Abstract
We report herein our institutional experience in the treatment of diffuse intrinsic pontine glioma (DIPG) with a hypofractionated external-beam radiotherapy schedule. Between April 1996 and January 2004, 22 patients (age 2.9–12.5 years) with newly diagnosed DIPG were treated by hypofractionated radiation therapy delivering a total dose of 45 Gy in daily fractions of 3 Gy, given over 3 weeks. No other treatment was applied concomitantly. Fourteen of the 22 patients received the prescribed dose of 45 Gy in 15 fractions of 3 Gy, and 2 patients received a total dose of 60 and 45 Gy with a combination of two different beams (photons and neutrons). In five cases the daily fraction was modified to 2 Gy due to intolerance, and one patient died due to serious intracranial hypertension after two fractions of 3 Gy and one of 2 Gy. Among 22 children, 14 patients showed clinical improvement, usually starting in the second week of treatment. No grade 3 or 4 acute toxicity from radiotherapy was observed. No treatment interruption was needed. In six patients, steroids could be discontinued within 1 month after the end of radiotherapy. Median time to progression and median overall survival were 5.7 months and 7.6 months, respectively. External radiotherapy with a radical hypofractionated regimen is feasible and well tolerated in children with newly diagnosed DIPG. However, this regimen does not seem to change overall survival in this setting. It could represent a short-duration alternative to more protracted regimens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brainstem gliomas represent 10–15% of all childhood brain tumors [1], and despite multiple therapeutic efforts in recent years to improve results, prognosis remains dismal and no progress has been achieved in treatment of children with such tumors in the last 20 years [2]. Radiation remains the gold-standard treatment for diffuse intrinsic pontine gliomas (DIPG); by contrast, chemotherapy has not shown any benefit. Most prospective studies have investigated alternative radiation schedule options, such as hyperfractionation with escalating doses, and also in combination with chemotherapy. Several studies have concluded that conventional external-beam radiotherapy to a dose of 54 Gy in 30 fractions in 6 weeks is the mainstay of treatment [3–8] with median time to progression and overall survival of 6 and 9 months, respectively [8, 9]. The goal of the current study is to determine the feasibility and tolerance in terms of acute toxicity of a hypofractionated radiotherapy schedule with total dose of 45 Gy in daily fractions of 3 Gy, given over 3 weeks. This study also aims to estimate treatment efficacy in terms of progression-free survival and overall survival.
Materials and methods
Between April 1996 and January 2004, 22 children aged less than 18 years with DIPG were treated with a hypofractionated radiation therapy, through a local Institutional Review Board (IRB) approved protocol. Information was given according to French law. Parents or guardians gave their informed consent for treatment, data recording, and chart review. The diagnosis of DIPG was based on high-quality, gadolinium-enhanced magnetic resonance imaging (MRI) with T1 and T2 sequences showing involvement of at least 50% of the pons by a hyperintense T2 infiltrating tumor. Biopsy was required only in case of atypical MRI appearance or prolonged duration of symptoms, i.e., over 3 months. If hydrocephalus was found on imaging with signs of raised intracranial pressure, cerebrospinal fluid diversion was recommended before initiation of radiotherapy. No performance criteria were required for entry to the study.
Treatment was initiated as soon as possible after diagnosis, i.e., within less than 3 weeks. The treatment consisted of external radiotherapy, using two opposed lateral photon beams with energy of 4 and 20 MV, with a hypofractionated schedule delivering a total dose of 45 Gy in single daily fractions of 3 Gy, given over 3 weeks, with both fields treated at the same session. For radiotherapy planning, computed tomography with optional intravenous contrast medium was performed, using a thermoplastic mask to maintain the patient’s head. Patients younger than 4 years received general anesthesia for realization of the planning three-dimensional (3D) scan and all radiotherapy sessions. The planned target volume included the tumor as defined by the T2-weighted MRI images with a margin of 1–1.5 cm. Margins were adjusted for bony structures and tentorium. Pituitary gland was protected by a shield from the dose of 40 Gy. Treatment volumes were documented by dosimetric plans of some axial, sagittal, and coronary scanner views and by digital reconstructed radiography (DRR). Dosimetric calculation of the dose distribution was carried out for all patients.
Once a week during radiotherapy, patients were seen in the clinic by the radiooncologist, who collected data about tolerance and acute toxicity. No systematic blood count surveillance was applied; monthly follow-up was applied after the radiation therapy and shared by pediatricians and radiation oncologists. Steroids and setrons were authorized before and during radiation therapy. After radiation therapy, cerebral MRI was performed every 3 months. The goal was to decrease steroids as clinically tolerated after completion of irradiation.
All MRI files were reviewed by two independent investigators (L.N., K.B.). Radiological progressions/responses were defined according to standard criteria [10].
