Abstract
Radiotherapy is the only treatment definitely indicated for diffuse pontine gliomas (DIPG). Findings on the role of EGFR signaling in the onset of childhood DIPG prompted the use of nimotuzumab, an anti-EGFR monoclonal antibody. Assuming a potential synergy with both radiotherapy and vinorelbine, a pilot phase 2 protocol was launched that combined nimotuzumab with concomitant radiation and vinorelbine. An amendment in July 2011 introduced re-irradiation at relapse. The primary endpoint for first-line treatment was objective response rate (CR + PR + SD) according to the RECIST. This report concerns the outcome of this strategy as a whole. Vinorelbine 20 mg/m2 was administered weekly, with nimotuzumab 150 mg/m2 in the first 12 weeks of treatment; radiotherapy was delivered from weeks 3 to 9, for a total dose of 54 Gy. Vinorelbine 25 mg/m2 and nimotuzumab were given every other week thereafter until the tumor progressed or for up to 2 years. Re-irradiation consisted of 19.8 Gy, fractionated over 11 days. Baseline and latest MRIs were assessed blindly by an outside neuroradiologist. Twenty five children (mean age 7.4 years) were enrolled as of August 2009 (median follow-up 29 months). A response was observed in 24/25 patients (96 %). The nimotuzumab/vinorelbine combination was very well tolerated, with no acute side-effects. Eleven of 16 locally-relapsing patients were re-irradiated. One-year PFS and OS rates were 30 ± 10 % and 76 ± 9 %, respectively; 2-year OS was 27 ± 9 %; the median PFS and OS were 8.5 and 15 months, respectively. This strategy generated interesting results and warrants further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with diffuse pontine gliomas (DIPG) have characteristic MRI findings [1] and histological confirmation has often been judged unnecessary. For the past 30 years, focal radiotherapy has remained the only standard treatment [2]. In our 20-year experience [3], we have attempted numerous strategies but failed to improve the prognosis, consistently with the literature [4, 5]. Response to treatment, variously assessed in terms of tumor size reduction, is reportedly prognostic [6, 7].
Gilbertson et al. [8] and Zarghooni et al. [9] reported finding ERBB1/EGFR overexpression and amplification in DIPG, suggesting that this tyrosine-kinase-receptor might be a promising therapeutic target. Warren studied 13 post-mortem DIPG specimens, identifying a genetic and histological intratumoral heterogeneity, with areas of high-grade tumor showing gene amplifications, and focal areas of EGFR positivity at immunohistochemistry [10]. As emerges from recent molecular profiling studies [11–13], EGFR gene alterations are seen in a minority of cases, but EGFR protein overexpression/activation is frequently evident. Ballester et al. [14] recently reported EGFR overexpression on a multitissue array of 24 postmortem DIPG samples, and concluded that EGFR dysregulation may have an important role in the development of DIPG, and also as a therapeutic target.
Nimotuzumab is a humanized IgG1 monoclonal anti-ERBB1/EGFR antibody suitable for treating DIPG. It binds to the extracellular domain of ERBB1/EGFR with a moderate affinity and high specificity, blocking EGF binding, preventing receptor dimerization/activation, and sparing normal tissues with a low EGFR density any severe cytotoxicity [15, 16, 17]. Its capacity to cross the blood–brain barrier was studied in xenografted mice, revealing a time-dependent increase in nimotuzumab uptake into the tumor: nimotuzumab was able to spread inside brain tumors and bind to a specific target, namely the extracellular region of EGFR [16, 17]. Its passage through the blood–brain barrier was also studied using radio-immunoscintigraphy to assess EGFR expression in patients with high-grade glioma enrolled in a phase I/II trial [18]: after radiation plus nimotuzumab, immunoscintigraphy showed a monoclonal antibody uptake by residual lesions.
