Abstract
Background
Metformin has good anti-hyperglycemic effectiveness, but does not induce hypoglycemia,is very safe, and has become the preferred drug for the treatment of type 2 diabetes. Recently, the other effects of metformin, such as being anti-inflammatory and delaying aging, have also attracted increased attention.
Methods and Results
The relevant literatures on pubmed and other websites for reading, classification and sorting, and did not involve any animal experiments.
Conclusion
Metformin has anti-inflammatory effects through multiple routes, which provides potential therapeutic targets for certain inflammatory diseases, such as neuroinflammation and rheumatoid arthritis. In addition, inflammation is a key component of tumor occurrence and development ; thus, targeted inflammatory intervention is a significant benefit for both cancer prevention and treatment. Therefore, metformin may have further potential for inflammation-related disease prevention and treatmen. However, the inflammatory mechanism is complex; various molecules are connected and influence each other. For example, metformin significantly inhibits p65 nuclear translocation, but pretreatment with compound C, an AMPK inhibitor, abolishes this effect, and silencing of HMGB1 inhibits NF-κB activation . SIRT1 deacetylates FoxO, increasing its transcriptional activity . mTOR in dendritic cells regulates FoxO1 via AKT. The interactions among various molecules should be further explored to clarify their specific mechanisms and provide more direction for the treatment of inflammatory diseases, as well as cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
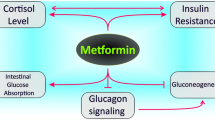
Metformin has been used since 1957 for the treatment of type 2 diabetes due to its good anti-hyperglycemic effectiveness, but does not induce hypoglycemia,high safety, low cost, and low rate of adverse reactions, and has become the medication of choice for first-line treatment. In addition, it can reduce the complications caused by diabetes and has several benefits unrelated to blood-sugar control, such as prolonged life and reduced risk for cancer and cardiovascular events [1,2,3,4,5,6]. It inhibits the occurrence and progression of colorectal cancer [7, 8], improves the survival rate of endometrial cancer patients [9], and reduces the incidence of breast cancer [10]. Regarding inflammatory diseases, metformin can target oxidative stress to downregulate transcription factor NF-κB-mediated pro-inflammatory signaling and reduce mucosal damage in inflammatory bowel disease [11], improve rheumatic disease [12, 13], and inhibit the expression of pro-inflammatory factors in neuroinflammation [14]. Inflammation is a key component of tumorigenesis. For example, chronic inflammation is considered the underlying mechanism causing DNA damage in gastric cancer [15]. However, the specific mechanisms of inflammation need further exploration. The possible relevant mechanisms of the anti-inflammatory effects of metformin are reviewed below (Fig. 1).
Suppression of pro-inflammatory cytokine release
Pro-inflammatory cytokines are present at all stages of the inflammatory response and play important roles in the occurrence and development of inflammation. Metformin inhibits pro-inflammatory cytokine release to have anti-inflammatory effects [16,17,18,19,20,21]. A study in rats of the potential protective effect of metformin on depression-like behavior and neuroinflammation induced by oxandrolone (OXA) showed that metformin reversed the upregulation of the pro-inflammatory factors interleukin 1 (IL-1) mRNA, IL-6 mRNA, and tumor necrosis factor alpha (TNF-α) mRNA in the hypothalamus and hippocampus [16]. Another study found that metformin inhibited IL-1-induced release of IL-6 and IL-8 and NF-κB activity, thereby hindering human blood-vessel-wall inflammation [22]. It has been found to reverse the increased expression of the pro-inflammatory cytokines IL-17, IL-18, and IL-6 in mouse models of aldosterone-induced myocarditis [20], and to inhibit the extracellular signal-regulated kinase 1/2-early growth response factor-1 (ERK1/2-Egr-1) pathway in human monocytes, thereby suppressing lipopolysaccharide (LPS)-induced TNF and tissue factor (TF) production and thus alleviating the induction effect of TNF on chemotaxis proteins and interleukins [23]. In a TBI model, metformin reduced ERK1/2 and p38 mitogen-activated protein kinase (p38 MAPK) phosphorylation levels [24], indicating that it may inhibit the inflammatory response induced by microglial activation via the NF-κB-MAPK signaling pathway.
The release of NF-κB is a crucial initial step in inflammation. NF-κB is an important nuclear transcription factor in cells that is involved in inflammatory, immune, and stress responses, as well as apoptosis regulation. Its overactivation is associated with many human diseases, such as rheumatoid arthritis, as well as inflammatory changes in heart and brain diseases [22, 24]. Metformin inhibits the phosphorylation and nuclear translocation of the NF-κB subunit p65 and suppresses the degradation of its inhibitory protein I καB, causing NF-κB to be sequestered in the cytoplasm and unable to translocate to the nucleus to participate in inducing an inflammatory response [17, 18, 24, 25]. Most studies have shown that metformin promotes the synthesis of the anti-inflammatory factor IL-10 [16, 26]; however, one study [27] found that it and its analogues had no effect on IL-10 secretion, but reduced IL-12 p40 and IL-6 secretion. In addition, that study found that metformin significantly inhibited five plasma cytokines (CCL11, CCL22, IL-2, IL-4, and stromal-cell-derived factor 1). CCL11 is independent of BMI and diabetes, however, BMI is associated only with CCL22 and stromal-cell-derived factor 1αβ, indicating that the anti-inflammatory effect of metformin is partly independent of its comprehensive control of blood sugar, lipids, and body weight.
Activation of adenosine 5′-monophosphate-activated protein kinase
Adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK) is a highly conserved serine/threonine protein kinase that is closely associated with bioenergy metabolism regulation and is widely expressed in various cells. AMPK is a heterotrimeric serine/threonine kinase composed of three subunits, one catalytic subunit and two regulatory subunits. The metformin-activated AMPK pathway mainly reduces the expression of inflammatory cytokines and chemokines, including CCL2, CXCL10, and CXCL11, by acting on various downstream proteins such as histone deacetylase (HDAC) and peroxisome-proliferator-activated receptor co-activation factor 1α (PGC-1α) [21, 28].
