Abstract
To clarify the antioxidant, anti-glycation and immunomodulatory capacities of fermented blue-green algae Aphanizomenon flos-aquae (AFA), hot aqueous extract suspensions made from 10% AFA were fermented by Lactobacillus plantarum AN7 and Lactococcus lactis subsp. lactis Kushiro-L2 strains isolated from a coastal region of Japan. The DPPH and O2− radical scavenging capacities and Fe-reducing power were increased in the fermented AFA. The increased DPPH radical scavenging capacity of the fermented AFA was fractionated to mainly < 3 kDa and 30–100 kDa. The increased O2− radical scavenging capacities were fractionated to mainly < 3 kDa. Anti-glycation activity in BSA-fructose model rather than BSA-methylglyoxal model was increased by the fermentation. The increased anti-glycation activity was fractionated to mainly 30–100 kDa. The NO concentration in the murine macrophage RAW264.7 culture media was high with the fermented AFA. The increased immunomodulation capacity was also fractionated to mainly 30–100 kDa. These results suggest that the fermented AFA is a more useful material for health foods and supplements.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A large quantity of blue-green algae, the cyanobacterium Aphanizomenon flos-aquae (AFA) inhabits Upper Klamath Lake in south-central Oregon in the United States [1]. AFA is rich in polyunsaturated fatty acids, proteins, vitamins, and minerals [2]. Additionally, the hypocholesterolaemia and anti-diabetes properties of AFA have been reported [3]. Although AFA inhabiting other regions is regarded as undesirable microalgae due to its toxins (such as aphantoxins), AFA from Upper Klamath Lake does not have toxins and is used for human health food supplements [4].
Reactive oxygen species (ROS), such as superoxide anion radicals (O2−), hydrogen peroxide (H2O2), hydroxyl radicals, and singlet oxygen, are generated in the bodies of living organisms [5]. These oxygen species react with important cell components, such as DNA, proteins, lipids, and small cellular molecules, and induce a wide range of common diseases and age-related degenerative conditions [6, 7]. A correlation between ROS and age-related diseases, such as cardiovascular disease, inflammatory conditions, and neurodegenerative diseases, such as Alzheimer’s disease and cancer, has been reported [8, 9]. To prevent diseases and aging caused by ROS, the antioxidative capacities of various foods have been studied [10].
Glycation is a non-enzymatic reaction of reducing sugars with amino acids and/or proteins in processed food (Maillard reaction) and in vivo [11, 12]. Advanced glycation end products (AGE), such as carboxylmethyl lysine and carboxylethyl lysine are generated irreversibly after various intermediates such as glyoxal, methylglyoxal (MGO), and 3-deoxyglucosone [13]. AGEs are thought to induce diabetes and other diabetes- and age-related illnesses such as retinopathy, cataracts, arteriosclerosis, and renal dysfunction [14]. Due to the AGE synthesis contain oxidation process, already there are reports about food materials have both antioxidant and anti-glycation activities with some phenolic compounds [15].
Nitric oxide (NO) is an ROS that is an endogenously synthesised free radical and a member of the gaseous signalling molecules widely known as gasotransmitters [16]. NO directly modifies its intracellular targets due to its ability to passively permeate the cellular membrane, and it plays important roles in inflammation, life-style, neurological and age-related diseases as mentioned above [17, 18]. The anti-inflammatory effects of various plant foods and ethnic medicinal plants and their chemical compounds were evaluated by the inhibitory effect on NO secretion induced by Escherichia coli lipopolysaccharide (LPS) in murine RAW264.7 macrophages [18, 19]. Alternatively, stimulatory effects on NO production have been reported in other food materials, particularly in exopolysaccharide (EPS) containing probiotic lactic acid bacteria (LAB), polyunsaturated fatty acids, and oligo- and poly-saccharides in RAW264.7 cells [20,21,22].
The antioxidant properties of AFA have been reported [23,24,25]. The immunomodulation capacities of AFA also have been reported; though, the mechanisms are still not clear [26, 27]. Some LABs isolated from coastal regions can increase the antioxidant (\({\text{O}}_{2}^{ - }\)radical scavenging, and protection of macrophages and epithelial-like cells against H2O2), anti-inflammation, and anti-glycation capacities of suspensions and/or aqueous extract solutions of some edible algae during fermentation [28,29,30,31].
In the present study, we sought to clarify the effect of LAB fermentation on the antioxidant, anti-glycation and immunomodulatory capacities of AFA. Hot aqueous extract suspensions made from dry-powdered AFA were fermented by Lactobacillus plantarum and Lactococcus lactis subsp. lactis strains isolated from a coastal region in Japan. These LAB strains showed higher stress (acid, bile and salinity) resistances compared with ones of their type strain [19, 32]. Then, the in vitro antioxidant and anti-glycation properties and immunomodulation effects on NO secretion from RAW264.7 cells of the non-fermented and fermented AFA suspensions were determined.
