van den Bent MJ, Hegi ME, Mirimanoff RO, Weller M, Cairncross JG, Eisenhauer E, Belanger K, Brandes AA, Allgeier A, Lacombe D, Stupp R (2008) Nomograms for predicting survival of patients with newly diagnosed glioblastoma: prognostic factor analysis of EORTC and NCIC trial 26981-22981/CE.3. Lancet Oncol 9:29–38. doi:10.1016/S1470-2045(07)70384-4
Hopkins WG (2007) A spreadsheet for deriving a confidence interval, mechanistic inference and clinical inference from a p value. Sportscience 11:16–20
Costa B, Caeiro C, Guimarães I, Martinho O, Jaraquemada T, Augusto I, Castro L, Osório L, Linhares P, Honavar M, Resende M, Braga F, Silva A, Pardal F, Amorim J, Nabiço R, Almeida R, Alegria C, Pires M, Pinheiro C, Carvalho E, Lopes JM, Costa P, Damasceno M, Reis RM (2010) Prognostic value of MGMT promoter methylation in glioblastoma patients treated with temozolomide-based chemoradiation: a Portuguese multicentre study. Oncol Rep 23:1655–1662. doi:10.3892/or_00000808
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558. doi:10.1002/sim.1186
Brell M, Tortosa A, Verger E, Gil JM, Vinolas N, Villa S, Acebes JJ, Caral L, Pujol T, Ferrer I, Ribalta T, Graus F (2005) Prognostic significance of O6-methylguanine-DNA methyltransferase determined by promoter hypermethylation and immunohistochemical expression in anaplastic gliomas. Clin Cancer Res 11:5167–5174. doi:10.1158/1078-0432.CCR-05-0230
Schaich M, Kestel L, Pfirrmann M, Robel K, Illmer T, Kramer M, Dill C, Ehninger G, Schackert G, Krex D (2009) A MDR1 (ABCB1) gene single nucleotide polymorphism predicts outcome of temozolomide treatment in glioblastoma patients. Ann Oncol 20:175–181. doi:10.1093/annonc/mdn548
Brandes AA, Franceschi E, Tosoni A, Blatt V, Pession A, Tallini G, Bertorelle R, Bartolini S, Calbucci F, Andreoli A, Frezza G, Leonardi M, Spagnolli F, Ermani M (2008) MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol 26:2192–2197. doi:10.1200/JCO.2007.14.8163
Cao VT, Jung TY, Jung S, Jin SG, Moon KS, Kim IY, Kang SS, Park CS, Lee KH, Chae HJ (2009) The correlation and prognostic significance of MGMT promoter methylation and MGMT protein in glioblastomas. Neurosurgery 65:866–875; discussion 875. doi:10.1227/01.NEU.0000357325.90347.A1
Clarke JL, Iwamoto FM, Sul J, Panageas K, Lassman AB, DeAngelis LM, Hormigo A, Nolan CP, Gavrilovic I, Karimi S, Abrey LE (2009) Randomized Phase II trial of chemoradiotherapy followed by either dose-dense or metronomic temozolomide for newly diagnosed glioblastoma. J Clin Oncol 27:3861–3867. doi:10.1200/JCO.2008.20.7944
Dunn J, Baborie A, Alam F, Joyce K, Moxham M, Sibson R, Crooks D, Husband D, Shenoy A, Brodbelt A, Wong H, Liloglou T, Haylock B, Walker C (2009) Extent of MGMT promoter methylation correlates with outcome in glioblastomas given temozolomide and radiotherapy. Br J Cancer 101:124–131. doi:10.1038/sj.bjc.6605127
Eoli M, Menghi F, Bruzzone MG, De Simone T, Valletta L, Pollo B, Bissola L, Silvani A, Bianchessi D, D’Incerti L, Filippini G, Broggi G, Boiardi A, Finocchiaro G (2007) Methylation of O6-methylguanine DNA methyltransferase and loss of heterozygosity on 19q and/or 17p are overlapping features of secondary glioblastomas with prolonged survival. Clin Cancer Res 13:2606–2613. doi:10.1158/1078-0432.CCR-06-2184
Felsberg J, Rapp M, Loeser S, Fimmers R, Stummer W, Goeppert M, Steiger HJ, Friedensdorf B, Reifenberger G, Sabel MC (2009) Prognostic significance of molecular markers and extent of resection in primary glioblastoma patients. Clin Cancer Res 15:6683–6693. doi:10.1158/1078-0432.CCR-08-2801
Gerstner ER, Yip S, Wang DL, Louis DN, Iafrate AJ, Batchelor TT (2009) MGMT methylation is a prognostic biomarker in elderly patients with newly diagnosed glioblastoma. Neurology 73:1509–1510. doi:10.1212/WNL.0b013e3181bf9907
Glas M, Happold C, Rieger J, Wiewrodt D, Bahr O, Steinbach JP, Wick W, Kortmann R, Reifenberger G, Weller M, Herrlinger U (2009) Long-term survival of patients with glioblastoma treated with radiotherapy and lomustine plus temozolomide. J Clin Oncol 27:1257–1261. doi:10.1200/JCO.2008.19.2195
Hegi ME, Diserens A, Godard S, Dietrich P, Regli L, Ostermann S, Otten P, Van Melle G, de Tribolet N, Stupp R (2004) Clinical trial substantiates the predictive value of O-6-methylguanine-DNA methyltransferase promoter methylation in glioblastoma patients treated with temozolomide. Clin Cancer Res 10:1871–1874. doi:10.1158/1078-0432.CCR-03-0384