HDAC activation
Silent information regulation 2 homolog 1 (SIRT1) is an NAD+-dependent deacetylase with histone protein/protein as a substrate, and is a key enzyme associated with energy metabolism and lifespan signaling. Immunohistochemistry results have confirmed that AMPK activation increases p-SIRT1 expression levels, and knockdown of AMPK or use of the AMPK inhibitor compound C abolishes metformin activity [29]. This is consistent with numerous studies that found that metformin activates AMPK to upregulate SIRT1 and has an anti-inflammatory effect [29,30,31,32,33]. SIRT1 inhibits transcriptionally active NF-κB [34, 35]. After pretreatment with the SIRT1 activator, TNF-α-induced cellular NF-κB transcription is reduced. By contrast, pretreatment with SIRT1 inhibitor or HDAC I and II inhibitors increases TNF-α-induced NF-κB activity. Co-transfection experiments that used p300 to acetylate RelA/p65 in vivo have revealed that SIRT1 directly deacetylates RelA/p65 lysine 310 to act with the p65 subunit rather than p50 to repress NF-κB gene expression [34]. SIRT1 also positively activates AMPK. Lan et al. expressed the AMPK upstream kinase LKB1, wild-type SIRT1, and catalytically inactive SIRT1 in HEK293T cells. After wild-type SIRT1 activation with an activator, LKB1 acetylation was significantly reduced, however, inactive SIRT1 and shRNA knockout SIRT1 increased LKB1 acetylation several fold, with different LKB1 fragments interacting with SIRT1, showing that SIRT1-mediated Lys-48 is a key site for upstream kinase LKB1 activation, phosphorylation, and cytosolic localization by AMPK [36]. In a study of the endotoxin-induced endothelial pro-inflammatory response [35], pretreatment of human umbilical vein endothelial cells (HUVECs) with metformin and the AMPK activator AIRCA phosphorylating AMPKα at threonine 172 and serine 498 of HDAC5 induced nuclear export of phosphorylated HDAC5. Thus, metformin inhibited the upregulation of vascular cell adhesion molecule-1 (VCAM1) adhesion molecules induced by LPS and TNF-α and downregulation of Krupp-like factor 2 (KLF2), thus improving the endotoxemia-induced endothelial pro-inflammatory response. However, after AMPK knockdown, the inhibitory effect of metformin on inflammation was abolished.
PGC-1α activation
PGC-1α is a class of nuclear co-activators and its expression is regulated by various factors. It interacts with multiple transcription factors to regulate the transcription efficiency of target genes and participates in activities including multiple mitochondrial metabolic pathways, immunity, and inflammation. Metformin activates the AMPK-PGC-1α pathway [37, 38]. Increasing or restoring PGC-1α inhibits inflammatory cytokines [37, 39,40,41]. Hang et al. [37] found that pAMPK/AMPK ratios varied in different regions of the mouse brain, being significantly higher in the ventral midbrain regions than in the cortex and other regions. They also found that levels of PGC-1α, a downstream target of AMPK, were well correlated with AMPK activity levels in different brain regions. In their study, PGC-1α significantly reduced the normally higher ventral midbrain pAMPK/AMPK ratio caused by parkin defects in the Parkinson’s disease (PD)-related gene, and Paris, a negative regulator of PGC-1α, was upregulated. Metformin treatment significantly restored the pAMPK/AMPK ratio and PGC-1α levels in the ventral brain of deficient mice.
Metformin also restores the nuclear translocation level of PGC-1α [42]. Immunofluorescence and histochemical analyses have demonstrated that PGC-1α expression levels in microglia in the brains of humans and mice that have suffered an ischemic stroke are altered in a time-dependent manner and peak on the first day. To further determine the role of PGC-1α in microglia, investigators have performed transient middle cerebral artery occlusion (tMCAO) surgery after inducing PGC-1α overexpression in PGC-1α mice (mPGC-1α) and PGC-1αf/f mice (littermate control mice carrying the PGC-1α allele) and found that PGC-1α mediates neuroprotection after stroke. Quantification of 40 cytokines/chemokines in microglia using protein-array analysis and verified by FACS analysis revealed that the levels of many pro-inflammatory cytokines such as IL-1, CCL5, IL-6, IL-17, and TNF-α, were significantly decreased and IL-1 maturation and release were mainly regulated by NLRP3, consistent with the suppression of NLRP3 activation in mPGC-1αmice. ChIP-Seq analysis and plasmid transfection experiments have confirmed that PGC-1αand ERR jointly regulate ULK1 expression in microglia and reduce the expression of pro-inflammatory factors, thus inhibiting neuroinflammation [39].
Activation of the tumor suppressor gene p53
p53 is a tumor suppressor gene that normally monitors or slows cell division to keep it in the normal range. When it undergoes a mutation resulting in an altered spatial conformation, it loses its regulatory effect and transforms into an oncogene. Recent studies have shown that p53 also plays a role in inflammation [43,44,45,46,47]. Metformin activates AMPK acting at p53 serine 15 and serine 20, enhancing p53 activity and having an anti-inflammatory effect [43]. Treatment of SKM-1 cells with metformin results in increased AMPK phosphorylation and p53 expression. Metformin-induced upregulation of p53 expression is attenuated after AMPK knockdown [48]. When comparing cytokine concentrations in streptomycin-induced diabetic mice without the p53 gene and in wild-type mice, IL-6, IL-11, IL-12, and other pro-inflammatory factors are significantly increased in p53 gene-deficient mice than in control mice. Knockout of the p53 gene in mice results in neutrophils and macrophages responding more frequently to LPS stimulation, showing stronger pro-inflammatory cytokine induction and NF-κB DNA binding activity [44]. The above studies showed that p53 expression is inversely associated with pro-inflammatory factors [44, 45] and the regulation by metformin is inseparable from AMPK. Furthermore, p53 inhibits the activity of three different NF-κB binding sites, slowing its mediated transcription; NF-κB-induced cytokine-encoding gene expression is enhanced upon p53 failure [47]. In addition, it inhibits the transcriptionally active NF-κB by competing for a complex of limited p300 and CREB-binding protein (CBP) coactivating proteins [46].
FoxO1 inhibition and FoxO3a activation
Both FoxO1 and FoxO3 are important members of the Forkhead box protein (FoxO) family. Their activity is regulated by various modification processes such as phosphorylation and acetylation and both participate in a variety of physiopathological processes. Recently, several studies have shown that FoxO plays an important role in inflammation and immune cells [49,50,51]. However, FoxO family expression is mainly regulated by the AMPK signaling pathway [52,53,54]. After knockdown of FoxO1, the expression levels of pro-inflammatory factors such as TNF-α, IL-1β, MIP2, and IFN-β significantly decreased [55] but significantly increased with FoxO1 overexpression, leading to macrophage activation and promotion of the inflammatory response [56, 57]. In terms of macrophage apoptosis, inhibiting FoxO1 activity promotes macrophage apoptosis and suppresses the inflammatory response [58]. FoxO1 activation promotes macrophage proliferation and the inflammatory response [59, 60]. FoxO3a directly enhances its own transcriptional activity by phosphorylating AMPK. Knockdown of FoxO3a results in significantly increased NF-κB activity and inflammatory factors [61, 62]. FoxO3a also reduces the pro-inflammatory cytokine TNF-α and promotes the production of anti-inflammatory cytokines such as IL-10 through protein–DNA interactions directly regulating transforming growth factor 1 (TGF-β1) and indirectly controlling TGF-β1 [63].
