Abstract
Glioblastoma has a poor prognosis with median survival of 12–14 months. Long-term survivors (LTS), alive at least 2 years from diagnosis, comprise 13% of this population. This study aims to provide a clinical profile of LTS at two institutions in Melbourne, Australia. Histological diagnosis of glioblastoma from 1st January 2006 to 31st December 2012 were identified from pathology/oncology databases. Demographic, treatment and survival characteristics were recorded (follow-up to 31st December 2015). Relevant inter-group statistics were used to identify differences between LTS and those surviving less than 2 years. Survival estimated by Kaplan–Meier. 776 patients were identified with 154 surviving > 2 years. Compared with patients surviving < 2 years, LTS were more likely to be younger (median age 56 vs. 65 years, p < .001), have ECOG 0–2 (97 vs. 65%, p < .001), gross tumour resection (91 vs. 61%, p < .001), and receive chemoradiotherapy (94 vs. 40%, p < .001). Most common presenting symptoms amongst LTS were headache (42%), seizure (28%) and speech disturbance (16%). Of LTS, 111 patients (72%) progressed at a median of 20.1 months from diagnosis, with 46% undergoing a second craniotomy. The most common non-surgical second line treatments were temozolomide (41%), followed by radiotherapy (12%). One-third of LTS received three or more lines of treatment, and 10% underwent three or more craniotomies. LTS of glioblastoma (20%) are more likely to be younger, have unilateral tumours, good performance status and undergo multimodality treatment. These data may assist in predicting LTS at diagnosis and understanding their clinical journey to facilitate planning of treatment and supportive care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma is the most common primary brain tumour in adults. Despite the advances in surgical and radiotherapy techniques, and the introduction of chemotherapy as standard treatment, the prognosis of glioblastoma remains poor with a median survival of 12–14 months [1].
In 2005, the National Cancer Institute of Canada and European Organization for Research and Treatment of Cancer published a seminal study showing an improvement in overall survival with the addition of concurrent oral temozolomide to standard radiotherapy [1]. After a median of 5 years follow-up, addition of chemotherapy to radiotherapy improved 3 year survival from 4.4 to 16% [2].
Long-term survivors defined as those alive at least 2 years from diagnosis, comprise 13% of all patients diagnosed with glioblastoma [3]. These patients may experience changes in neurocognition and significant functional dependence [4, 5]. Early prediction of those patients who are likely to be long-term survivors and a full understanding of their clinical course can thus assist clinicians in providing tailored treatment and support to patients and their families.
The aim of this retrospective study is to (1) identify clinical predictive factors for longer survival among patients diagnosed with glioblastoma and (2) describe the clinical experience of long-term survivors of glioblastoma.
Methods
Consecutive adult patients with newly diagnosed and histologically confirmed glioblastoma (WHO grade IV glioma) from 1 January 2006 to 31 December 2012 treated at two institutions in Melbourne (Australia) were retrospectively identified from pathology and oncology databases (and cross-referenced between each database to ensure all eligible patients were captured), and followed up until 31 December 2015. Secondary glioblastoma was included. Patients without a histological confirmation of glioblastoma were excluded.
De-identified data were collected from the medical records including patient demographic and clinical characteristics, date of histological diagnosis, surgical and oncological treatment information and date of death or last follow-up. Performance status, based on Eastern Cooperative Oncology Group (ECOG) score [6], was assessed post-surgery at initial contact with the medical or radiation oncologist. This was either recorded directly or estimated by the Principal Investigator (LG). Tumour location and laterality were determined based on review of pre-operative imaging studies (Magnetic Resonance images or Computed Tomography scans), with deep lesions defined as those involving the corpus callosum, thalamus or brainstem.
The study was approved by the Institutional Human Ethics Committee at St Vincent’s Hospital, Melbourne, and The Alfred Hospital, Melbourne.
Statistical analyses
Descriptive statistics were used to describe patient demographic and clinical characteristics. Categorical variables are presented as observed counts and weighted percentages, and continuous variables as median with the corresponding range. Survival was estimated by the Kaplan–Meier method. Overall survival (OS) was defined as the time from surgery to death or censored at the date of last follow-up.