Materials and methods
Chemicals
( + )-Catechin, Folin–Ciocalteu phenol reagent, the stable 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical phenazine methosulphate (PMS), 3-(2-pyridyl)-5,6-di(p-sulfophenyl)1,2,4-triazine disodium salt (ferrozine), β-nicotinamide adenine dinucleotide (NADH), nitroblue tetrazolium salt (NBT), methylglyoxal (MGO), and lipopolysaccharide (LPS) from Escherichia coli O111 B4 were purchased from Sigma-Aldrich (St. Louis, MO). Potassium ferricyanide, trichloroacetic acid (TCA), bovine serum albumin (BSA), D-fructose (Fru) and Griess-Romijn nitrite reagent were purchased from FUJIFILM Wako Pure Chemicals (Osaka, Japan).
Freeze-dried powder of Aphanizomenon flos-aquae (AFA) harvested from Upper Klamath Lake was obtained from Dr’s Choice Co., Tokyo, Japan. The other reagents were of analytical grade.
Screening of AFA fermentative lactic acid bacteria
AFA (5 g) was suspended with 200 mL of distilled water (DW) and autoclaved (121 °C for 15 min, 2.5% AFA-S). Twenty-nine Lactobacillus plantarum and 10 Lactococcus lactis subsp. lactis isolated from coastal regions [19, 28, 31, 33] were pre-incubated in 3 mL of de Man, Rogosa, and Sharpe (MRS) broth (Oxoid, Basingstoke, UK) and incubated at 37 °C for 24 h. Then, 0.03 mL of the pre-culture was inoculated into 3 mL of the 2.5% AFA-S. After 4 days fermentation at 37 °C, the pH value was measured using a pH meter (LAQUA Twin B-711, Horiba, Kyoto, Japan). Strains of L. plantarum and L. lactis that showed the lowest pH values were selected as starters for further experiments.
Fermentation of 10% AFA-S
AFA powder (20 g) was suspended with 200 mL of DW, the pH was adjusted to 7.0 with 0.1 mol/mL NaOH, and the suspension was autoclaved (10% AFA-S). 5 mL of 10% AFA-S (5 mL) was fermented by inoculation of the pre-culture (0.05 mL) of the two selected LAB strains. After 0, 1, 2, 4, and 7 days fermentation, the pH values and viable counts with MRS agar plates were determined.
To analyse glucose, lactic acid, and acetic acid in the 7 day fermented 10% AFA-S by high-performance liquid chromatography (HPLC), the sample was centrifuged at 3000 × g at 4 °C for 5 min and the supernatant was filtered using a 0.2 µm pore filter. The HPLC conditions were as follows: column, ICSep ICE-ORH-801 (Tokyo Chemical Industry Co., Ltd., Tokyo, Japan); operating temperature, 35 °C; elution, 0.005 mol/L of sulfuric acid (H2SO4); flow rate, 0.8 mL/min. Eluted compounds were detected by a refractive index (RI) detector.
Among the major minerals (Na, K, Ca, Mg, Fe, Cu, and Mn), K, Ca, Fe, Cu, and Mn were measured using commercially available kits from the series of Reagent Set for Water Analyzer (No. 36, No. 48, No. 41B, No. 50, and No. 28, respectively, Kyoritsu Chemical-Check Lab., Corp., Tokyo, Japan). Na was measured using a Na-ion meter (LAQUA Twin B-721, Horiba, Kyoto, Tokyo). Mg was determined using a commercial diagnosis kit (Magnesium B-Test Wako, FUJIFILM Wako Pure Chemical).
After diluting the sample solution with nine volumes of distilled water, the free amino acid content was determined by an amino acid analyser (L-8900, Hitachi, Tokyo, Japan), as previously reported [34]. This high-performance liquid chromatography (HPLC) analysis method can also detect some amino-acid-related compounds, such as ammonia, ethanolamine, and aminobutyric acids.
Total phenolic compound content (TPC) of fermented 10% AFA-S
For experiments to determine phenolic content and antioxidant properties, intact and fermented 10% AFA-S was centrifuged at 3000 × g at 4 °C for 10 min. The experiments to determine TPC, antioxidant properties, and anti-glycation properties were conducted in triplicate.
Then, 0.03 mL of a diluted supernatant and 0.06 mL of 10% Folin–Ciocalteu solution were placed in a 96-well microplate. After 3 min, 0.12 mL of 10% sodium carbonate was added. The mixture stood for 60 min at ambient temperature and the absorbance was measured at 750 nm using a grating microplate reader (SH-1000 Lab; Corona Electric, Hitachinaka; Ibaraki, Japan). The phenolic content is expressed as catechin equivalents (CatEq)/g dry sample.