Overall survival rates were estimated using the Kaplan–Meier method from date of diagnosis to death or to date of last follow-up visit for patients who were still alive. Progression-free survival rates were estimated with the above-mentioned method from date of diagnosis to time of documented failure (as documented by clinical deterioration and/or radiological progression).
Results
Median age at diagnosis was 5.9 years (range 2.9–12.5 years). There were 10 girls and 12 boys. The median interval between onset of symptoms and diagnosis was 35 days (range 4–60 days). Clinical presentation included cerebellar signs (73%), cranial nerve paralysis (68%), hemiparesis (50%), headache (36%), and vomiting (9%) as signs of raised intracranial hypertension. In four patients, diagnosis was confirmed by biopsy, showing grade III astrocytoma in three patients and grade IV glioblastoma multiforme in one case. All patients received steroids at start and during radiotherapy. Hydrocephalus was present in one case, and the treatment consisted of insertion of a ventriculoperitoneal shunt before initiation of radiation therapy. Radiotherapy started within 13 days of diagnosis (range 1–29 days) (Table 1). Fourteen of 22 patients (64%) received the prescribed dose of 45 Gy in 15 fractions of 3 Gy, given over 3 weeks. Two of the 22 patients (9%) received a hypofractionated schedule of radiotherapy combining two different beams, photons and neutrons, with total dose of 60 Gy and 45 Gy. The remaining six patients showed worse tolerance of hypofractionated treatment, and reduction of the daily fraction dose to 2 Gy was needed.
Efficacy
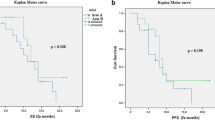
Clinical improvement was observed in 14 of the 16 patients (87%) treated with the hypofractionated schedule, mostly starting in the second week of radiotherapy. In 6 of 16 patients (37%), steroids could be discontinued within 1 month after the end of radiotherapy. Median progression-free survival was 5.7 months (range 1.4–15.8 months) (Fig. 1). Median overall survival was 7.6 months (range 0.3–21 months) (Fig. 2). Median time to death from first progression was 3 months (range 0.3–9.13 months).
Toxicity
Eight patients required increase of corticosteroid dosage during treatment mainly because of headache and vomiting suggesting increase of intracranial pressure. In five of the eight cases, the daily fraction of radiation was modified to 2 Gy per fraction to a total dose ranging between 45 and 54 Gy, and three of them showed general clinical improvement. One patient developed serious intracranial hypertension with death after two fractions of 3 Gy and one fraction of 2 Gy. This evolution could not be directly related to the radiation therapy. No other grade 3 or 4 acute toxicity from radiotherapy was observed. No treatment interruption was needed.
Discussion
Although magnetic resonance imaging has improved and facilitated diagnosis of diffuse brainstem glioma [11], no progress has been observed in treatment of children with these tumors in the last 20 years [2], and their prognosis remains very poor. Conventionally fractionated external-beam focal radiotherapy to dose of 54 Gy to 60 Gy in 6 weeks remains the standard treatment for diffuse brainstem glioma [8]. Without radiation, median survival is approximately 20 weeks (4.7 months) [12]. Radiation treatment leads to a significant, albeit temporary, clinical neurological improvement of patients and can permit reduction or cessation of steroidal treatment that is usually introduced at diagnosis. Conventional radiotherapy results in median time to progression and survival of 6 and 9 months, respectively [8].
In this current study, the hypofractionated radiation schedule proved to be well tolerated with valuable shortening of overall treatment time. Of patients, 87% showed general clinical/neurological improvement, mostly starting in the second week of radiotherapy, with complete reversal of initial symptoms at the end of radiation. In 6 of 16 patients (37%), use of steroids could be temporary discontinued. However, in 36% of our patients (8/22), steroid therapy had to be increased as a result of progressive neurologic symptoms. In the literature, use of steroids is not well reported, but transient interruption ranges probably between 38% and 63% [13, 14].
In our study, median time to progression, median overall survival, and time between progression and death were 5.7, 7.6, and 3 months, respectively. According to a recently published review of clinical trials, median time to progression, overall survival, and time between progression and death ranged from 5 to 8.8, 7 to 16, and 1 to 4.5 months, respectively, with conventional accelerated, or hypofractionated schedules and other experimental treatment combinations [2]. A Dutch pilot study [15] reported the feasibility and tolerance of a hypofractionation schedule with 13 fractions of 3 Gy or 6 fractions of 5.5 Gy, given over 3 weeks. This study, conducted on a smaller number of patients (nine), but with similar characteristics to the patient population of our study, showed similar results with symptom improvement in all patients within 2 weeks after start of radiotherapy, without development of grade 3 or 4 toxicity. In this pilot study, median time to progression and overall survival were comparable to our study, i.e., 4.9 and 8.6 months, respectively.