In a univariate analysis, variables associated with OS were assessed using the log-rank test, with p values < 0.05 were considered statistically significant [7]. Significant predictors from the univariate analyses were then included in a multivariate analysis using a Cox proportional hazards model to determine their relative effect on OS, after adjustment for other clinically important variables. Patients with missing data were excluded from multivariate analysis.
Subgroup analyses examining differences in the clinical, management and outcome characteristics of patients with glioblastoma who survived more or less than 2 years, and between those surviving more than 2 years and more than 5 years (extreme survivors) were conducted. Relevant inter-group test statistics were applied (chi square for categorical variables, independent samples t test for continuous variables, or Fisher’s exact test when sample size was less than five) to identify statistical differences between the survival cohorts. Age was considered a continuous variable. The remainder, including ECOG, were considered categorical variables. A logistic regression was performed to ascertain independent predictors for long-term survivors and extreme survivors.
All analyses were performed using R programing software (version 3.4.0). Statistical tests were two sided and p values < 0.05 were considered statistically significant.
Results
Patient characteristics
Seven hundred and seventy-six patients with glioblastoma were identified during the 7-year study period, and all were included in the analysis, with a median follow-up time of 71.5 months (range 36–121 months). The demographic and clinical characteristics of all patients is shown in Table 1. The median age was 63 years (range 20–91 years), with 46% aged over 65 years. 80% were unilateral tumours, and 18% were bilateral. Most patients had an ECOG performance status of 0–2 post-operatively. Two-thirds underwent gross tumour resection. Following surgical intervention, 10% did not receive any oncological intervention and 16% received radiotherapy alone. Half of the cohort received combined chemoradiotherapy.
Survival and prognostic factors
The median overall survival for all patients estimated by Kaplan Meier was 11.0 months (CI 9.7–12.3). Significant predictors for longer survival from the univariate analysis shown in Table 2 were included in a multivariate analysis. The multivariate analysis included 497 patients with complete data available. Results for improved overall survival are shown in Table 3. The significant factors on multivariate analysis were age (HR 0.98, CI 0.97–0.99, p < .0001), ECOG performance status (HR 0.52, CI 0.36–0.74, p = .0003), gross tumour resection (HR 0.57, CI 0.45–0.73, p < .0001) and extent of oncological treatment: radiotherapy alone (HR 0.24, CI 0.16–0.36, p < .0001), chemoradiotherapy (HR 0.08, CI 0.05–0.12, p < .0001). There was no significant interaction between ECOG performance status and treatment (p = .28). There was a correlation between age and the following factors: ECOG performance status (p < .001), surgery (p = .3) and extent of oncological treatment (p < .001). When age was removed from the multivariate analysis, ECOG performance status (HR 0.51, CI 0.36–0.73, p = .0002), gross tumour resection (HR 0.57, CI 0.44–0.73, p < .0001) and extent of oncological treatment: radiotherapy alone (HR 0.18, CI 0.13–0.30, p < .0001), chemoradiotherapy (HR 0.07, CI 0.04–0.10, p < .0001), remained significant.
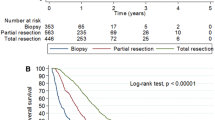
Long-term survivor cohort 154 patients (20%) survived 2 or more years following diagnosis and were deemed long-term survivors. Amongst long-term survivors, there was equal distribution of male and female gender. Most tumours were unilateral, with 47% right sided and 40% left sided. Over 90% underwent surgical resection with 49% undergoing maximal tumour debulking and 42% undergoing partial debulking. Over 80% completed radiotherapy with concurrent temozolomide followed by adjuvant temozolomide and 12% received radiotherapy with concurrent temozolomide only. One patient underwent radiotherapy alone due to patient preference, and less than 5% received chemotherapy alone.
The most common presenting symptoms were headache (42%), seizure (28%) and speech disturbance (16%). Confusion was present in 11% and memory loss in 6%. Anticonvulsants were prescribed in 79%, most commonly phenytoin (73%). Twenty-one patients (14%) had a prior history of glioma (WHO grade II or III). Over half of these patients were asymptomatic with disease detected on routine surveillance scans, and were subsequently histologically confirmed. There was no statistically significant difference in median overall survival based on past history of glioma.
