Abstract
Extended survival of 3 or more years is rare in patients with glioblastoma (GBM) but is becoming more common. Clinical outcome has not been well studied. We reviewed GBM patients at Memorial Sloan-Kettering Cancer Center between 2001 and 2003 who were seen for two or more visits. Patient characteristics and long-term clinical outcomes were reviewed for patients who had survived 3 or more years following diagnosis. Thirty-nine (11%) of 352 GBM patients were identified as long-term survivors. Median survival was 9.15 years (range: 3–18 years). Median age was 47 years (range: 16–69); 13% were 65 years or older. Median KPS was 90 (range: 50–100). One long-term survivor underwent biopsy alone; 19 patients each had either complete or subtotal resection. All received focal radiotherapy (RT) with a median dose of 5940 cGy; 18% received concurrent temozolomide. Adjuvant chemotherapy was administered to 35 (90%). Twelve patients (31%) remained in continuous remission. Twenty-seven had tumor progression a median of 29.2 months after diagnosis (range: 1.2–167 months); 18 had multiple relapses. Median KPS at last follow-up was 70 (range: 40–100); 85% of long-term survivors had at least one significant neurologic deficit. Eleven (28%) had clinically significant RT-induced leukoencephalopathy, 9 (23%) developed RT necrosis and 9 (23%) treatment-related strokes. Treatment-related complications occurred a median of 2.7 years from diagnosis (range: 0.9–11.5 years). Long-term survivors remain rare, but are found across all age groups despite multiple recurrences; clinically significant delayed complications of treatment are common.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma (GBM) is the most common primary brain tumor in adults and accounts for approximately 50% of gliomas and 12–15% of all intracranial tumors [1]. Despite multimodal treatment including surgery, radiation, and chemotherapy, the prognosis for the vast majority of patients remains poor, with median survival of 14 months [2]. However, with recent advances in therapy, an increasing number of GBM patients will survive for 36 months or longer [2–7]. Although a number of studies have evaluated potential prognostic factors that might predict long-term survival including age, gender, performance status at diagnosis, and chromosomal abnormalities such as loss of chromosome 19q [8], the clinical outcome of long-term GBM survivors has not been studied carefully; however, other brain tumor populations where long-term survival is more common have a significant risk of neurologic sequelae related to therapy and the tumor itself [9–11]. Understanding long-term clinical outcomes are critical to optimizing the medical management and function of these patients. Therefore, we studied patients with GBM who were alive three or more years following their original diagnosis.
Patients and methods
We reviewed the Department of Neurology patient database at Memorial Sloan-Kettering Cancer Center (MSKCC) to identify all GBM patients with at least two clinical evaluations between January 1, 2001 and December 31, 2003 (N = 352). Patients from this initial cohort who met the following criteria were included in the study: 3 years from initial pathologic diagnosis, pathology reviewed at MSKCC, no evidence of oligodendroglial differentiation on pathology review, no history of prior low-grade glioma either pathologically or radiographically. Pathology for all cases was re-reviewed and confirmed to represent GBM at MSKCC. Charts and available imaging of the selected patients were reviewed in detail. The Institutional Review Board at MSKCC reviewed and approved this retrospective study. Follow-up extended through July 31, 2007.
Results
Patient characteristics
Thirty-nine patients (11%) met the defined criteria for long-term survivors. Median age at diagnosis was 47 years (range: 14–69 years); 13% were 65 years of age or older. At diagnosis, the median Karnofsky Performance Score (KPS) was 90 (range: 50–100). Symptoms developed a median of 1.22 months (range: 0.1–9.4 months) before diagnosis of GBM. Twenty-two patients (56%) presented with headache, 11 (28%) with seizure, six (16%) with aphasia, five (13%) with hemiparesis, three (8%) with visual deficits and two (5%) with confusion. Tumors were located in the frontal lobe in 51%, temporal lobe in 26%, parietal lobe in 5%, occipital lobe in 5%, fronto-temporal in 8%, and temporo-parietal lobe in 5%. Nineteen patients had a tumor in the right hemisphere and 18 had left hemisphere tumors; bilateral disease crossing the corpus callosum was observed in two patients (5%).