DPPH radical-scavenging capacity
Sample diluted solution (0.1 mL) and ethanol (0.1 mL) were put into a 96-well microplate and absorbance at 517 nm (Abs1) was measured using the microplate reader. Next, 1 mmol/L DPPH radical was added and incubated at 37 °C for 30 min and the absorbance (Abs2) was measured again. The DPPH radical-scavenging capacity was calculated using the following formula:
\({\text{O}}_{2}^{ - }\)radical-scavenging capacity
The sample solution (0.1 mL) was treated with 0.05 mL of 250 mmol/L phosphate buffer (pH 7.2), 2 mmol/L NADH (0.025 mL), and 0.5 mmol/L NBT (0.025 mL), while the absorbance at 560 nm was measured as a blank value. After 5 min incubation at room temperature (20–24 °C) with 0.025 mL of 0.03 mmol/L PMS, the absorbance was measured again. The radical-scavenging capacity was calculated using the above formula.
Ferric-reducing power
For each 0.05 mL of the sample solution, 0.025 mL of 0.1 mol/L phosphate buffer (pH 7.2) and 0.025 mL of 10 g/L potassium ferricyanide were placed in a 96-well microplate. After incubation at 37 °C for 60 min, 0.025 mL of 10% TCA and 0.1 mL of distilled water were added, and the absorbance was measured at 700 nm (Abs1). Next, 0.025 mL of 0.1% FeCl3 was added to the mixture and the absorbance was measured again (Abs2). The ferrous-reducing power was calculated using the following formula:
Anti-glycation property
The anti-glycation assays in the BSA-Fru and BSA-MGO models were determined using the method of [35] with slight modification [28]. BSA-Fru and BSA-MGO models evaluate all stages and the middle stage, respectively, of protein glycation. 1.5 mol/L Fru or 60 mmol/L (0.5 mL) was mixed with 0.5 mL of AES and 0.5 mL of sodium phosphate buffer (50 mmol/L, pH 7.4, with 0.02% sodium azide) in screw-capped test tubes and kept at 37 °C for 2 h. BSA (30 mg/mL, 0.5 mL) was added to each test tube, and the mixtures were incubated at 37 °C for 5 days. Fluorescent AGEs were monitored on a multiple microplate reader (SH-9000; Corona Electric) using 340 nm as the excitation wavelength and 420 nm (for BSA-Fru) or 380 nm (for BSA-MGO) as the emission wavelength. Percentage of the AGE inhibition was calculated by the following equation:
F0 and F5d represent fluorescent intensity after the reaction for 0 and 5 days, respectively.
Effects on nitric oxide (NO) secretion by RAW264.7 murine macrophages
The sample suspensions were heated in boiling water for 20 min. After cooling, the suspensions were 1/2 serial-diluted with Dulbecco’s modified Eagle’s medium (DMEM; Nissui Pharmaceutical, Tokyo, Japan) containing 5% v/v foetal bovine serum (FBS). To determine the immune stimulation and anti-inflammation properties, murine macrophage like RAW264.7 cells (TIB-71; American Type Culture Collection, Manassas, VA) were used [36].
RAW264.7 cells were suspended in the aforementioned medium (6 log cells/mL) and seeded into a 96-well microplate (0.1 mL/well). After incubation at 37 °C for 2 h in an atmosphere of 5% CO2, the medium was replaced with fresh medium (0.1 mL) and the sample containing medium (0.1 mL) was added. After a 20 h incubation, the nitric oxide (NO) concentration in the cultured medium was determined with 10% (w/v) Griess-Romijn nitrite reagent as described previously (Shikano et al., 2018). To confirm the cell toxicity, after the incubation, the viability of the RAW264.7 cells with and without the samples were determined with a commercial kit containing a reducing indicator: WST-8 (Cell Counting Kit-8, Dojindo Laboratories, Mashiki, Japan), according to the manual.
For the anti-inflammation assay, LPS solution (4 µg/mL) was added 2 h after the addition of the sample containing medium (0.01 mL/well for a final concentration of 0.19 µg/mL). The sample was incubated for 18 h, and the NO concentration was measured.
Measuring the TPC, antioxidant capacity, anti-glycation activity and induction capacity for the NO secretion of RAW264.7 cells of the ultrafiltered fractions of the intact and fermented 10% AFA-S
Portions (20 mL) of the supernatant of the intact and fermented 10% AFA-S were separated into six fractions (based on their molecular weights: > 300, 100–300, 30–100, 10–30, 3–10, and < 3 kDa), using an ultrafiltration system VIVASPIN 20 (Sartorius AG, Gottingen, Germany) [19]. The TPC, antioxidant capacities, and NO secretion in the culture of RAW264.7 cells were measured using the methods described above.