Vinorelbine is a semisynthetic vinca alkaloid capable of altering blood–brain barrier permeability [19, 20, 21]. We had already used it to treat DIPG, associated with radiation, achieving one 10-year survivor among 12 patients [3]. Vinorelbine has proved active against glioma both in vitro and in vivo [19, 20], and also in EGFR FISH-positive non-small-cell lung cancers [22]. Vinorelbine enhances the EGFR binding sites in both human breast and non-small-cell lung cancer cell lines [23, 24]. We combined nimotuzumab with vinorelbine, seeking synergistic effects on DIPG. In 2009 we designed a phase II pilot study to assess the effectiveness and safety of this combination, administered together with radiation. Given the results seen in a few DIPG patients treated at the MD Anderson center [25], we offered re-irradiation to relapsing patients from 2011 onwards. The results obtained in this study (including re-irradiated cases) were compared with those of the BSC-PED-05 international trial conducted in 2006, involving nimotuzumab alone with radiotherapy [6, 26].
Patients and methods
Study design and eligibility criteria
A non-randomized, open label phase II pilot study was conducted to assess the efficacy—in terms of objective response rate (CR + PR + SD) according to the RECIST criteria [27] (already used in previous trials involving nimotuzumab [6, 26])—of combining nimotuzumab and vinorelbine with radiation in newly-diagnosed DIPG. Patients from 2 to 21 years old were eligible. Our Institute ethical committee approved the trial. The diagnosis of DIPG was confirmed by central radiological review, and by a second neuroradiologist (MWM) blinded to patients’ clinical data, who also assessed response after induction treatment and confirmed disease status at patients’ last follow-up. Strict eligibility criteria included radiologically-verified DIPG (an intrinsic, pontine-based infiltrative lesion hypointense on T1- and hyperintense on T2-weighted sequences, involving at least 2/3 of the pons) [1]. Other inclusion criteria were: symptoms lasting less than 6 months, life expectancy >4 weeks; Karnofsky/Lansky performance status ≥40 %; no organ dysfunction; no pregnancy or breast-feeding.
Patients underwent baseline cranial MRI with gadolinium, to be repeated if treatment began more than 2 weeks later (spinal MRI was also performed in some cases, but was not compulsory).
Written and signed informed consent from parents or legal guardians was obtained before starting the treatment.
Nimotuzumab and vinorelbine infusions
Drug treatments were scheduled as shown in Fig. 1, with nimotuzumab infusion first, then vinorelbine infusion. Nimotuzumab doses were chosen after pediatric phase II and III trials had shown that it was safe [6, 26], while weekly vinorelbine doses were reduced to 80 % of those used in single drug schedules [28] because no data were available on the safety of this combination.
After a first follow-up examination at week 12, the therapy was continued every other week in cases without progressive disease; the dose of vinorelbine was raised to 25 mg/m2, while the nimotuzumab dose remained unchanged. The treatment lasted up to 104 weeks.
Radiotherapy
Irradiation was scheduled to begin in the 3rd week after starting the nimotuzumab and vinorelbine treatment. A total dose of 54 Gy was delivered, in 1.8 Gy daily fractions 5 days a week, with a 6 MV linear accelerator. To plan radiotherapy, CT images were acquired with a 2 mm slice thickness, with patients positioned ready for treatment, their heads immobilized with a custom-made thermoplastic mask. Each patient’s CT images were co-registered with T2-weighted, gadolinium-enhanced T1-weighted, and fluid-attenuated inversion recovery MRI sequences to identify the gross target volume (GTV) precisely. A three-dimensional conformal radiotherapy technique with 5 or 6 coplanar or non-coplanar beams was adopted.
Re-irradiation for local relapse
Simulation, contouring and planning procedures were much the same as for the first-line treatment. The GTV included all tumor grossly visible on MRI, embracing previously-irradiated areas as well as any new areas of progressive disease. The beam geometry for re-irradiation was chosen so as to avoid the entrance beam paths of the first-line treatment wherever possible. A total dose of 19.8 Gy was delivered in eleven 1.8 Gy daily fractions. There were no restrictions on the time elapsing between the initial radiotherapy and any re-irradiation. Parents were again asked to give their consent before any re-irradiation was administered.