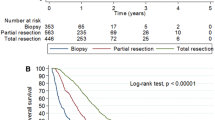
Median survival of the entire cohort is 9.15 years (range: 3–18 years) from GBM diagnosis (Fig. 1). At last follow-up, 23 patients were alive with a median follow-up of 4.7 years (range: 3.1–9.1 years). The cause of death was recurrent GBM in 13; two patients died of complications related to neurologic disability with no active tumor and one patient died of an unrelated cause (Table 1).
Initial treatment
All patients had surgery for definitive diagnosis. Nineteen patients each underwent complete or subtotal resection as defined by post-operative MRI or operative report (if imaging was not available). Of the 19 patients with incomplete resection, three had a re-resection within 3 months of the first surgery. Only one long-term survivor had a biopsy only. All patients received focal radiotherapy following surgery; the median dose delivered was 5940 cGy (range: 4500–6120 cGy). Seven patients (18%) received concurrent temozolomide (daily dose 75 mg/m2) during RT and one patient received concomitant therapy with the experimental farnesyl transferase inhibitor R115777. Four patients (10%) received no additional therapy following RT. Adjuvant chemotherapy was administered to 35 patients (90%). Nineteen (49%) received temozolomide, 13 (33%) received carmustine or lomustine, and two patients each received PCV (procarbazine, lomustine and vincristine) or intra-arterial cisplatin (3%).
Disease control
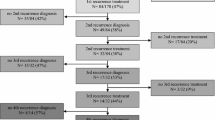
Twelve patients (31%) remained in continuous remission following initial treatment for a median of 5.4 years (range: 3.1–18.17 years). The median age of this group was 57 years (range: 29–69); seven of these patients had an initial complete resection. All remain alive except one patient who died 5.7 years after GBM diagnosis. The cause of death was attributed to neurologic deterioration following treatment-related stroke.
Twenty-seven patients (69%) had documented tumor recurrence a median of 29.2 months from original diagnosis (range: 1.2–167 months). Nine patients received treatment for a single relapse and 18 were treated for multiple relapses. Sixteen patients had one or more additional surgeries at relapse and 9 had a second course of radiotherapy. All 27 patients had one or more salvage drug therapies including temozolomide (27), BCNU (11), other chemotherapy (12), bevacizumab (3) and targeted molecular therapies (5).
Clinical outcome
Clinical outcome at last outpatient follow-up was determined from the medical record a median of 9 years after initial GBM diagnosis (range: 3–18 years). Median KPS at last follow-up was 70 (range: 40–100). Ten patients were employed (9 full-time), twelve were retired and 17 were unemployed largely as a result of neurologic disability. Twenty-one patients were independent in activities of daily living. On neurologic examination six patients had no deficits noted; the remaining 33 (85%) had significant neurologic deficits including gait abnormalities in 24 (8 wheel-chair bound), aphasia (12), visual field deficit (9), hearing loss (4), incontinence (3), cognitive difficulties (15) and hemiparesis (12).
Treatment-related toxicity
Eighteen patients (46%) developed one or more clinically significant treatment-related complications a median of 2.8 years after diagnosis of GBM (range: 0.9–11.5 years).
Radiation-induced leukoencephalopathy was observed on MRI in all 39 patients. However, only 11 patients (28%) developed related clinical symptoms attributed to leukoencephalopathy (no patient with active tumor was diagnosed with clinical leukoencephalopathy). Cognitive impairment or slowing, difficulties with recent memory, distractibility and fatigue were the most common complaints. These symptoms developed a median of 3.5 years following the initial GBM diagnosis (range: 1.25–11.57 years). Whereas the symptoms were described as mild in five patients, they were considered severe in the remaining six. One patient developed hearing loss and tinnitus attributed to radiation-induced ototoxicity.