Statistical analysis
Measured values (n = 3) are presented as mean ± standard error of the mean (SEM). One-way ANOVA was performed to assess differences among groups, and individual means were compared by Tukey’s post hoc test or Student’s t-test, using statistical software (Excel Statistic Ver. 6, Esumi, Tokyo, Japan). Significant differences were accepted at p < 0.05.
Results and discussion
Selected LAB strains
Among the 29 Lactobacillus plantarum stocked strains, L. plantarum AN7 (Accession No: LC384876, Fig. 1A) isolated from traditional fermented horse mackerel with rice made in Noto Peninsula, Ishikawa [32] showed the clearest pH lowering in 2.5% AFA-S, from 6.3 to 5.1. Among the 10 Lactococcus lactis subsp. lactis strains, L. lactis Kushiro-L2 (Accession No: LC333954, Fig. 1B) isolated from Shirarutoroko Lake, Hokkaido in the present study, showed the clearest pH lowering from 6.3 to 5.6. These two LAB strains were selected for further experiments.
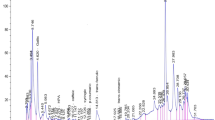
Images of Lactobacillus plantarum AN7 (A) and Lactococcus lactis subsp. lactis Kushiro-L2 (B) with a table top SEM (TM3030) and the cells in a 10% (w/v) Aphanizomenon flos-aquae suspension (AFA-S) observed under a phase-contrast microscope (C). D and F: Changes of the viable counts of the strains and pH, respectively, of the 10% AFA-S during 7 days fermentation at 37 °C. Values are mean and SEM (n = 3). F: HPLC chromatograph of 7 day fermented 10% AFA-S
Fermentation properties
After 7 days incubation of the selected LAB strains in 10% AFA-S, both LAB cells could be observed under a phase-contrast microscope (Fig. 1C). As shown in Fig. 1D, the inoculated L. plantarum AN7 increased immediately in 24 h and then decreased. Along with viable count, the pH value decreased in 24 h from 6.3 to 4.8, and then it was 5.1 at 7 days fermentation (Fig. 1E). In contrast, the inoculated L, lactis Kushiro-L2 increased slowly and the pH was lowered to 5.5.
By the HPLC analysis (Fig. 1F; Table 1), glucose and other some saccharides were detected in the intact 10% AFA-S and these were converted to mainly lactic acid, followed by acetic acid and a small amount of ethanol. These organic acid generations were high with L. plantarum AN7 rather than L. lactis Kushiro-L2. The concentration of the 10% AFA-S fermented with both LAB strains were similar with ones of L. plantarum AN7 fermented 10% AFA-S.
These results indicate that L. plantarum AN7 can typical lactic acid fermentation in 10% AFA-S, though the pH value was not so low. The prebiotic effect of blue extract and pure phycocyanin in AFA on Lactobacillus acidophilus has been reported [37]. However, it is possible that glucose in AFA might maintain the growth of various LAB, similarly to this study.
Mineral composition
Soluble minerals in 10% AFA-S are also summarized in Table 1. Even in intact AFA-S, the concentration of K (0.48 mg/mL), Cu (0.28 mg/mL), and Mn (0.22 mg/mL) were high. Specifically, the ratio of K to Na (K/Na) was 1.9. During the fermentation, the Na concentration was only 1.1 times increased. On the other hand, K and Ca were approximately 1.6 times increased by the fermentation and the K/Na ratio was increased to approximately 2.7. Ca was increased 2.4 and 1.9 times with L. plantarum AN7 and L. lactis Kushiro-L2, respectively.
The K/Na ratio is important for people who take diuretics to control hypertension and who suffer from excessive excretion of potassium [38, 39]. Minerals, Ca, Cu, and Mn are also important as constituents of bones, teeth, and soft tissues and are vital for overall mental and physical wellbeing [40,41,42]. From the K and Ca concentrations that might be liberated due to acidification with lactic acid and acetic acid, the fermented AFA-S rather than the non-fermented AFA-S is promising.
Free amino acids
AFA-S was rich in alanine (0.61 mg/mL), aspartic acid (0.54 mg/mL), and leucine (0.40 mg/mL), and these and total free amino acids tended to be high in the fermented AFA-S. Arginine was 0.16 and 0.18 mg/mL in intact 10% AFA-S and 10% AFA-S with L. plantarum AN7, respectively. It was not detected in 10% AFA-S fermented with L. lactis Kushiro-L2. It might be converted to ornithine.
The major free amino acids: alanine, aspartic acid, leucine, and glutamic acid, shown in this experiment are also major amino acids in various food materials. Besides taste, various food and medicinal functions of amino acids are known, e.g. liver protection, neural system improvement, and TCA cycle promoting compounds [43, 44]. Since the1980s, arginine-ornithine exchange pathways in Lactococcus lactis have been known [45]. Various functions of both arginine and ornithine, such as improvement of gut function and liver protection, are also known [46, 47]. Furthermore, the antioxidant properties of alanine have been reported [48].