Kamiryo T, Tada K, Shiraishi S, Shinojima N, Kochi M, Ushio Y (2004) Correlation between promoter hypermethylation of the O6-methylguanine-deoxyribonucleic acid methyltransferase gene and prognosis in patients with high-grade astrocytic tumors treated with surgery, radiotherapy, and 1-(4-amino-2-methyl-5-pyrimidinyl)methyl-3-(2-chloroethyl)-3-nitrosourea-based chemotherapy. Neurosurgery 54:349–357; discussion 357
Karayan-Tapon L, Quillien V, Guilhot J, Wager M, Fromont G, Saikali S, Etcheverry A, Hamlat A, Loussouarn D, Campion L, Campone M, Vallette FM, Gratas-Rabbia-Re C (2010) Prognostic value of O6-methylguanine-DNA methyltransferase status in glioblastoma patients, assessed by five different methods. J Neurooncol 97:311–322. doi:10.1007/s11060-009-0031-1
Metellus P, Coulibaly B, Nanni I, Fina F, Eudes N, Giorgi R, Barrie M, Chinot O, Fuentes S, Dufour H, Ouafik L, Figarella-Branger D (2009) Prognostic impact of O6-methylguanine-DNA methyltransferase silencing in patients with recurrent glioblastoma multiforme who undergo surgery and carmustine wafer implantation: a prospective patient cohort. Cancer 115:4783–4794. doi:10.1002/cncr.24546
Park CK, Park SH, Lee SH, Kim CY, Kim DW, Paek SH, Kim DG, Heo DS, Kim IH, Jung HW (2009) Methylation status of the MGMT gene promoter fails to predict the clinical outcome of glioblastoma patients treated with ACNU plus cisplatin. Neuropathology 29:443–449. doi:10.1111/j.1440-1789.2008.00998.x
Rivera AL, Pelloski CE, Gilbert MR, Colman H, De La Cruz C, Sulman EP, Bekele BN, Aldape KD (2010) MGMT promoter methylation is predictive of response to radiotherapy and prognostic in the absence of adjuvant alkylating chemotherapy for glioblastoma. Neuro Oncol 12:116–121. doi:10.1093/neuonc/nop020
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO, European Organisation for Research, Treatment of Cancer Brain Tumour, Radiation Oncology Groups, National Cancer Institute of Canada Clinical Trials Group (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466. doi:10.1016/S1470-2045(09)70025-7
Watanabe T, Katayama Y, Komine C, Yoshino A, Ogino A, Ohta T, Fukushima T (2005) O6-methylguanine-DNA methyltransferase methylation and TP53 mutation in malignant astrocytomas and their relationships with clinical course. Int J Cancer 113:581–587. doi:10.1002/ijc.20625
Weller M, Felsberg J, Hartmann C, Berger H, Steinbach JP, Schramm J, Westphal M, Schackert G, Simon M, Tonn JC, Heese O, Krex D, Nikkhah G, Pietsch T, Wiestler O, Reifenberger G, von Deimling A, Loeffler M (2009) Molecular predictors of progression-free and overall survival in patients with newly diagnosed glioblastoma: a prospective translational study of the German Glioma Network. J Clin Oncol 27:5743–5750. doi:10.1200/JCO.2009.23.0805
Esteller M (2006) Epigenetics provides a new generation of oncogenes and tumour-suppressor genes. Br J Cancer 94:179–183. doi:10.1038/sj.bjc.6602918
Pegg AE, Dolan ME, Moschel RC (1995) Structure, function, and inhibition of O6-alkylguanine-DNA alkyltransferase. Prog Nucleic Acid Res Mol Biol 51:167–223
Bobustuc GC, Baker CH, Limaye A, Jenkins WD, Pearl G, Avgeropoulos NG, Konduri SD (2010) Levetiracetam enhances p53-mediated MGMT inhibition and sensitizes glioblastoma cells to temozolomide. Neuro Oncol. doi:10.1093/neuonc/noq044
Quinn JA, Jiang SX, Reardon DA, Desjardins A, Vredenburgh JJ, Rich JN, Gururangan S, Friedman AH, Bigner DD, Sampson JH, McLendon RE, Herndon JE II, Walker A, Friedman HS (2009) Phase II trial of temozolomide plus O6-benzylguanine in adults with recurrent, temozolomide-resistant malignant glioma. J Clin Oncol 27:1262–1267. doi:10.1200/JCO.2008.18.8417
Acknowledgments
The authors would like to thank Dr. Michael Stoto at the Harvard School of Public Health for his instruction and constructive feedback on this manuscript.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Olson, R.A., Brastianos, P.K. & Palma, D.A. Prognostic and predictive value of epigenetic silencing of MGMT in patients with high grade gliomas: a systematic review and meta-analysis. J Neurooncol 105, 325–335 (2011). https://doi.org/10.1007/s11060-011-0594-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-011-0594-5