Radiological evaluation for inclusion and response
T2-weighted sequences in all three planes were needed to accurately estimate the tumor’s size. Response to treatment was assessed using RECIST criteria [27], considering the diameters measured on T2-weighted images.
The largest tumor diameters in the 3 main directions were measured, and the largest of these was used to assess response. A general neurological examination was performed at the baseline and at least at the time of all neuroradiological assessments. If the treatment had to be stopped due to a patient’s overall deterioration, the case was classified as symptomatic PD and censored as such. MRI was repeated after the 12th week elapsing after starting the treatment (i.e. 3 weeks after completing the radiotherapy), then every 12 weeks, and when the treatment was stopped (after 104 weeks). MRI was repeated every 4 months during the 3rd/4th years, twice a year in the 5th/6th years, and yearly thereafter.
Statistical methods
The primary endpoint of the study was the objective response rate according to the RECIST [complete response (CR) plus partial response (PR) plus stable disease (SD)] as assessed at week 12. For the combined treatment to be considered promising, a response rate of around 90 % or more would be desirable; if the response rate was 70 % (as obtained by radiation with nimotuzumab alone [6]), then the combined treatment would be unacceptable. A sample of 28 patients would have a power of 86 % for detecting a 20 % increase in response rate—from 70 to 90 % using a 1-sided exact test for single proportions with a 5 % alpha level. The best cut-off for distinguishing between the two rates would be 24 responses (86 %) for the higher rate to be accepted as more likely. The exact 95 % confidence interval of the proportion of responses was also calculated, based on the binomial distribution. The secondary endpoints were overall survival (OS) and progression-free survival (PFS), estimated with the Kaplan–Meier method and compared with the log rank test. The analyses were conducted on the intention-to-treat (ITT) population, i.e. all enrolled and eligible patients were included. PFS and OS times were calculated from the date of the first nimotuzumab/vinorelbine infusion to the date of any radiological or clinical progression or death due to any cause (whichever came first), and censored as at the date of latest follow-up for patients who were event-free and alive, respectively. The results were considered statistically significant whenever the P value was below 0.05.
Adverse events were classified using the Common Terminology Criteria for Adverse Events v4.0 (CTCAE).
Results
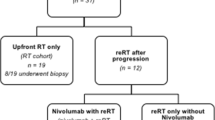
Figure 2 shows the patient flow after enrolment.
Thirty-one patients were initially considered eligible, but 6 were excluded after the external review (3 had atypical DIPG images, 2 had leptomeningeal metastases, and one had a Lansky score below 40 and rapidly deteriorating conditions that prevented any radiotherapy from being started).
The 25 eligible patients (15 males) were a median 6.1 years of age [interquartile range (IQR): 4.5–9.8; range: 2–17 years], a mean 7.4 years old, and 4 of them were under 4 years old. All patients had been symptomatic for less than 6 months prior to their DIPG being diagnosed, and 20 of them had begun referring symptoms less than 3 months earlier. The median duration of symptoms was 14 days (IQR: 14–21).
Four patients had undergone biopsies (at parents’ request in one case, and for enhancing areas possibly suggesting a non-glioma histology in three), resulting in a diagnosis of grade 2 diffuse astrocytoma (in 2) and grade 3 anaplastic astrocytoma (in 2). One anaplastic astrocytoma had cytoplasmic membrane expression for EGFR.
Four patients presented with hydrocephalus, which required third ventriculo-cisternostomy in 2 cases, and ventriculoperitoneal shunting in 2. Another patient without hydrocephalus had undergone ‘preventive’ ventriculoperitoneal shunting elsewhere. Four children required shunting during treatment, which involved a ventriculoperitoneal device in 3 cases, and third ventriculocisternostomy in one.
Neurological signs included cranial nerve palsy in all patients, accompanied by pyramidal deficits in 23, and cerebellar signs in 9. Eight patients had the whole triad.
Seventeen patients had spinal MRI at diagnosis (proving technically imperfect due to movement artifacts in 3 cases); the other 8 only underwent cranial assessment.