Antioxidant properties of whole 10% AFA-S
TPC in the AFA was 8 µmol CatEq/g dry samples and was little increased by the fermentation (Fig. 2A). It is closed to concentrations of the phenol group having amino acids phenylalanine and tyrosine content (Table 1).
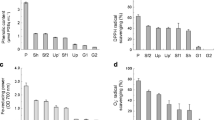
Total phenolic compound content (A), DPPH (B) and superoxide anion (C) radical scavenging capacities, and ferric reducing power (D) of 10% AFA-S fermented with L. plantarum AN7 and Lactococcus lactis Kushiro-L2. *Catechin equivalents/g dry sample. B-D: 63 µL AFA-S/mL. Values are mean and SEM (n = 3). a–c: Values with different letters are significantly different at p < 0.05
In the antioxidant tests, the concentration of the intact sample that had 40–50% of max reaction value was used to determine the effect of the fermentation. DPPH radical scavenging capacity was increased by fermentation with L. lactis Kushiro-L2 but not with L. plantarum AN7 (Fig. 2B). The O2− scavenging capacity was high with L. plantarum AN7 compared with L. lactis Kushiro-L2 (Fig. 2C). The Fe-reducing power was slightly high with fermentation by L. lactis Kushiro-L2 (Fig. 2D). In all antioxidant assays, the 10% AFA-S fermented with the mixed culture of AN7 and Kushiro-L2 showed high capacities compared with the intact one. In the following experiments, intact and fermented mixed-cultures of 10% AFA-S were used.
Antioxidant properties of the ultrafiltered fractions
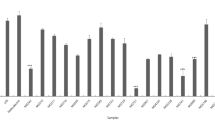
Figure 3 shows the TPC and antioxidant properties of the ultrafiltered fractions. Most of TPC was smaller than 3 kDa, followed by 30–100 kDa and 300 kDa < compounds (Fig. 3A). During the fermentation, the low molecular TPC was little increased and the high molecular TPC was little decreased.
Before the fermentation, the DPPH radical scavenging capacity was highest in 30–100 kDa, followed by 10–30 and 300 < kDa fractions (Fig. 3B). During the fermentation, the capacity was increased significantly in the < 3 kDa and 30–100 kDa fractions. The capacity of 10–30 kDa and 300 < kDa fractions disappeared with the fermentation. O2− radical scavenging compounds of intact AFA-S might be fractionated to < 3 kDa, 3–10 kDa and 30–100 kDa (Fig. 3C). In the case of 100 kDa and higher fractions, O2− radical or other NBT-oxidizable substances might be shown. The capacity in < 3 kDa, 10–30 kDa, and 100–300 kDa fractions were increased by the fermentation. The Fe-reducing power was high in the < 3 kDa and followed by 30–100 and 300 < kDa fractions (Fig. 3D). These were little decreased by the fermentation.
As mentioned in the introduction, the antioxidant properties of AFA with phycocyanin have been reported [23,24,25,26,27]. However, there are no reports about the activities of AFA fermented with LAB, though antioxidant, anti-inflammation, and anti-glycation capacities of some LAB-fermented edible algae had been reported [28,29,30,31].
From the results shown in Fig. 2, fermented AFA can be used more as an antioxidative food or supplement material. DPPH has been used extensively as a free radical to evaluate the reduction of substances in various foods including edible algae because it is simple and affordable [28, 31]. In previous reports about brown algae that containing phenolic compounds: phloroglucinols as antioxidant compounds show high DPPH radical scavenging capacity and Fe-reducing power [28, 31, 33]. In these reports, TPC and the scavenging capacity were lowered by the fermentation. It might due to decomposition by the LAB. Therefore, the scavenging capacity of < 3 kDa and 30–100 kDa fractions shown in Fig. 3B were not correlated with the TPC. The isolation and identification of the active compounds in the fractions are needed for future studies.
In most organisms, \({\text{O}}_{2}^{ - }\) radicals are converted to hydrogen peroxide (H2O2) by superoxide dismutase [49]. In the absence of transition metal ions, hydrogen peroxide is stable. However, hydroxyl radicals can be formed by the reaction of O2− with H2O2 in the presence of metal ions, usually ferrous or copper [50]. Hydroxyl free radicals are much more reactive and toxic than O2−. Therefore, the O2− radical-scavenging activity shown in Fig. 2C suggests that the fermented 10% AFA-S can scavenge not only O2−, but also H2O2 and hydroxyl radicals. This scavenging capacity (%) was well and moderately correlated with lactic acid and acetic acid concentrations shown in Table 1, approximately R2 = 0.998 and 0.861, respectively. Furthermore, increasing the scavenging capacity in the < 3 kDa fraction shown in Fig. 3C may be due to mainly lactic acid. Lactate salts are known as antioxidative food additives [51]. Additionally, Groussard et al. [52] reported that lactate has O2− scavenging activity in both water and plasma. Further studies on NBT-oxidizable products in the high molecular fractions are needed.