Radiation necrosis was diagnosed in 9 patients (23%) a median of 3.65 years after GBM diagnosis (range: 0.75–9.4 years). In 6 patients, the diagnosis was confirmed and treated by surgical resection; in 3 patients, radiation necrosis was diagnosed by clinical exam and imaging and steroids were used to manage symptoms. No patient with radiation necrosis had received concurrent temozolomide with RT; however, three had received salvage re-irradiation at recurrence. Presenting symptoms included new or increasing frequency of seizures (4), new hemiparesis (3), new headache (2), memory difficulties (2) and cerebellar ataxia (1).
Nine long-term survivors (23%) subsequently developed a total of 10 strokes at a median of 4.03 years (range: 0–17 years) following GBM diagnosis. Seven strokes were ischemic, three were hemorrhagic. One patient developed a sinus venous thrombosis associated with a hemorrhagic infarct. One stroke was a direct complication of surgical resection of the GBM. The remaining 8 strokes were attributed to RT-induced vasculopathy. The median age at onset of the stroke was 60 years (range: 30.4–73.8 years).
Discussion
Most patients with GBM have a relatively short survival but some have prolonged survival well beyond 3 years. Recent advances in available therapies are expected to result in larger proportions of patients living longer, thus the functional state of these long-term survivors is critically important. Our review suggests that long-term survival in GBM patients, although uncommon, may be increasing. Approximately 11% of GBM patients seen at MSKCC between 2001 and 2003 survived more than 3 years. This percentage is significantly higher than a report published in the late 1990s, which showed 2.2% of GBM patients surviving longer than 3 years [7]. Our observed long-term survival rate may reflect selection bias, but newer therapies such as temozolomide and bevacizumab may also account for this shift [4, 12, 13]. In particular, 16% of patients treated with concomitant and adjuvant temozolomide can be expected to survive 3 years after diagnosis [14].
Our results suggest that there may be two subgroups of long-term GBM survivors: one group that remains in continuous remission following initial therapy and another group that can be salvaged following one or more relapses. The group that remained in continuous remission represented slightly less than one-third of our patients; this group had a slightly higher rate of initial complete resection but was also older than the overall group. However, there are no obvious differences in clinical characteristics between these two groups. In contrast, slightly more than two-thirds of our long-term survivors had received treatment for one or more relapses. Most of these relapses occurred relatively late, generally more than 2 years following initial GBM diagnosis. Patients in both groups challenge many commonly held assumptions about GBM. Although uncommon, a small group of patients with GBM appear to have been cured of their disease; however, a recent report suggests that GBM patients remain at risk for very late relapses [15]. Some of long-term survivors were older where a poor prognosis is the expected (>10 years) outcome. Furthermore, there are patients who can enjoy prolonged survival despite multiple tumor recurrence. A recent study identified MGMT promoter hypermethylation as a prognostic marker for prolonged survival [16]. Future studies characterizing the molecular and genetic features of GBM tissue from long-term survivors may identify signatures that could be used to predict outcome or risk of recurrence for newly diagnosed patients.
The clinical outcome of long-term GBM survivors raises new challenges for patients, caregivers and physicians. Neurologic sequelae in long-term survivors can be linked both to the direct effect of the tumor as well as the chronic and delayed side effects of treatment. The majority of our patients had at least one demonstrable neurologic deficit and the overall median KPS had declined from initial diagnosis, suggesting that these neurologic sequelae accumulate over time and have a significant impact on day-to-day function. A small but in depth study of 10 long-term GMB survivors supports our findings; they found mild neurologic and frequent cognitive deficits and all patients had treatment-related leukoencephalopathy [17]. While both tumor location at diagnosis and recurrence, as well as treatment-related toxicity may contribute to a decline in functional status, our data would suggest that at least one-half of the patients had chronic symptoms as a result of their therapy.