Transcription factor 3 (ATF-3) activation
ATF-3 has a leucine zipper structure and belongs to the ATF/CREB family of transcription factors. It is a key regulator in the stress response and involved in various physiopathological processes. It negatively regulates the expression of pro-inflammatory genes [66, 67]. Expression of pro-inflammatory factors IL-6 and IL-8 is significantly upregulated in human bronchial epithelioid cells when lacking ATF-3, and nuclear expression of NF-κB p65 and p-p-p65 protein increase the degradation of p65 repressor IkBa and increase the levels of p-IkBa protein, indicating that ATF-3 plays a role in regulating p65 phosphorylation status [66]. Furthermore, ATF-3 directly binds to NF-κB p65 and inhibits the expression of inflammatory response cytokines induced by the NF-κB signaling pathway [64], or directly binds to the promoter regions of IL-6 and IL-12b to regulate the inflammatory response [67].
In LPS-induced inflammation in mouse macrophages, metformin increases ATF-3 expression and suppresses pro-inflammatory factors in a dose-dependent manner. After treatment with metformin, LPS-induced enrichment of NF-κB at the IL-6 and TNF-α promoter is replaced by ATF-3. Furthermore, ATF-3 competes with NF-κB for the promoter binding of TNF-α and IL-6 to inhibit NF-κB signaling. Knockdown of ATF-3 results in the inhibition of the phosphorylation of pro-inflammatory cytokines and abolishes MAPK activity. Phosphorylation of threonine 172 is very important for AMPK activity; however, after knockdown of AMPK, the effect of metformin on ATF-3 and pro-inflammatory factors is attenuated. In one study [65], leptin-deficient mice were used to model obesity and type 2 diabetes. The mice were treated with oral metformin for 3 weeks and the levels of pro-inflammatory cytokines and ATF-3 in plasma and tissues were analyzed. Significantly increased AMPK and significantly decreased plasma pro-inflammatory cytokine levels were observed in treated mice compared to control mice. The above studies confirm that metformin has anti-inflammatory effects, at least in part, through AMPK-ATF-3-dependent mechanisms.
NLRP3 inflammasome inhibition
The NLRP3 inflammasome contains three domains: NYD, NACHT, and LRR. The inflammasome has a high-molecular-weight multiprotein cytosolic assembly composed of receptors and sensors, an important component of innate immunity, and is closely associated with many diseases such as rheumatoid arthritis [68]. When stimulated by agonists, the NLRP3 inflammasome can activate caspase-1, thus promoting the maturation of cellular interleukins and other cytokines. Metformin affects the NLRP3 inflammasome mainly through the AMPK-dependent and AMPK-independent pathways. Yang et al. [69] found that metformin acts on the NLRP3 inflammasome through the AMPK/mTOR pathway and inhibits the recruitment and activation of the pro-inflammatory protein caspase-1 by NLRP3 inflammasome, thus preventing the conversion of IL-1 and IL-18 precursors into mature cytokines. Furthermore, AMPK can activate autophagy-negative regulation to inhibit the NLRP3 inflammasome [70]. In addition, the inhibition of the NLRP3 inflammasome can be independent of the metformin-activated AMPK pathway, reduce DNA polymerase (DNA POL) activity by reducing the ATP content and inhibiting mitochondrial DNA (mt-DNA) synthesis, reduce the cytosol of oxidized mt-DNA (Ox-mtDNA), and inhibit NLRP3 inflammasome activation as well as caspase-1 and IL-1β cleavage and release [71].
Mammalian target of rapamycin signaling pathway inhibition
Mammalian target of rapamycin (mTOR) is a class of filament/threonine kinase and has an important role in eukaryotic cell signaling. Cytokine expression in T cells is affected by mTOR stability. mTOR participates in immune suppression, affecting transcription and protein synthesis, and is an important regulator of cell growth, proliferation, and the immune response, mainly acting through the formation of the mTORC1 and mTORC2 complexes. Metformin has been widely recognized as an anti-inflammatory agent that acts through the AMPK-mTOR pathway [69, 72,73,74]. It directly modulates mTOR [75, 76] independently of the AMPK pathway, but may be dependent on the ability of Rag GTPases to induce the transfer of mTORC1 to the intracellular lumen occupied by Rheb. Therefore, direct inhibition of mTORC1 signaling inhibits inflammation [75].
High-mobility group box 1 protein inhibition
High-mobility group box 1 (HMGB1) contains three major functional domains: an A-box domain at the N-terminal, a B-box domain with cytokine activity at the middle, and the C-terminal, an acidic tail domain composed of 30 acidic amino acids [77]. Horiuchi et al. [78] studied metformin in vitro and in vivo using a compound containing a metformin-like structure for affinity chromatography; mass spectrometry showed that the HMGB1 protein bound to metformin. Furthermore, full-length HMGB1, three functional domains, A-free junction and acid-free HMGB1 recombinant protein were obtained for pulldown experiments. The HMGB1 mutants containing an acidic tail structure clearly bound to the compound whereas mutants lacking the acidic tail did not. The association between recombination of full-length HMGB1 and the compound was concentration-dependently inhibited only in the presence of the acidic tail mutants. The results confirm that metformin binds directly to the acidic tail of the C terminal end of HMGB1, thereby inhibiting p38 phosphorylation and the cytokine-like activity of HMGB1.
The high-mobility family protein B1, a multifunctional protein jointly involved with DNA in the regulation of gene expression, is also an alarm protein for cellular inflammation that induces the inflammatory response through its cytokine-like activity [78,79,80,81]. In one study [82], the development of neuroinflammatory responses was monitored using magnetic resonance imaging (MRI) and immunohistochemistry after injecting a single redox isoform of HMGB1 directly into the cerebral cortex. The results indicated that disulfide HMGB1 (ds-HMGB1) and complete reduction of HMGB1 (fr-HMGB1) acted as pro-inflammatory mediators that promoted blood–brain barrier disruption and a local inflammatory response. Targeting HMGB1 can alleviate a variety of disease injuries [78, 83,84,85] such as sepsis-induced acute liver, kidney, and lung injuries. The use of anti-HMGB1 monoclonal antibodies inhibits the activation of parkinsonian microglia and the expression of inflammatory cytokines such as IL-1 and IL-6 [80], alters murine sepsis models and early inflammatory factor profiles, improves the sepsis survival rate [86], and blocks the activation of NF-κB, p38, and Erk1/2 [81]. HMGB1 is associated with toll-like receptor (TLR) [79, 87,88,89], late glycosylation end product receptor (RAGE) [90], C-X-X motif chemokine receptor 4 (CXCR4), and N-methyl-d-aspartate receptor (NMDAR), which play important roles in inflammation [91]. HMGB1 also binds to the complement system C1q to activate the classical pathway in an antibody-independent manner, exacerbating the sterile inflammatory environment [92]. Furthermore, metformin inhibits HMGB1 mRNA expression and nuclear translocation to hinder the associated inflammatory response [93, 94]. In rabbit AF stem cells, LPS-induced HMGB1 release from the nucleus to the cytoplasm causes increased release of inflammatory cytokines, and treatment with metformin inhibits HMGB1 nuclear translocation and the expression of inflammatory factors TNF-α, IL-6, IL-1, and I L-1β [93].