Most non-enzymatic antioxidant activities, such as the scavenging of free radicals and the inhibition of peroxidation, are mediated by redox reactions [53]. Compounds with reducing power are electron donors that can reduce the oxidized intermediates of lipid peroxidation processes and thereby act as primary and secondary antioxidants. The reducing power shown in Figs. 2D and 3D tended to be similar with the TPC result (Figs. 2A, 3A) and was slightly increased in < 3 kDa.
Anti-glycation properties
BSA was used in this study to determine the anti-glycation property. Serum albumin can be glycated at multiple sites [12]. In the BSA-Fru model (Fig. 4A), anti-glycation activity of the 10% AFA suspension was increased clearly with the fermentation. The activity in fermented AFA was dose dependent manner. Before fermentation, the anti-glycation activity was mainly fractionated to < 3 kDa, 300 < kDa, and 30–100 kDa fractions (Fig. 4B). During the fermentation, the activity was increased only in 30–100 kDa fraction. On the other hand, it was deceased in 100 and higher kDa fractions. In the BSA-MGO model (Fig. 4C), the anti-glycation activity was shown with dose response manner and effect of the fermentation on the anti-glycation activity was small. The activity was mainly fractionated to < 3 kDa and 30–100 kDa (Fig. 4D).
Due to the AGE synthesis contain oxidation process, already there are reports about food materials have both antioxidant and anti-glycation activities with some phenolic compounds [15, 28, 29]. The anti-glycation activity of AFA suspension shown in BSA-MGO was correlated with TPC (Fig. 3A, r2 = 0.905) and Fe-reducing power (Fig. 3D, r2 = 0.933). On the other hand, in the case of BSA-Fru model, although the correlation with any antioxidant capacities shown in Fig. 3 was not shown (r2 < 0.489), the activity increasing in 30–100 kDa fraction during the of the fermentation was agreed with DPPH radical scavenging capacity (Fig. 3B).
Effects on NO secretion from murine macrophage RAW264.7 cells of whole 10% AFA-S
Without LPS and intact 10% AFA-S (Fig. 5A), the NO secretion in the culture media was increased with a concentration response at 2.0 µL/mL and less sample. The NO secretion at 1.0–3.9 µL/mL samples was increased by the fermentation. In the higher sample concentration, the induction was reverse concentration dependent. To confirm the reverse dependence, the cell toxicity of the high sample concentration was determined using WST-8 (Fig. 5B). Only 125 µL/mL sample of intact 10% AFA-S lowered 27% in viability, though this cell toxicity was not observed in the fermented 10% AFA-S. The LPS induced NO was decreased by 16 µL/mL and higher samples (Fig. 5C). The inhibition activity of the 10% AFA-S was not affected by the fermentation.
Effect of intact AFA-S (open columns) or AFA-S fermented with L. plantarum AN7 and L. lactis L2 (closed columns) on NO secretion from murine macrophage RAW264.7 cells without (A) or with (C) LPS. (B) Cell viability in the high concentration samples confirmed by WST-8. Values are mean and SEM (n = 3). *p < 0.05, **p < 0.01. Values in (B) with different superscript letters are significantly different at p < 0.05
To determine the existence of fermented products with an induction capacity to NO secretion, the NO with the ultrafiltered fractions without LPS was determined. The NO secretion without LPS was induced clearly by 100 kDa and higher molecular fractions (Fig. 6). The induction was not high in the high sample concentration (Fig. 6C); rather than the less concentration (Fig. 6A, B). This induction activity of the high molecular fractions was decreased by the fermentation. Furthermore, the high molecular fractions of fermented AFA-S dependent on the concentration. After the fermentation, the induction activity in 30–100 kDa fraction was very high. It can be considered that immunomodulation activity induced by the fermentation was correlated with DPPH radical scavenging (Fig. 3B) and anti-glycation, in BSA-Fru model (Fig. 3B), activities.