The implications of our study are important to the development of new therapies. Clearly, too few GBM patients achieve long-term survival with current therapies and more effective regimens are needed to improve outcome for most patients. However, technological advances such as improvements in radiation therapy planning may be invaluable to spare patients from some radiation-induced sequelae. It will be critical to assess the long-term outcome of patients treated with concurrent temozolomide and radiotherapy with particular attention to cognitive function and development of leukoencephalopathy. It is possible that patients treated with concurrent temozolomide and radiotherapy may be at increased risk of neurologic sequelae as a result of the toxicity associated with a combined modality regimen or because of the increased potential for prolonged survival. Patients who become long-term survivors need aggressive surveillance to diagnose new or worsening neurologic symptoms and interventions to optimize their neurologic function.
References
DeAngelis LM (2001) Brain tumors. N Engl J Med 344:114–123
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Schmidinger M, Linzmayer L, Becherer A, Fazeny-Doemer B, Fakhrai N, Prayer D, Killer M, Ungersboeck K, Dieckmann K, Marosi C (2003) Psychometric- and quality-of-life assessment in long-term glioblastoma survivors. J Neuro-oncol 63:55–61
Chandler KL, Prados MD, Malec M, Wilson CB (1993) Long-term survival in patients with glioblastoma multiforme. Neurosurgery 32:716–720 (discussion 720)
Morita M, Rosenblum MK, Bilsky MH, Fraser RA, Rosenfeld MR (1996) Long-term survivors of glioblastoma multiforme: clinical and molecular characteristics. J Neuro-oncol 27:259–266
Salford LG, Brun A, Nirfalk S (1988) Ten-year survival among patients with supratentorial astrocytomas grade III and IV. J Neurosurg 69:506–509
Scott JN, Rewcastle NB, Brasher PM, Fulton D, Hagen NA, MacKinnon JA, Sutherland G, Cairncross JG, Forsyth P (1998) Long-term glioblastoma multiforme survivors: a population-based study. Can J Neurol Sci 25:197–201
Burton EC, Lamborn KR, Feuerstein BG, Prados M, Scott J, Forsyth P, Passe S, Jenkins RB, Aldape KD (2002) Genetic aberrations defined by comparative genomic hybridization distinguish long-term from typical survivors of glioblastoma. Cancer Res 62:6205–6210
Ammirati M, Vick N, Liao YL, Ciric I, Mikhael M (1987) Effect of the extent of surgical resection on survival and quality of life in patients with supratentorial glioblastomas and anaplastic astrocytomas. Neurosurgery 21:201–206
Scheibel RS, Meyers CA, Levin VA (1996) Cognitive dysfunction following surgery for intracerebral glioma: influence of histopathology, lesion location, and treatment. J Neuro-oncol 30:61–69
Taylor BV, Buckner JC, Cascino TL, O’Fallon JR, Schaefer PL, Dinapoli RP, Schomberg P (1998) Effects of radiation and chemotherapy on cognitive function in patients with high-grade glioma. J Clin Oncol 16:2195–2201
Muller H, Brock M, Ernst H (1985) Long-term survival and recurrence-free interval in combined surgical, radio- and chemotherapy of malignant brain gliomas. Clin Neurol Neurosurg 87:167–171
Salcman M, Scholtz H, Kaplan RS, Kulik S (1994) Long-term survival in patients with malignant astrocytoma. Neurosurgery 34:213–219 (discussion 219–220)
Stupp R, Hegi ME, Mason WP, et al. (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol [Epub ahead of print]
Bahr O, Herrlinger U, Weller M, Steinback JP (2009) Very late relapses in glioblastoma long-term survivors. J Neurol [Epub ahead of print]
Krex D, Klink B, Hartmann C et al (2007) Long-term survival with glioblastoma multiforme. Brain 130:2596–2606
Steinbach JP, Blaicher H-P, Herrlinger U et al (2006) Surviving glioblastoma for more than 5 years: the patient’s prospective. Neurology 66:239–242
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hottinger, A.F., Yoon, H., DeAngelis, L.M. et al. Neurological outcome of long-term glioblastoma survivors. J Neurooncol 95, 301–305 (2009). https://doi.org/10.1007/s11060-009-9946-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-009-9946-9