Oxidative stress pathway inhibition
Oxidative stress and inflammation are interrelated processes; oxidative stress is an imbalance between oxidation and antioxidant effects in the body. When the antioxidant system cannot adequately act on reactive oxygen clusters, a large number of oxidation intermediates emerge, such as leukotriene (LT), thromboxane A2 (TXA2), and other pro-inflammatory mediators. Reactive oxygen clusters degrade the inhibitory subunit IκBα of NF-κB leading to increased release of NF-κB to promote inflammation [94,95,96]. Metformin has anti-inflammatory effects through antioxidants [20, 79, 97]. Alhaider et al. [79] found that metformin restores the mRNA levels of antioxidant genes such as GST, NQO1, and CAT in a streptomycin-induced rat diabetic nephropathy model, thus inhibiting the expression of TNF-α and IL-6 pro-inflammatory genes. Metformin inhibits the aldosterone-induced oxidative stress response, silences the cytoplasmic adaptor molecule TRAF31 interacting protein 2 (TRAF3IP2) in the oxidative stress response, and inhibits the expression of pro-inflammatory factors such as IL-6, IL-17, and IL-18 [20]. Metformin reduces the production of reactive oxygen species and pro-inflammatory factors caused by multiple pathways,such as hyperglycemia-triggered mitochondrial dysfunction, generation of advanced glycation end-products(AGEs) and the activation of protein kinases (PKC), improves endothelial dysfunction and cardiac function, and slows down diabetes-related cardiovascular events[4, 5].
Conclusion
Metformin has anti-inflammatory effects through multiple routes, which provides potential therapeutic targets for certain inflammatory diseases, such as neuroinflammation and rheumatoid arthritis. In addition, inflammation is a key component of tumor occurrence and development [98,99,100,101,102]; thus, targeted inflammatory intervention is a significant benefit for both cancer prevention and treatment. Therefore, metformin may have further potential for inflammation-related disease prevention and treatmen. However, the inflammatory mechanism is complex; various molecules are connected and influence each other. For example, metformin significantly inhibits p65 nuclear translocation, but pretreatment with compound C, an AMPK inhibitor, abolishes this effect, and silencing of HMGB1 inhibits NF-κB activation [77, 85]. SIRT1 deacetylates FoxO, increasing its transcriptional activity [103]. mTOR in dendritic cells regulates FoxO1 via AKT [104]. The interactions among various molecules should be further explored to clarify their specific mechanisms and provide more direction for the treatment of inflammatory diseases, as well as cancer.
References
(1998) Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 352(9131): 854–865. (Erratum in: Lancet 1998 352(9139):1558)
Holman R, Paul S, Bethel M, Matthews D, Neil H (2008) 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 359:1577–1589. https://doi.org/10.1056/NEJMoa0806470
Evans JM, Donnelly LA, Emslie-Smith AM et al (2005) Metformin and reduced risk of cancer in diabetic patients. BMJ 330:1304–1305. https://doi.org/10.1136/bmj.38415.708634
Salvatore T, Pafundi PC, Galiero R, Rinaldi L, Caturano A, Vetrano E, Aprea C, Albanese G, Di Martino A, Ricozzi C, Imbriani S, Sasso FC (2020) Can metformin exert as an active drug on endothelial dysfunction in diabetic subjects?. Biomedicines 9(1):3. https://doi.org/10.3390/biomedicines9010003
Salvatore T, Galiero R, Caturano A, Vetrano E, Rinaldi L, Coviello F, Di Martino A, Albanese G, Marfella R, Sardu C, Sasso FC (2021) Effects of metformin in heart failure: from pathophysiological rationale to clinical evidence. Biomolecules 11(12):1834. https://doi.org/10.3390/biom11121834
Salvatore T, Pafundi PC, Morgillo F, Di Liello R, Galiero R, Nevola R, Marfella R, Monaco L, Rinaldi L, Adinolfi LE, Sasso FC (2020) Metformin: an old drug against old age and associated morbidities. Diabetes Res Clin Pract 160:108025. https://doi.org/10.1016/j.diabres.2020.108025
Yang YX, Hennessy S, Lewis JD (2004) Insulin therapy and colorectal cancer risk among type 2 diabetes mellitus patients. Gastroenterology 127:1044–1050. https://doi.org/10.1053/j.gastro.2004.07.01
Zhang ZJ, Zheng ZJ, Kan H, Song Y, Cui W, Zhao G et al (2011) Reduced risk of colorectal cancer with metformin therapy in patients with type 2 diabetes: a meta-analysis. Diabetes Care 34:2323–2328. https://doi.org/10.2337/dc11-0512
Ko EM, Walter P, Jackson A, Clark L, Franasiak J, Bolac C et al (2014) Metformin is associated with improved survival in endometrial cancer. Gynecol Oncol 132:438–442. https://doi.org/10.1016/j.ygyno.2013.11.021
Kasznicki J, Sliwinska A, Drzewoski J (2014) Metformin in cancer prevention and therapy. Ann Transl Med 2:57. https://doi.org/10.3978/j.issn.2305-5839.2014.06.01
Abhimanu P, Sneh V, Kumar VL (2017) Metformin maintains mucosal integrity in experimental model of colitis by inhibiting oxidative stress and pro-inflammatory signaling. Biomed Pharmacother 94:1121–1128. https://doi.org/10.1016/j.biopha.2017.08.020
Gharib M, Elbaz W, Darweesh E, Sabri NA, Shawki MA (2021) Efficacy and safety of metformin use in rheumatoid arthritis: a randomized controlled study. Front Pharmacol 12:726490. https://doi.org/10.3389/fphar.2021.726490
Salvatore T, Pafundi PC, Galiero R, Gjeloshi K, Masini F, Acierno C, Di Martino A, Albanese G, Alfano M, Rinaldi L, Sasso FC (2020) Metformin: a potential therapeutic tool for rheumatologists. Pharmaceuticals (Basel, Switzerland) 13(9):234. https://doi.org/10.3390/ph13090234
Han B, Jiang W, Cui P, Zheng K, Dang C, Wang J, Li H, Chen L, Zhang R, Wang QM, Ju Z, Hao J (2021) Microglial PGC-1α protects against ischemic brain injury by suppressing neuroinflammation. Genome Med 13(1):47. https://doi.org/10.1186/s13073-021-00863-5
Ernst PB, Gold BD (2000) The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu Rev Microbiol 54:615–640. https://doi.org/10.1146/annurev.micro.54.1.615
Hammad AM, Ibrahim YA, Khdair SI, Hall FS, Alfaraj M, Jarrar Y, Abed AF (2021) Metformin reduces oxandrolone- induced depression-like behavior in rats via modulating the expression of IL-1β, IL-6, IL-10 and TNF-α. Behav Brain Res 414:113475. https://doi.org/10.1016/j.bbr.2021.113475