The immunomodulation activity of extracted compounds from AFA, such as polysaccharides, chromoprotein-related compounds (phycobilins and phycobiliproteins), and low molecular (< 5 kDa) fraction, had been reported [54,55,56]. As shown in Fig. 5, AFA might have immune-promoting activity in lower concentrations and anti-inflammatory activity in high concentrations. Furthermore, the immune-modulating activity provided as NO secretion without LPS was increased by the fermentation (Fig. 5A). These phenomena were also observed for a green loofah Luffa cylindrica suspension [20]. Because some selected LAB strains have immune-modulating activity [19, 57], it is thought that the increasing NO secretion was the result of additives or the synergistic effect of AFA and LAB cells. However, as shown in Fig. 6, the immunomodulation activity of 100 kDa and higher molecular fractions was decreased by the fermentation and it was increased significantly in the 30–100 kDa fraction. This result suggests that the active compounds in fermented AFA-S are different from high molecular compounds including cell membranes and < 3 kDa compounds, already reported and mentioned above. In this fraction, the DPPH radical scavenging capacity was increased by the fermentation (Fig. 3B). The isolation and identification of the active compounds in the 30–100 kDa fraction, including phycobiliprotein-related compounds, are also needed.
The results of this study suggest that the fermented AFA-S can play antioxidant, anti-glycation and immunomodulation roles in food and supplement materials. These activities are higher than in the non-fermented AFA-S. Future studies regarding the isolation of metabolites generated during fermentation and their biological effects on cultured macrophages and in vivo are needed.
Conclusion
To clarify the effect of LAB fermentation on the antioxidant and immunomodulatory capacities of AFA, hot aqueous extract suspensions made from dry-powdered AFA (10% AFA-S) were fermented by L. plantarum AN7 and L. lactis Kushiro-L2 strains isolated from a coastal region in Japan. Then, the in vitro antioxidant properties and immunomodulation effects on NO secretion from RAW264.7 cells of the non-fermented and fermented 10% AFA-S were determined. The DPPH and O2− radical scavenging capacities and Fe-reducing power were increased in the mixed culture of AN7 and Kushiro-L2. The DPPH radical scavenging capacity of the fermented AFA-S was fractionated to mainly < 3 kDa and 30–100 kDa. The O2− radical scavenging capacities of the fermented AFA-S were fractionated to mainly < 3 kDa. Anti-glycation activity in BSA-fructose model rather than BSA-methylglyoxal model was increased by the fermentation. The increased anti-glycation activity was fractionated to mainly 30–100 kDa. Without LPS, 2.0 µL/mL and less non-fermented 10% AFA-S increased the NO secretion in the murine macrophage RAW264.7 culture media with a concentration response. The NO secretion activity was increased by the fermentation. The increased immunomodulation capacity in the fermented AFA-S was also fractionated to mainly 30–100 kDa. These results suggest that fermented AFA-S is a more useful material for health foods and supplements. Further study on the active compounds in < 3 kDa and 30–100 kDa fractions are needed in the future.
References
Parker CH, Stutts WL, Degrasse SL (2015) Development and validation of a liquid chromatography-tandem mass spectrometry method for the quantitation of microcystins in blue-green algal dietary supplements. J Agric Food Chem 63:10303–10312
Bishop WM, Zubeck M (2012) Evaluation of microalgae for use as nutraceuticals and nutritional supplements. Nutr Food Sci 2:1000147
Kushak C, Drapeau EM, Van Cott HH (2000) Winter, favorable effects of blue-green algae Aphanizomenon flos-aquae on rat plasma lipids. J Am Nutr Assoc 2:59–65
Carmichael WW, Drapeau C, Anderson DM (2000) Harvesting of Aphanizomenon flosaquae Ralfs ex Born. & Flah. var. flosaquae (Cyanobacteria) from Klamath Lake for human dietary use. J Appl Phycol 12:585–595
Taveme YJ, Merkus D, Bogers AJ, Halliwell B, Duncker DJ, Lyons TW (2018) Reactive oxygen species: Radical factors in the evolution of animal life: a molecular timescale from earth’s earliest history to the rise of complex life. Bioessays 40:https://doi.org/10.1002/bies.201700158
He LL, Wang X, Wu XX, Wang XX, Kong YM, Wang X et al (2015) Protein damage and reactive oxygen species generation induced by the synergistic effects of ultrasound and methylene blue. Spectrochim Acta A 134:361–366
Nissanka N, Moraes CT (2018) Mitochondrial DNA damage and reactive oxygen species in neurodegenerative disease. FEBS Lett 592:728–742