At the first MRI evaluation, 2/25 tumors had shrunk enough to qualify as PRs. Considering a lower cut-off than the RECIST criteria in subsequent analyses, 5 patients had a more than 20 % reduction in tumor size, 10 had smaller reductions, and 7 were stable after this first treatment phase. One child’s tumor increased in size by 53 %, accompanied by worsening clinical symptoms: this case was later classified as a pseudoprogression, which lasted 4 months before shrinking. One child with stable local disease developed spinal dissemination. The response rate was therefore 96 % (95 % confidence interval 79.7–100.0 %; P = 0.002).
Symptom improvement enabled steroid weaning and suspension before the radiotherapy came to an end in 13 patients, and from 1 to 8 weeks (median 3) after it ended in 10 cases. Steroids were administered for another 4 months in one child due to a pseudoprogression (mentioned above), and steroid weaning was unfeasible in one child due to persistent hydrocephalus despite a ventriculoperitoneal shunt.
This series of patients was followed up for a median 29 months (IQR: 19–42 months).
Twenty patients progressed, 16 locally (one with dissemination too), 4 with dissemination. One child was lost to follow-up with stable tumor and symptoms. One child died after 7 months due to infectious complications of a shunt, without any disease progression. 11/12 of the children progressing locally after July 2011 were re-irradiated, while one patient’s parents refused this re-treatment. 4/5 with dissemination were irradiated on the metastases. None of the patients had unexpected side-effects or worsening neurological symptoms during re-irradiation, which induced a tumor volume shrinkage in 7 cases. In 10, symptoms improved enough to enable steroid suspension. Survival after re-irradiation ranged from 6 weeks to 14 months (median 6 months). Eighteen of the 25 children have died so far, 17 due to their tumor, one due to infection (as mentioned earlier).
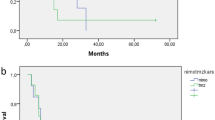
The median PFS was 8.5 months, the median OS 15 months. The 1- and 2-year PFS rates (±standard error) were 30 ± 10 % and 12 ± 7 %, respectively, while for OS they were 76 ± 9 % and 27 ± 9 %, respectively (Fig. 3). The median PFS for the 11 locally re-irradiated children was 8.3 months as opposed to 8.5 for the other 14; their median OS times were 16 and 13.3 months, respectively (P ns), whereas the median OS for the 5 children with local relapses who were not re-irradiated was 12 months (P = 0.03).
We compared our sample’s PFS and OS rates with those of the patients accrued in the international BSC-PED-05 trial administering nimotuzumab with standard local radiotherapy for the primary treatment of DIPG [6], since there were no major clinical differences between the two patient groups (data not shown). The images were centrally reviewed using the same inclusion criteria and response/progression was assessed by the same external neuroradiologist unaware of the patients’ clinical course (MWM). In the BSC-PED-05 trial, the median PFS and OS had been 5.8 months (P = 0.002) and 9.4 months (P = 0.003), respectively, i.e. both differed statistically from the rates recorded in our series (Fig. 4).
We also checked for parameters influencing prognosis, e.g. sex, age under/over 4 years, duration of symptoms, need for shunting at diagnosis, and RECIST tumor shrinkage below/above 20 % after induction. For the 7 patients whose tumor had shrunk more than 20 %, the 1- and 2-year OS rates were 100 % and 50 ± 20 %, while for the 18 patients whose tumors had shrunk less, they were 67 ± 11 % and 17 % ± 10 %, respectively (P = 0.05). A better OS emerged for children under 4 years old at diagnosis (their 2-year OS rate being 75 ± 22 % vs. 15 ± 9 % for the older patients; P = 0.04), and for patients who were shunted (the 1-year OS rate was 60 ± 22 % as opposed to 22 ± 10 % for those not shunted; P = 0.01).
Toxicity
None of the patients had hematological toxicity beyond grade 1. One had grade 3 hypokalemia while on dexamethasone medication. Another had appendicitis preventing the treatment’s continuation for 1 week. Three patients developed fever (apparently unrelated to any infection) after infusions, which persisted for several hours and regressed spontaneously within 24 h. Two others had acute respiratory infections during radiotherapy, which was consequently suspended for 3 consecutive days. One child developed iron deficiency anemia 20 months after starting the treatment. The case of pseudoprogression and the onset of hydrocephalus have already been mentioned above.