Koh SJ, Kim JM, Kim IK, Ko SH, Kim JS (2014) Anti-inflammatory mechanism of metformin and its effects in intestinal inflammation and colitis-associated colon cancer. J Gastroenterol Hepatol 29(3):502–510. https://doi.org/10.1111/jgh.12435
Docrat TF, Nagiah S, Chuturgoon AA (2021) Metformin protects against neuroinflammation through integrated mechanisms of miR-141 and the NF-ĸB-mediated inflammasome pathway in a diabetic mouse model. Eur J Pharmacol 903:174146. https://doi.org/10.1016/j.ejphar.2021.174146
Tsuchiya Y, Osaki K, Kanamoto M, Nakao Y, Takahashi E, Higuchi T, Kamata H (2017) Distinct B subunits of PP2A regulate the NF-κB signalling pathway through dephosphorylation of IKKβ. IκBα and RelA. FEBS Lett. 591(24):4083–4094
Mummidi S, Das NA, Carpenter AJ, Kandikattu H, Krenz M, Siebenlist U, Valente AJ, Chandrasekar B (2016) Metformin inhibits aldosterone-induced cardiac fibroblast activation, migration and proliferation in vitro, and reverses aldosterone + salt-induced cardiac fibrosis in vivo. J Mol Cell Cardiol 98:95–102. https://doi.org/10.1016/j.yjmcc.2016.07.006
Chung MM, Nicol CJ, Cheng YC, Lin KH, Chen YL, Pei D, Lin CH, Shih YN, Yen CH, Chen SJ, Huang RN, Chiang MC (2017) Metformin activation of AMPK suppresses AGE-induced inflammatory response in hNSCs. Exp Cell Res 352(1):75–83. https://doi.org/10.1016/j.yexcr.2017.01.017
Isoda K, Young JL, Zirlik A et al (2006) Metformin inhibits proinflammatory responses and nuclear factor-kappaB in human vascular wall cells. Arterioscler Thromb Vasc Biol 26:611–617. https://doi.org/10.1161/01.ATV.0000201938.78044.75
Arai M, Uchiba M, Komura H et al (2010) Metformin, an antidiabetic agent, suppresses the production of tumor necrosis factor and tissue factor by inhibiting early growth response factor-1 expression in human monocytes in vitro. J Pharmacol Exp Ther 334:206–213. https://doi.org/10.1124/jpet.109.164970
Tao L, Li D, Liu H, Jiang F, Xu Y, Cao Y, Gao R, Chen G (2018) Neuroprotective effects of metformin on traumatic brain injury in rats associated with NF-κB and MAPK signaling pathway. Brain Res Bull 140:154–161. https://doi.org/10.1016/j.brainresbull.2018.04.008
Ba W, Xu Y, Yin G, Yang J, Wang R, Chi S, Wang Y, Li C (2019) Metformin inhibits pro-inflammatory responses via targeting nuclear factor-κB in HaCaT cells. Cell Biochem Funct 37(1):4–10. https://doi.org/10.1002/cbf.3367
Zhou Z, Tang Y, Jin X, Chen C, Lu Y, Liu L, Shen C (2016) Metformin inhibits advanced glycation end products-induced inflammatory response in murine macrophages partly through ampk activation and RAGE/NFκB pathway suppression. J Diabetes Res 2016:4847812. https://doi.org/10.1155/2016/4847812
Cameron AR, Morrison VL, Levin D, Mohan M, Forteath C, Beall C, McNeilly AD, Balfour DJ, Savinko T, Wong AK, Viollet B, Sakamoto K, Fagerholm SC, Foretz M, Lang CC, Rena G (2016) Anti-inflammatory effects of metformin irrespective of diabetes status. Circ Res 119(5):652–665. https://doi.org/10.1161/CIRCRESAHA.116.308445
Ye J, Zhu N, Sun R, Liao W, Fan S, Shi F, Lin H, Jiang S, Ying Y (2018) Metformin inhibits chemokine expression through the AMPK/NF-κB signaling pathway. J Interferon Cytokine Res 38(9):363–369. https://doi.org/10.1089/jir.2018.0061
Zhou X, Chen J, Chen L, Feng X, Liu Z, Hu L, Zeng Z, Jia X, Liang M, Shi B, Yi G, Liu J (2017) Negative regulation of Sirtuin 1 by AMP-activated protein kinase promotes metformin-induced senescence in hepatocellular carcinoma xenografts. Cancer Lett 28(411):1–11. https://doi.org/10.1016/j.canlet.2017.09.027
Sun Z, Li J, Luo G, Liu W, He Y, Wang F, Qian Y, Fan C (2021) Pharmacological activation of SIRT1 by metformin prevented trauma-induced heterotopic ossification through inhibiting macrophage mediated inflammation. Eur J Pharmacol 909:174386. https://doi.org/10.1016/j.ejphar.2021.174386
Wang XD, Yu WL, Sun Y (2021) Activation of AMPK restored impaired autophagy and inhibited inflammation reaction by up-regulating SIRT1 in acute pancreatitis. Life Sci 277:119435. https://doi.org/10.1016/j.lfs.2021.119435
Arunachalam G, Lakshmanan AP, Samuel SM, Triggle CR, Ding H (2016) Molecular interplay between microRNA-34a and sirtuin1 in hyperglycemia-mediated impaired angiogenesis in endothelial cells: effects of metformin. J Pharmacol Exp Ther 356(2):314–323. https://doi.org/10.1124/jpet.115.226894
Takano K, Kakuki T, Kaneko Y, Kohno T, Kikuchi S, Himi T, Kojima T (2017) Histone deacetylase inhibition prevents cell death induced by loss of tricellular tight junction proteins in temperature-sensitive mouse cochlear cells. PLoS ONE 12(8):e0182291. https://doi.org/10.1371/journal.pone.0182291
Yeung F, Hoberg et al (2004) Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 23(12):2369–2380. https://doi.org/10.1038/sj.emboj.7600244
Tian R, Li R, Liu Y et al (2019) Metformin ameliorates endotoxemia-induced endothelial pro-inflammatory responses via AMPK-dependent mediation of HDAC5 and KLF2. Biochim Biophys Acta 1865(6):1701–1712. https://doi.org/10.1016/j.bbadis.2019.04.009
Lan F, Cacicedo JM, Ruderman N, Ido Y (2008) SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem 283(41):27628–27635. https://doi.org/10.1074/jbc.M805711200
Hang L, Thundyil J, Goh GWY, Lim KL (2019) AMP kinase activation is selectively disrupted in the ventral midbrain of mice deficient in parkin or PINK1 expression. Neuromolecular Med 21(1):25–32
Li Q, Jia S, Xu L, Li B, Chen N (2019) Metformin-induced autophagy and irisin improves INS-1 cell function and survival in high-glucose environment via AMPK/SIRT1/PGC-1α signal pathway. Food Sci Nutr 7(5):1695–1703. https://doi.org/10.1002/fsn3.1006
Han B, Jiang W, Cui P, Zheng K, Dang C, Wang J, Li H, Chen L, Zhang R, Wang QM, Ju Z, Hao J (2021) Microglial PGC-1α protects against ischemic brain injury by suppressing neuroinflammation. Genome Med 13(1):47. https://doi.org/10.1186/s13073-021-00863-5
Fu X, Jiao J, Qin T, Yu J, Fu Q, Deng X, Ma S, Ma Z. (2021) A new perspective on ameliorating depression-like behaviors: Suppressing neuroinflammation by upregulating PGC-1α. Neurotox Res 39(3):872– 885. doi:10.1007/s12640–020–00292-z.