As shown in Table 1, compared with patients surviving less than 2 years, long-term survivors were more likely to be younger (median age 56 vs. 65 years, p < .0001), have ECOG 0–2 (97 vs. 64%, p < .0001), undergo gross tumour resection (91 vs. 61%, p < .0001), and receive chemoradiotherapy (94 vs. 40%, p < .0001). Median overall survival was three times longer in the long-term survivor cohort, compared with the entire cohort (38.3 vs. 11.0 months, p < .0001). There was no significant difference between the two cohorts in terms of gender or laterality. The significant independent predictors for long-term survivors on logistic regression were younger age (p < .0001), gross tumour resection (p = .002) and receipt of chemoradiotherapy (p < .001). ECOG was not a significant independent predictor.
Of all long-term survivors, thirty-two patients (20%) survived at least 5 years, termed extreme survivors. Compared with patients surviving at least 2 years, extreme survivors were more likely to be younger (median age 47 vs. 59 years, p = .02). There were no other statistically significant differences between the two groups (see Table 4).
Treatment received at recurrence is outlined in Table 5. Data at recurrence is available for one hundred and eleven patients (72%). An additional 14 patients did not recur during the follow up period. The median time to progression was 20.1 months from diagnosis. Almost half underwent a second craniotomy. Thirty-six patients received further treatment following their second craniotomy. Three patients received further post-operative radiotherapy, 27 patients received further post-operative chemotherapy and five patients were enrolled in a clinical trial. Temozolomide was the most common non-surgical second line treatment (41%), followed by radiotherapy (12%). Enrolment in a clinical trial following recurrence was associated with a statistically significant difference in survival post recurrence (clinical trial 24.7 months vs. no clinical trial 14.37 months, p = .049 on Mann–Whitney test) (see Table 5).
Seventy-two patients progressed following second line treatment. Of these, 70% received third line treatment, mostly chemotherapy (46%) followed by surgery (23%). Thirty-six patients progressed following third line treatment, with 50% receiving treatment. With each recurrence, a lower proportion of patients underwent surgery or received oncological intervention. Overall, 10% of long-term survivors underwent three or more craniotomies.
Discussion
This study details the clinical characteristics of, to our knowledge, the largest group of long-term survivors of glioblastoma.
There is currently a lack of consensus on a definition for “long-term survivor” in glioblastoma with survival from diagnosis ranging from 18 months to 5 years. In this study, survival of 2 years from diagnosis was used as this is twice the median survival for patients with glioblastoma and corresponds with a halving of the cumulative relative survival rate [8]. Other reported studies have used 3 or 5 years post diagnosis. Extreme survivors, defined as those surviving at least 5 years, have also been reported.
An important outcome of this study is the examination of the characteristics of long-term survivors of glioblastoma that may be identified at baseline. Field et al. (2014) reported the predictors for long-term survivors, surviving more than 24 months, compared with short term survivors, defined as those surviving less than 6 months [9]. Their analysis included 269 patients, with those long-term survivors more likely to be younger, have improved performance status, and have undergone gross macroscopic resection. Further to this, they found frontal tumours, unifocal disease, multiple operations and participation in a clinical trial to be more likely in long-term survivors. However, the only independent predictors for survival were age, performance status, extent of resection and clinical trial participation [9]. Similarly, other authors have determined in comparisons of long-term survivors, defined as surviving over 3 years, with non-long-term survivor controls, that long-term survivors were significantly younger, had better performance status, and were more likely to undergo gross total resection and adjuvant chemotherapy [10, 11]. These studies however, included only small numbers of long-term survivors (less than 20 patients) and did not address the independent predictors for survival. Meanwhile, Krex et al. (2007) described the characteristics of 55 long-term survivors and found they were young and had a good performance status at diagnosis; however, no comparison was made to determine if these were significant predictors [12].