Discussion
In recent decades, we and others have tried different therapeutic strategies for DIPG, but failed to improve patients’ prognosis. Based on previously-published scientific grounds [8–15], as mentioned earlier, a phase II study by Bode et al. [6] tested the efficacy of nimotuzumab in 45 children with recurrent high-grade gliomas, achieving disease control in 17/45 (11 with DIPG) after 8 weeks, and responders had a significantly longer median OS than non-responders according to the RECIST criteria. These promising results set the scene for the international trial BSC-PED-05 in children with newly-diagnosed DIPG. Nimotuzumab 150 mg/m2 was given weekly for 12 weeks as induction therapy, associated with radiotherapy, then every other week for consolidation until the disease progressed. The results were comparable with those obtained in the two previous decades using other chemotherapy regimens with irradiation, but with virtually no toxicity [26].
Erlotinib, a small-molecule EGFR inhibitor was also used as a target-specific therapy in DIPG, concomitantly with radiation: 30 patients treated in a phase I trial achieved a median PFS of 8 months and a median OS of 12 months [29]. Gefitinib, another EGFR-inhibitor administered during radiotherapy in children with newly-diagnosed DIPG, led to a 24-month OS of 19.6 %, and 3 patients were still progression-free after a more than 36-month follow-up [30]. Finally, vandetanib, a small-molecule inhibitor of both EGFR and VEGFR2 (vascular endothelial growth factor receptor 2), was administered concurrently with radiotherapy and as maintenance, alone or with dasatinib, in newly-diagnosed DIPG patients in two successive phase I trials: the 1-year OS was 37.5 % for vandetanib alone, and 52 % when it was combined with dasatinib [31, 32]. All these studies showed that concomitant treatment with EGFR inhibitors and radiation was feasible in DIPG, but had little influence on patients’ outcome.
Our results in terms of OS were never previously reported in the context of a trial involving a central imaging review, which confirmed that all patients had DIPG [5]. So far, no differences had been seen in DIPG patient outcomes after using standard- versus high-dose, or conventional versus hyperfractionated radiotherapy schedules, so conventionally fractionated radiotherapy, with a total dose of 54 Gy delivered over 6 weeks, came to be adopted as a standard radiotherapy schedule [33]. Recently-published experiences with hypofractionated radiotherapy focused on whether the results were as good as those achieved with a shorter treatment schedule, but the reported median OS rates were 8 and 9 months, i.e. at the lower end of the range emerging from the literature [34, 35].
Early response to the combination treatment was a prognostic parameter in our series, as in other reports [6, 7, 36], so the likelihood of a longer PFS and OS was correlated with an early effect of the treatment. A better prognosis was seen for younger patients, as already reported by Broniscer et al. [37], and for children needing a shunt. This latter finding may be an indirect sign of a better prognosis for larger tumors [7, 36] (the most likely cause of obstructive hydrocephalus).
The feasibility of re-irradiation points to a real chance of a longer life expectancy after relapse that warrants assessment in appropriate, larger clinical trials. Wolff et al. [38] also obtained a statistically significant improvement in EFS with re-irradiation than with any other strategy adopted for relapsing DIPG.
Based on our safety and treatment outcome data, we propose our approach as one arm for future possible randomized DIPG trials challenging the role of this whole strategy on aspects where we cannot separate the exact contributions of each individual agent.