Yang X, Xu S, Qian Y, Xiao Q (2017) Resveratrol regulates microglia M1/M2 polarization via PGC-1α in conditions of neuroinflammatory injury. Brain Behav Immun 64:162–172. https://doi.org/10.1016/j.bbi.2017.03.003
Matsiukevich D, Piraino G, Lahni P, Hake PW, Wolfe V, O’Connor M, James J, Zingarelli B. Metformin ameliorates gender-and age-dependent hemodynamic instability and myocardial injury in murine hemorrhagic shock. Biochim Biophys Acta Mol Basis Dis. 2017 Oct;1863(10 Pt B):2680–2691
Maclaine NJ, Hupp TR (2009) The regulation of p53 by phosphorylation: a model for how distinct signals integrate into the p53 pathway. Aging (Albany NY) 1(5):490–502. https://doi.org/10.18632/aging.100047
Gudkov AV, Gurova KV, Komarova EA (2011) Inflammation and p53: a tale of two stresses. Genes Cancer 2(4):503–516. https://doi.org/10.1177/1947601911409747
Zheng SJ, Lamhamedi-Cherradi SE, Wang P, Xu L, Chen YH (2005) Tumor suppressor p53 inhibits autoimmune inflammation and macrophage function. Diabetes 54(5):1423–1428. https://doi.org/10.2337/diabetes.54.5.1423
Komarova EA, Krivokrysenko V, Wang K, Neznanov N, Chernov MV, Komarov PG, Brennan ML, Golovkina TV, Rokhlin OW, Kuprash DV, Nedospasov SA, Hazen SL, Feinstein E, Gudkov AV (2005) p53 is a suppressor of inflammatory response in mice. FASEB J 19(8):1030–1032. https://doi.org/10.1096/fj.04-3213fje
Webster GA, Perkins ND (1999) Transcriptional cross talk between NF-κB and p53. Mol Cell Biol 19(5):3485–3495. https://doi.org/10.1128/MCB.19.5.3485
Zhou X, Kuang Y, Liang S, Wang L (2019) Metformin inhibits cell proliferation in SKM-1 cells via AMPK-mediated cell cycle arrest. J Pharmacol Sci 141(4):146–152. https://doi.org/10.1016/j.jphs.2019.10.003
Harada Y, Harada Y, Elly C, Ying G, Paik JH, DePinho RA, Liu YC (2010) Transcription factors Foxo3a and Foxo1 couple the E3 ligase Cb1-b to the induction of Foxp3 expression in induced regulatory T cells. J Exp Med 207(7):1381–1391. https://doi.org/10.1128/MCB.19.5.3485
Kerdiles YM, Stone EL, Beisner DR, McGargill MA, Ch’en IL, Stockmann C, Katayama CD, Hedrick SM (2010) Foxo transcription factors control regulatory T cell development and function. Immunity 33(6):890–904. https://doi.org/10.1016/j.immuni.2010.12.002
Han C, Guo L, Sheng Y et al (2020) FoxO1 regulates TLR4/MyD88/MD2-NF-κB inflammatory signalling in mucosal barrier injury of inflammatory bowel disease. J Cell Mol Med 24:3712–3723. https://doi.org/10.1111/jcmm.15075
Sanchez AM, Candau RB, Bernardi H (2014) FoxO transcription factors: Their roles in the maintenance of skeletal muscle homeostasis. Cell Mol Life Sci 71(9):1657–1671. https://doi.org/10.1007/s00018-013-1513-z
Barthel A, Schmoll D, Krüger KD, Roth RA, Joost HG (2002) Regulation of the forkhead transcription factor FKHR (FOXO1a) by glucose starvation and AICAR, an activator of AMP-activated protein kinase. Endocrinology 143:3183–3186. https://doi.org/10.1210/endo
Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, Brunet A (2007) The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem 282(41):30107–30119. https://doi.org/10.1074/jbc.M705325200
Zhou M, Zhang Y, Chen X et al (2015) PTEN-Foxo1 signaling triggers HMGB1-mediated innate immune responses in acute lung injury. Immunol Res 62(1):95–105. https://doi.org/10.1007/s12026-015-8639-z
Miao H, Ou J, Zhang X et al (2015) Macrophage CGI-58 deficiency promotes IL-1beta transcription by activating the SOCS3-FOXO1 pathway. Clin Sci (Lond) 128(8):493–506. https://doi.org/10.1042/CS20140414
Chung S, Ranjan R, Lee YG et al (2015) Distinct role of FoxO1 in M-CSF- and GM-CSF-differentiated macrophages contributes LPS-mediated IL-10: implication in hyperglycemia. J Leukoc Biol 97(2):327–339. https://doi.org/10.1189/jlb.3A0514-251R
Russe OQ, Moser CV, Kynast KL et al (2014) LPS inhibits caspase 3-dependent apoptosis in RAW264 7 macrophages induced by the AMPK activator AICAR. Biochem Biophys Res Commun 447(3):520–525. https://doi.org/10.1016/j.bbrc.2014.04.008
Garg NK, Tyagi RK, Singh B et al (2016) Nanostructured lipid carrier mediates effective delivery of methotrexate to induce apoptosis of rheumatoid arthritis via NF-kappaB and FOXO1. Int J Pharm 499(1–2):301–320. https://doi.org/10.1016/j.ijpharm.2015.12.061
Liu Y, Jiang J, Wang X et al (2013) miR-582–5p is upregulated in patients with active tuberculosis and inhibits apoptosis of monocytes by targeting FOXO1. PLoS ONE 8(10):e78381. https://doi.org/10.1371/journal.pone
Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, Brunet A (2007) The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem 282(41):30107–30119. https://doi.org/10.1074/jbc.M705325200
Lin L, Hron JD, Peng SL (2004) Regulation of NF-kappaB, Th activation, and autoinflammation by the forkhead transcription factor Foxo3a. Immunity 21(2):203–213. https://doi.org/10.1016/j.immuni.2004.06.016
Lee JC, Espéli M, Anderson CA, Linterman MA, Pocock JM, Williams NJ, Roberts R, Viatte S, Fu B, Peshu N, Hien TT, Phu NH, Wesley E, Edwards C, Ahmad T, Mansfield JC, Gearry R, Dunstan S, Williams TN, Barton A, Vinuesa CG; UK IBD Genetics Consortium, Parkes M, Lyons PA, Smith KG (2013) Human SNP links differential outcomes in inflammatory and infectious disease to a FOXO3-regulated pathway. Cell 155(1):57–69. https://doi.org/10.1016/j.cell.2013.08.034