The major advantage of hypofractionated radiotherapy is the shorter overall treatment time. This represents reduced outpatient department transfers, less prolonged hospital stays with a possible reduction of patient burden, maintaining the same efficacy as conventionally fractionated external-beam radiotherapy. Considering the dismal prognosis in this disease, the above-mentioned advantages become relevant for the quality of life of patients.
Shorter overall treatment time is economically advantageous, and this kind of treatment can improve resource usage. It also ensures that, in the future, good treatment quality can be provided even in difficult financial circumstances.
Conclusions
External radiotherapy with a radical hypofractionated regimen is feasible and well tolerated in children with newly diagnosed brainstem glioma with no grade 3 and 4 toxicities. Additionally, the results are comparable to standard regimens, therefore this type of treatment can represent a short-duration alternative to more protracted regimens. It can offer quick clinical improvement with reduction of patient burden, and also treatment costs, at a time when medical resources are under close political and economical surveillance. However, this study is able to show good clinical responses in this new environment in favor of the patient, which must remain the main clinical and ethical interest of therapeutics. Outside of clinical trials, a hypofractionated radiotherapy schedule delivering a total dose of 45 Gy in 15 fractions of 3 Gy over 3 weeks could be considered in patients with rapid neurological worsening, linked to worse prognosis. Use of such a schedule in combination with radiosensitizing agents would need further investigation.
References
Smith MA, Freidlin B, Ries LA et al (1998) Trends in reported incidence of primary malignant brain tumors in children in the United States. J Natl Cancer Inst 90:1269–1277
Hargrave D, Bartels U, Bouffet E (2006) Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol 7:241–248
Lee F (1975) Radiation of infratentorial and supratentorial brainstem tumors. J Neurosurg 43:65–68
Halperin EC (1985) Pediatric brain stem tumors: pattern of treatment failure and their implications for radiotherapy. Int J Radiat Oncol Biol Phys 11:1293–1298
Littman P, Jarret P, Bilaniuk LT et al (1980) Pediatric brainstem gliomas. Cancer 45:2787–2792
Eifel PJ, Cassady JR, Belli JA (1987) Radiation therapy of tumors of the brainstem and midbrain in children: experience of the Joint Center for Radiation Therapy and Children’s Hospital Medical Center (1971–1981). Int J Radiat Oncol Biol Phys 13:847–852
Freeman CR, Suissa S (1986) Brain stem tumors in children: results of a survey of 62 patients treated with radiotherapy. Int J Radiat Oncol Biol Phys 12:1823–1828
Donaldson SS, Laningham F, Fisher PG (2006) Advances toward an understanding of brainstem gliomas. J Clin Oncol 24:1266–1272
Mandell LR, Kadota R, Freeman C et al (1999) There is no role for hyperfractionated radiotherapy in the management of children with newly diagnosed diffuse intrinsic brainstem tumors: results of a pediatric oncology group phase III trial comparing conventional versus hyperfractionated radiotherapy. Int J Radiat Oncol Biol Phys 43:959–964
Gnekow AK (1995) Recommendations of the brain tumor subcommittee for the reporting of trials. SIOP brain tumor subcommittee. International Society of Pediatric Oncology. Med Pediatr Oncol 24:104–108
Albright AL, Packer RJ, Zimmerman RA et al (1993) Magnetic resonance scans should replace biopsies for the diagnosis of diffuse brain stem gliomas: a report from the children’s cancer group. Neurosurgery 33:1026–1029
Langmoen IA, Lundar T, Storm-Mathisen I et al (1991) Management of pediatric pontine gliomas. Childs Nerv Syst 7:13–15
Freeman CR, Krischer JP, Sanford RA et al (1993) Final results of a study of escalating doses of hyperfractionated radiotherapy in brain stem tumors in children: a pediatric oncology group study. Int J Radiat Oncol Biol Phys 27:197–206
Kretschmar CS, Tarbell NJ, Barnes PD et al (1993) Pre-irradiation chemotherapy and hyperfractionated radiation therapy 66 Gy for children with brain stem tumors. A phase II study of the pediatric oncology group, Protocol 8833. Cancer 72:1404–1413
Janssens GO, Gidding CE, van Lindert EJ et al (2009) The role of hypofractionation radiotherapy for diffuse intrinsic brainstem glioma in children: a pilot study. Int J Radiat Oncol Biol Phys 73:722–726
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Negretti, L., Bouchireb, K., Levy-Piedbois, C. et al. Hypofractionated radiotherapy in the treatment of diffuse intrinsic pontine glioma in children: a single institution’s experience. J Neurooncol 104, 773–777 (2011). https://doi.org/10.1007/s11060-011-0542-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-011-0542-4