References
Fischbein NJ, Prados MD, Wara W, Russo C, Edwards MS, Barkovich AJ (1996) Radiologic classification of brain stem tumors: correlation of magnetic resonance imaging appearance with clinical outcome. Pediatr Neurosurg 24:9–23
Donaldson SS, Laningham F, Fisher PG (2006) Advances toward an understanding of brainstem gliomas. J Clin Oncol 24:1266–1272
Massimino M, Spreafico F, Biassoni V et al (2008) Diffuse pontine gliomas in children: changing strategies, changing results? A mono-institutional 20-year experience. J Neurooncol 87:355–361
Hargrave D, Bartels U, Bouffet E (2006) Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol 7:241–248
Jansen MH, van Vuurden DG, Vandertop WP, Kaspers GJ (2012) Diffuse intrinsic pontine gliomas: a systematic update on clinical trials and biology. Cancer Treat Rev 38:27–35
Bode U, Massimino M, Bach F et al (2012) Nimotuzumab treatment of malignant gliomas. Expert Opin Biol Ther. 12:1649–1659
Poussaint TY, Kocak M, Vajapeyam S et al (2011) MRI as a central component of clinical trials analysis in brainstem glioma: a report from the Pediatric Brain Tumor Consortium (PBTC). Neuro Oncol 13:417–427
Gilbertson RJ, Hill DA, Hernan R et al (2003) ERBB1 is amplified and overexpressed in high-grade diffusely infiltrative pediatric brain stem glioma. Clin Cancer Res 9:3620–3624
Zarghooni M, Bartels U, Lee E et al (2010) Whole-genome profiling of pediatric diffuse intrinsic pontine gliomas highlights platelet-derived growth factor receptor alpha and poly (ADP-ribose) polymerase as potential therapeutic targets. J Clin Oncol 28:1337–1344
Warren KE, Killian K, Suuriniemi M, Wang Y, Quezado M, Meltzer PS (2012) Genomic aberrations in pediatric diffuse intrinsic pontine gliomas. Neuro Oncol 14:326–332
Wu G, Broniscer A, McEachron TA et al (2012) Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet 44:251–253
Schwartzentruber J, Korshunov A, Liu XY et al (2012) Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 482:226–231
Sturm D, Witt H, Hovestadt V et al (2012) Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell 22:425–437
Ballester LY, Wang Z, Shandilya S et al (2013) Morphologic characteristics and immunohistochemical profile of diffuse intrinsic pontine gliomas. Am J Surg Pathol 37:1357–1364
Massimino M, Bode U, Biassoni V, Fleischhack G (2011) Nimotuzumab for pediatric diffuse intrinsic pontine gliomas. Expert Opin Biol Ther 11:247–256
Dietil M. Scientifically based report on the blood–brain-barrier in malignant tumors. Expert Opinion—BBB—malignant brain tumors. BBB-09-11-16:1–9, Institute for Pathology, Charite´-University Medicine Berlin
Fichtner I, Nowak CH. Report: biodistribution of nimotuzumab in xenografetd mice. Experimental Pharmacology & Oncology, Berlin-Buch gmbh, 22 April 2010
Crombet Ramos T, Figueredo J, Catala S et al (2006) Treatment of high-grade glioma patients with the humanized anti-epidermal growth factor receptor (EGFR) antibody h-R3. Cancer Biol Ther 5:375–379
Hanley ML, Elion GB, Colvin OM et al (1998) Therapeutic efficacy of vinorelbine against pediatric and adult central nervous system tumors. Cancer Chemother Pharmacol 42:479–482
Mouchard-Delmas C, Gourdier B, Vistelle R, Wiczewski M (1995) Modification of the blood-brain barrier permeability following intracarotid infusion of vinorelbine. Anticancer Res 15:2593–2596
Binet S, Fellous A, Lataste H, Krikorian A, Couzinier JP, Meininger V (1989) In situ analysis of the action of Navelbine on various types of microtubules using immunofluorescence. Semin Oncol 16(Suppl 4):5–8
Crinò L, Cappuzzo F, Zatloukal P et al (2008) Gefitinib versus vinorelbine in chemotherapy-naive elderly patients with advanced non-small-cell lung cancer (INVITE): a randomized, phase II study. J Clin Oncol 26:4253–4260