Kwon JW, Kwon HK, Shin HJ, Choi YM, Anwar MA, Choi S (2015) Activating transcription factor 3 represses inflammatory responses by binding to the p65 subunit of NF-κB. Sci Rep 28(5):14470. https://doi.org/10.1038/srep14470
Kim J, Kwak HJ, Cha JY, Jeong YS, Rhee SD, Kim KR, Cheon HG (2014) Metformin suppresses lipopolysaccharide (LPS)-induced inflammatory response in murine macrophages via activating transcription factor-3 (ATF-3) induction. J Biol Chem 289(33):23246–23255. https://doi.org/10.1074/jbc.M114.577908
Wu YP, Cao C, Wu YF et al (2017) Activating transcription factor 3 represses cigarette smoke-induced IL6 and IL8 expression via suppressing NF-κB activation. Toxicol Lett 270:17–24. https://doi.org/10.1016/j.toxlet.2017.02.002
Gilchrist M, Thorsson V, Li B et al (2006) Systems biology approaches identify ATF3 as a negative regulator of Toll-like receptor 4. Nature 441:173–178. https://doi.org/10.1038/nature04768
Mangan MSJ, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E (2018) Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov 17:588–606. https://doi.org/10.1038/nrd.2018.97
Yang F, Qin Y, Wang Y, Meng S, Xian H, Che H, Lv J, Li Y, Yu Y, Bai Y, Wang L (2019) Metformin inhibits the NLRP3 inflammasome via AMPK/mTOR-dependent effects in diabetic cardiomyopathy. Int J Biol Sci 15(5):1010–1019. https://doi.org/10.7150/ijbs.29680
Zhong Z, Sanchez-Lopez E, Karin M (2016) Autophagy, NLRP3 inflammasome and auto-inflammatory/immune diseases. Clin Exp Rheumatol 34(4 Suppl 98):12–16
Xian H, Liu Y, Rundberg Nilsson A, Gatchalian R, Crother TR, Tourtellotte WG, Zhang Y, Aleman-Muench GR, Lewis G, Chen W, Kang S, Luevanos M, Trudler D, Lipton SA, Soroosh P, Teijaro J, de la Torre JC, Arditi M, Karin M, Sanchez-Lopez E (2021) Metformin inhibition of mitochondrial ATP and DNA synthesis abrogates NLRP3 inflammasome activation and pulmonary inflammation. Immunity 54(7):1463–1477. https://doi.org/10.1016/j.immuni.2021.05.004
Wu K, Rui T, Jing H et al (2018) Metformin alleviated endotoxemia-induced acute lung injury via restoring AMPK-dependent suppression of mTOR. Chem Biol Interact 291:1–6. https://doi.org/10.1016/j.cbi.2018.05.018
Tzatsos A, Kandror KV (2006) Nutrients suppress phosphatidylinositol 3-kinase/Akt signaling via raptor-dependent mTOR-mediated insulin receptor substrate 1 phosphorylation. Mol Cell Biol 26(1):63–76. https://doi.org/10.1128/MCB.26.1.63-76.2006
Gwinn DM, Shackelford DB, Egan DF et al (2008) AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30:214–226. https://doi.org/10.1016/j.molcel.2008.03.003
Kalender A, Selvaraj A, Kim SY, Gulati P, Brûlé S, Viollet B, Kemp BE, Bardeesy N, Dennis P, Schlager JJ, Marette A, Kozma SC, Thomas G (2010) Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab 11(5):390–401. https://doi.org/10.1016/j.cmet.2010.03.014
Brugarolas J, Lei K, Hurley RL, Manning BD, Reiling JH, Hafen E, Witters LA, Ellisen LW, Kaelin WG Jr (2004) Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev 18(23):2893–2904. https://doi.org/10.1101/gad.1256804
Huang W, Tang Y, Li L (2010) HMGB1, a potent proinflammatory cytokine in sepsis. Cytokine 51(2):119–126. https://doi.org/10.1016/j.cyto.2010.02.021
Horiuchi T, Sakata N, Narumi Y, Kimura T, Hayashi T, Nagano K, Liu K, Nishibori M, Tsukita S, Yamada T, Katagiri H, Shirakawa R, Horiuchi H (2017) Metformin directly binds the alarmin HMGB1 and inhibits its proinflammatory activity. J Biol Chem 292(20):8436–8446. https://doi.org/10.1074/jbc.M116.769380
Alhaider AA, Korashy HM, Sayed-Ahmed MM, Mobark M, Kfoury H, Mansour MA (2011) Metformin attenuates streptozotocin-induced diabetic nephropathy in rats through modulation of oxidative stress genes expression. Chem Biol Interact 192(3):233–242. https://doi.org/10.1016/j.cbi.2011.03.014
Sasaki T, Liu K, Agari T, Yasuhara T, Morimoto J, Okazaki M, Takeuchi H, Toyoshima A, Sasada S, Shinko A, Kondo A, Kameda M, Miyazaki I, Asanuma M, Borlongan CV, Nishibori M, Date I (2016) Anti-high mobility group box 1 antibody exerts neuroprotection in a rat model of Parkinson’s disease. Exp Neurol 275(Pt 1):220–231. https://doi.org/10.1016/j.expneurol.2015.11.003
Jiang Y, Chen R, Shao X, Ji X, Lu H, Zhou S, Zong G, Xu H, Su Z (2018) HMGB1 silencing in macrophages prevented their functional skewing and ameliorated EAM development: nuclear HMGB1 may be a checkpoint molecule of macrophage reprogramming. Int Immunopharmacol 56:277–284. https://doi.org/10.1016/j.intimp.2018.01.013
Aucott H, Lundberg J, Salo H, Klevenvall L, Damberg P, Ottosson L, Andersson U, Holmin S, Erlandsson HH (2018) Neuroinflammation in response to intracerebral injections of different HMGB1 redox isoforms. J Innate Immun 10(3):215–227. https://doi.org/10.1159/000487056
Chen L, Lu Q, Deng F, Peng S, Yuan J, Liu C, Du X (2020) miR-103a-3p could attenuate sepsis-induced liver injury by targeting HMGB1. Inflammation 43(6):2075–2086. https://doi.org/10.1007/s10753-020-01275-0