Our study included 154 long-term survivors and compared these with a case control matched population of 622 patients. In this large cohort, the independent predictors for improved survival confirmed the findings of others and included being younger age, improved performance status, gross tumour resection and receipt of concurrent chemoradiotherapy. The differences between long-term survivors and those surviving less than 2 years were also confirmatory. However, the only significant independent predictors for long-term survivors at diagnosis were age, gross tumour resection and receipt of chemoradiotherapy. Interestingly, performance status was not a significant independent predictor and may be due to the interaction between performance status and treatment received.
Within this group of long-term survivors, there were 32 extreme survivors. This constitutes approximately 4% of all patients diagnosed with glioblastoma in this cohort, and is in keeping with other population studies [3]. The only significant factor for determining extreme survivors amongst long-term survivors was younger age.
These findings suggest that younger patients treated aggressively may subsequently become long-term survivors and should be identified early in their disease course. However, it is important to note that despite age being an independent predictor, 15% of long-term survivors were aged over 70 years, with three of these elderly patients surviving at least 5 years.
This study also aimed to document the clinical experience of long-term survivors of glioblastoma. Long-term survivors can present with a wide variety of neurological symptoms. The symptoms recorded in this study were similar in nature and rates to those seen in previous works [11, 13], in particular headache which has been reported in up to 56% [13]. Symptom comparison between short-term survivors and long-term survivors was not analysed as part of this study. Long-term survivors may also experience multiple relapses and undergo multiple lines of therapy [11, 13]; however, treatment practices at first recurrence varies between institutions.
Rates of craniotomies at first recurrence in long-term survivors are reported at approximately 50% [11, 13], and has been shown to improve post-recurrence survival and overall survival if complete resection is achieved [14,15,16]. In our study, the median post-recurrence survival of those that underwent craniotomy at first recurrence and those that did not was not significantly different. However, a significant proportion of patients received further treatment following their second craniotomy, suggesting a contribution of these treatments to survival. In 2014, Nava et al. (2014) published a large cohort study also showing that survival was not improved with resection at first recurrence, yet there was a survival advantage with second line stereotactic radiosurgery or chemotherapy [17]. Similarly, Ringel et al. (2016) retrospectively reviewed 503 patients, all of whom underwent re-resection at first recurrence and demonstrated an improvement in survival with the addition of chemotherapy from 8.5 to 13.4 months [18]. In our study, there was no significant difference in post-recurrence survival with the addition of chemotherapy. Our study also showed a statistically significant improvement in post-recurrence survival if patients were enrolled in a clinical trial over standard treatment. This has been suggested by Shahar et al. (2012) in a cohort of recurrent glioblastoma irrespective of survival status [19].
There has been little published data addressing the psychosocial and cognitive outcomes of long-term survivors of glioblastoma. Results from three studies with a combined total of 66 patients have shown that these patients are at risk of psychological symptoms and neurocognitive impairment [13, 20, 21]. Despite this, quality of life is relatively preserved [20, 21].
Whilst this study has not addressed these aspects directly, these data will be used as part of a larger project which will explore the psychosocial and neurocognitive outcomes and the impact they have on the survivorship experience. This will assist in documenting in further detail the clinical experience of this small but important patient population.
Limitations
This retrospective analysis relied heavily on documentation. Where specifics were missing, parameters such as ECOG performance status were estimated from the medical notes available. Documentation of ECOG performance status was missing in 27% of patients. Similarly, extent of surgical resection was often difficult to determine from operation reports and thus post-operative imaging (a more objective parameter) was used. Finally, documentation of mental state and cognitive functioning at the time of diagnosis was not consistently recorded and could not be reliably interpreted from the notes. As such, it was not possible to include recursive partitioning analysis in this study.
Molecular subtyping was not routinely performed between 2006 and 2012, and as such, this parameter could not be explored in our study. Both institutions are quaternary referral centres for the management of brain tumours. Our results may have limited generalizability to other sites with different practice patterns and patient populations. Nonetheless, this study reports upon large numbers of patients whose clinical characteristics provide an important baseline against which future patient cohorts can be compared.
Conclusions
In summary, long-term survivors of glioblastoma (20%) are more likely to be younger, have good performance status and undergo multimodality treatment. They experience an individualised clinical course that may involve multiple craniotomies and lines of drug therapy. These data may assist in predicting long-term survivors at diagnosis and understanding their clinical experience to facilitate planning of treatment and supportive care. The quality of life and quality of survival of this patients group is an important outcome that must be fully elucidated in future studies.