Depenbrock H, Shirvani A, Rastetter J, Hanauske AR (1995) Effects of vinorelbine on epidermal growth factor-receptor binding of human breast cancer cell lines in vitro. Invest New Drugs 13:187–193
Tsai CM, Chiu CH, Chang KT et al (2012) Gefitinib enahances cytotoxicities of antimicrotubile agents in non-small cell lung cancer cells exhibiting no sensitizing epidermal growth factor mutation. J Thorac Oncol 7:1218–1227
Fontanilla HP, Pinnix CC, Ketonen LM et al (2012) Palliative reirradiation for progressive diffuse intrinsic pontine glioma. Am J Clin Oncol 35:51–57
Fleischhack G, Siegler N, Zimmermann M, et al (2010) Concomitant therapy of nimotuzumab and standard radiotherapy for the treatment of newly diagnosed diffuse intrinsic pontine gliomas in children and adolescents. 14th international symposium of pediatric neuro-oncology, Vienna, Austria 20–23 June 2010 [abstract]. Neurooncology 12:ii8
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Casanova M, Ferrari A, Spreafico F et al (2002) Vinorelbine in previously treated advanced childhood sarcomas: evidence of activity in rhabdomyosarcoma. Cancer 94:3263–3268
Geoerger B, Hargrave D, Thomas F et al (2011) Innovative Therapies for Children with Cancer pediatric phase I study of erlotinib in brainstem glioma and relapsing/refractory brain tumors. Neuro Oncol 13:109–118
Pollack IF, Stewart CF, Kocak M et al (2011) A phase II study of gefitinib and irradiation in children with newly diagnosed brainstem gliomas: a report from the Pediatric Brain Tumor Consortium. Neuro Oncol 13:290–297
Broniscer A, Baker JN, Tagen M et al (2010) Phase I study of vandetanib during and after radiotherapy in children with diffuse intrinsic pontine glioma. J Clin Oncol 28:4762–4768
Broniscer A, Baker SD, Wetmore C et al (2013) Phase I trial, pharmacokinetics, and pharmacodynamics of vandetanib and dasatinib in children with newly diagnosed diffuse intrinsic pontine glioma. Clin Cancer Res 19:3050–3058
Lewis J, Lucraft H, Gholkar A (1997) UKCCSG study of accelerated radiotherapy for paediatric brain stem gliomas. United Kingdom Childhood Cancer Study Group. Int J Radiat Oncol Biol Phys 38:925–929
Janssens GO, Jansen MH, Lauwers SJ et al (2009) The role of hypofractionation radiotherapy for diffuse intrinsic brainstem glioma in children: a pilot study. Int J Radiat Oncol Biol Phys 73:722–726
Negretti L, Bouchireb K, Levy-Piedbois C et al (2011) Hypofractionated radiotherapy in the treatment of diffuse intrinsic pontine glioma in children: a single institution’s experience. J Neurooncol 104:773–777
Sedlacik J, Winchell A, Kocak M, Loeffler RB, Broniscer A, Hillenbrand CM (2013) MR Imaging assessment of tumor perfusion and 3D segmented volume at baseline, during treatment, and at tumor progression in children with newly diagnosed diffuse intrinsic pontine glioma. Am J Neuroradiol 34:1450–1455
Broniscer A, Laningham FH, Sanders RP, Kun LE, Ellison DW, Gajjar A (2008) Young age may predict a better outcome for children with diffuse pontine glioma. Cancer 113:566–572
Wolff JE, Rytting ME, Vats TS, Zage PE, Ater JL, Woo S et al (2012) Treatment of recurrent diffuse intrinsic pontine glioma: the MD Anderson Cancer Center experience. J Neurooncol 106:391–397
Acknowledgments
This work was supported by the Associazione Bianca Garavaglia Onlus (Busto Arsizio Italy), the Fondo di Giò Onlus (Trieste, Italy), and the Associazione Italiana per la Ricerca Sul Cancro (AIRC); nimotuzumab was given on compassionate grounds by Oncoscience AG.
Conflict of interest
There are no other conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Massimino, M., Biassoni, V., Miceli, R. et al. Results of nimotuzumab and vinorelbine, radiation and re-irradiation for diffuse pontine glioma in childhood. J Neurooncol 118, 305–312 (2014). https://doi.org/10.1007/s11060-014-1428-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-014-1428-z