Xu L, Hu G, Xing P, Zhou M, Wang D (2020) Paclitaxel alleviates the sepsis-induced acute kidney injury via lnc-MALAT1/miR-370–3p/HMGB1 axis. Life Sci 262:118505. https://doi.org/10.1016/j.lfs.2020.118505 (Erratum in: Life Sci. 2021 May 1;272:119159)
Yang P, Xiong W, Chen X, Liu J, Ye Z (2020) Overexpression of miR-129-5p mitigates sepsis-induced acute lung injury by targeting high mobility group box 1. J Surg Res 256:23–30. https://doi.org/10.1016/j.jss.2020.05.101
Stevens NE, Chapman MJ, Fraser CK, Kuchel TR, Hayball JD, Diener KR (2017) Therapeutic targeting of HMGB1 during experimental sepsis modulates the inflammatory cytokine profile to one associated with improved clinical outcomes. Sci Rep 7(1):5850. https://doi.org/10.1038/s41598-017-06205-z
Yan B, Chen F, Xu L, Xing J, Wang X (2017) HMGB1-TLR4-IL23-IL17A axis promotes paraquat-induced acute lung injury by mediating neutrophil infiltration in mice. Sci Rep 7(1):597. https://doi.org/10.1038/s41598-017-00721-8
Liu QY, Wang YX, Wu ZS, Shi ZW, Wu X, Chen X, Yang Z, Xu KZ (2018) High mobility group protein 1 reverses immune system paralysis in late-phase sepsis. Infect Immun 86(9):e00455-18. https://doi.org/10.1128/IAI.00455-18
Shi Y, Zhang L, Teng J, Miao W (2018) HMGB1 mediates microglia activation via the TLR4/NF-κB pathway in coriaria lactone induced epilepsy. Mol Med Rep 17(4):5125–5131. https://doi.org/10.3892/mmr.2018.8485
Wang J, Li R, Peng Z, Hu B, Rao X, Li J (2020) HMGB1 participates in LPS-induced acute lung injury by activating the AIM2 inflammasome in macrophages and inducing polarization of M1 macrophages via TLR2, TLR4, and RAGE/NF-κB signaling pathways. Int J Mol Med 45(1):61–80. https://doi.org/10.3892/ijmm.2019.4402 (Erratum in: Int J Mol Med. 2020 May;45(5):1628)
Wan W, Cao L, Khanabdali R, Kalionis B, Tai X, Xia S (2016) The emerging role of HMGB1 in neuropathic pain: a potential therapeutic target for neuroinflammation. J Immunol Res 2016:6430423. https://doi.org/10.1155/2016/6430423
Kim SY, Son M, Lee SE, Park IH, Kwak MS, Han M, Lee HS, Kim ES, Kim JY, Lee JE, Choi JE, Diamond B, Shin JS (2018) High-mobility group box 1-induced complement activation causes sterile inflammation. Front Immunol 11(9):705. https://doi.org/10.3389/fimmu.2018.00705
Han Y, Yuan F, Deng C, He F, Zhang Y, Shen H, Chen Z, Qian L (2019) Metformin decreases LPS-induced inflammatory response in rabbit annulus fibrosus stem/progenitor cells by blocking HMGB1 release. Aging (Albany NY) 11(22):10252–10265. https://doi.org/10.18632/aging.102453
Huang WS, Lin CT, Chen CN, Chang SF, Chang HI, Lee KC (2018) Metformin increases the cytotoxicity of oxaliplatin in human DLD-1 colorectal cancer cells through down-regulating HMGB1 expression. J Cell Biochem 119(8):6943–6952. https://doi.org/10.1002/jcb.26898
Dröge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82(1):47–95. https://doi.org/10.1152/physrev.00018.2001
Schreck R, Rieber P, Baeuerle PA (1991) Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J 10(8):2247–2258. https://doi.org/10.1002/j.1460-2075.1991
Abd-Elsameea AA, Moustaf AA, Mohamed AM (2014) Modulation of the oxidative stress by metformin in the cerebrum of rats exposed to global cerebral ischemia and ischemia/reperfusion. Eur Rev Med Pharmacol Sci 18(16):2387–2392
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420(6917):860–867. https://doi.org/10.1038/nature01322
Diakos CI, Charles KA, McMillan DC, Clarke SJ (2014) Cancer-related inflammation and treatment effectiveness. Lancet Oncol 15(11):e493-503. https://doi.org/10.1016/S1470-2045(14)70263-3
Kay J, Thadhani E, Samson L, Engelward B (2019) Inflammation-induced DNA damage, mutations and cancer. DNA Repair (Amst). 83:102673. https://doi.org/10.1016/j.dnarep.2019.102673
Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454(7203):436–444. https://doi.org/10.1038/nature07205
Zhong Z, Sanchez-Lopez E, Karin M (2016) Autophagy, inflammation, and immunity: a troika governing cancer and its treatment. Cell 166(2):288–298. https://doi.org/10.1016/j.cell.2016.05.051
Kobayashi Y, Furukawa-Hibi Y, Chen C, Horio Y, Isobe K, Ikeda K, Motoyama N (2005) SIRT1 is critical regulator of FOXO-mediated transcription in response to oxidative stress. Int J Mol Med 16(2):237–243
Brown J, Wang H, Suttles J, Graves DT, Martin M (2011) Mammalian target of rapamycin complex 2 (mTORC2) negatively regulates Toll-like receptor 4-mediated inflammatory response via FoxO1. J Biol Chem 286(52):44295–44305. https://doi.org/10.1074/jbc
Funding
This work is supported by Chongqing medical scientific research project (Grant No. 2021MSXM042), General program of Chongqing Natural Science Foundation (Grant No. cstc2020jcyj-msxmX0713)
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Research involving human and/or animal participants
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Feng, Y.Y., Wang, Z. & Pang, H. Role of metformin in inflammation. Mol Biol Rep 50, 789–798 (2023). https://doi.org/10.1007/s11033-022-07954-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07954-5