References
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996
Stupp R, Hegi ME, Mason WP et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomized phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10(5):459–466
Ostrom QT, Gittleman H, Farah P et al (2013) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol 15(Suppl 2):ii1–ii56
Meyers C, Boake C (1993) Neurobehavioral disorders experienced by brain tumor patients: rehabilitation strategies. Cancer Bull 45:362–364
Sizoo E, Dirven L, Reijneveld J et al (2014) Measuring health-related quality of life in high-grade glioma patients at the end of life using a proxy-reported retrospective questionnaire. J Neuroonc 116(2):283–290
Oken MM, Creech RH, Tormey DC et al (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5(6):649–655
Perneger TV (1998) What’s wrong with Bonferroni adjustments. BMJ 316(7139):1236–1238
Smoll NR, Schaller K, Gautschi OP (2013) Long-term survival of patients with glioblastoma multiforme (GBM). J Clin Neurosci 20(5):670–675
Field KM, Rosenthal MA, Yilmaz M et al (2014) Comparison between poor and long-term survivors with glioblastoma: review of an Australian dataset. Asia Pac J Clin Oncol 10(2):153–161
Scott JN, Rewcastle NB, Brasher PM et al (1999) Which glioblastoma multiforme patient will become a long-term survivor? A population-based study. Ann Neurol 46(2):183–188
Sonoda Y, Kumabe T, Watanabe M et al (2009) Long-term survivors of glioblastoma: clinical features and molecular analysis. Acta Neurochir 151(11):1349–1358
Krex D, Klink B, Hartmann C, German Glioma Network et al (2007) Long-term survival with glioblastoma multiforme. Brain 130(Pt 10):2596–2606
Hottinger AF, Yoon H, DeAngelis LM et al (2009) Neurological outcome of long-term glioblastoma survivors. J Neurooncol 95(3):301–305
Suchorska B, Weller M, Tabatabai G et al (2016) Complete resection of contrast-enhancing tumor volume is associated with improved survival in recurrent glioblastoma – results from the DIRECTOR trial. Neuro Oncol 18(4):549–556
Chen MW, Morsv AA, Liang S et al (2016) Re-do craniotomy for recurrent grade IV glioblastomas: Impact and outcomes from the national neuroscience institute Singapore. World Neurosurg 87:439–445
Ening G, Huynh MT, Schmieder K et al (2015) Repeat-surgery at Glioblastoma recurrence, when and why to operate? Clin Neurol Neurosurg 136:89–94
Nava F, Tramacere I, Fittipaldo A et al (2014) Survival effect of first- and second-line treatments for patients with primary glioblastoma: a cohort study form a prospective registry, 1997–2010. Neuro Oncol 16(5):719–727
Ringel F, Pape H, Sabel M et al (2016) Clinical benefit from resection of recurrent glioblastomas: results of a multicenter study including 503 patients with recurrent glioblastomas undergoing surgical resection. Neuro Oncol 18(1):96–104
Shahar T, Nossek E, Steinberg DM et al (2012) The impact of enrolment in clinical trials on survival of patients with glioblastoma. J Clin Neurosci 19(11):1530–1534
Steinbach JP, Blaicher HP, Herrlinger U et al (2006) Surviving glioblastoma for more than 5 years: the patient’s perspective. Neurology 66:239–242
Flechl B, Ackerl M, Sax C et al (2012) Neurocognitive and sociodemographic functioning of glioblastoma long-term survivors. J Neurooncol 109:331–339
Funding
This study was funded by St Vincent’s Research Endowment Fund Grant (81,779).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there is no conflict of interest.
Ethical approval
This study did not require participation from human patients. The study was approved by the Institutional Human Ethics Committee at St Vincent’s Hospital, Melbourne, and The Alfred Hospital, Melbourne.
Rights and permissions
About this article
Cite this article
Gately, L., McLachlan, SA., Philip, J. et al. Long-term survivors of glioblastoma: a closer look. J Neurooncol 136, 155–162 (2018). https://doi.org/10.1007/s11060-017-2635-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-017-2635-1