Birnbaum JH, Wanner D, Gietl AF, Saake A, Kündig TM, Hock C et al (2018) Oxidative stress and altered mitochondrial protein expression in the absence of amyloid-β and tau pathology in iPSC-derived neurons from sporadic Alzheimer’s disease patients. Stem Cell Res 27:121–130
Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK (2018) Reactive oxygen species in metabolic and inflammatory signalling. Circ Res 122:877–902
Franco R, Martinez-Soccio E (2017) Chemical rules on the assessment of antioxidant potential in food and food additives aimed at reducing oxidative stress and neurodegeneration. Food Chem 235:318–323
Kuda T, Yano T (2014) Mineral composition of seawater bittern nigari products and their effects on changing of browning and antioxidant activity in the glucose/lysine Maillard reaction. Appl Biochem Biotechnol 172:2989–2997
Roohk HV, Zaidi AR, Patel D (2018) Glycated albumin (GA) and inflammation: role of GA as a potential marker of inflammation. Inflamm Res 67:21–30
Rabbani N, Ashour A, Thornalley PJ (2016) Mass spectrometric determination of early and advanced glycation in biology. Glycoconjugate J 33:553–568
Neves D (2013) Advanced glycation end-products: a common pathway in diabetes and age-related erectile dysfunction. Free Radical Res 47:49–69
Crasci L, Lauro MR, Puglisi G, Panico A (2018) Natural antioxidant polyphenols on inflammation management: Anti-glycation activity vs metalloproteinases inhibition. Crit Rev Food Sci Nutr 58:893–904
Chinta KC, Saini V, Glasgaow JN, Mazorodze JH, Rahman MA, Reddy D et al (2016) The emerging role of gasotransmitters in the pathogenesis of tuberculosis. Nitric Oxide 59:28–41
Knott AB, Bossy-Wetzel E (2010) Impact of nitric oxide on metabolism in health and age-related disease. Diab Obes Metab 12:126–133
Ghasemi M, Mayasi Y, Hannoun A, Eslami SM, Carandang R (2018) Nitric oxide and mitochondrial function in neurological diseases. Neurosci 376:48–71
Kawahara M, Nemoto M, Nakata T, Kondo S, Takahashi H, Kimura B et al (2015) Anti-inflammatory properties of fermented soy milk with Lactococcus lactis subsp. lactis S-SU2 in murine macrophage RAW264.7 cells and DSS-induced IBD model mice. Int Immunopharm 26:295–303
Hirano S, Yokota Y, Eda M, Kuda T, Shikano A, Takahashi H et al (2017) Effect of Lactobacillus plantarum Tennozu-SU2 on Salmonella Typhimurium infection in human enterocyte-like HT-29-Luc cells and BALB/c mice. Probiotics Antimicro Prot 9:64–70
Han L, Yu J, Chen Y, Cheng D, Wang X, Wang C (2018) Immunomodulatory activity of docosahexenoic acid on RAW264.7 cells activation through GPR120-mediated signalling pathway. J Agric Food Chem 66:926–934
Shikano A, Kuda T, Takahashi H, Kimura B (2018) Effects of fermented green-loofah and green-papaya on nitric oxide secretion from murine macrophage RAW264.7 cells. Mol Biol Rep 45:1013–1021
Benedetti S, Benvenuti F, Pagliarani S, Francogli S, Scoglio S, Canestrari F (2004) Antioxidant properties of a novel phycocyanin extract from the blue-green alga Aphanizomenon flos-aquae. Life Sci 75:2353–2362
Benedetti S, Benvenuti F, Scoglio S, Canestrari F (2010) Oxygen radical absorbance capacity of phycocyanin and phycocyanobilin from the food supplement Aphanizomenon flos-aquae. J Med Food 13:223–227
Nuzzo D, Presti G, Picone P, Galizzi G, Gulotta E, Giuliano S et al (2018) Effects of the Aphanizomenon flos-aquae extract (Klamin®) on a neurodegeneration cellular model. Oxid Med Cell Longev. 9089016, https://doi.org/10.1155/2018/9089016
Hart AN, Zaske LA, Patterson KM, Drapeau C, Jensen GS (2007) Natural killer cell activation and modulation of chemokine receptor profile in vitro by an extract from the cyanophyta Aphanizomenon flos-aquae. J Med Food 10:435–441
Mohammed SA, Abdelhafez HM, Eid FA, Abdel-Raouf OM, Ibrahim RM (2016) The possible anti-inflammatory role of the blue green algae, Aphanizomenon flos-aquae on liver of adult male rats. J Biosci Appl Res 2:414–425
Eda M, Kuda T, Kataoka M, Takahashi H, Kimura B (2016) Anti-glycation properties of the aqueous extract solutions of dried algae products harvested and made in the Miura Peninsula, Japan, and effect of lactic acid fermentation on the properties. J Appl Phycol 28:3617–3624
Kuda T, Eda M, Kataoka M, Nemoto M, Kawahara M, Oshio S et al (2016) Anti-glycation properties of the aqueous extract solutions of dried algae products and effect of lactic acid fermentation on the proper. Food Chem 192:1109–1115
Nemoto M, Kuda T, Eda M, Yamakawa H, Takahashi H, Kimura B (2017) Protective effects of mekabu aqueous solution fermented by Lactobacillus plantarum Sanriku-SU7 on human enterocyte-like HT-29-luc cells and DSS-induced murine IBD model. Probiotics Antimicro Prot 9:48–55
Takei M, Kuda T, Eda M, Shikano A, Takahashi H, Kimura B (2017) Antioxidant and fermentation properties of aqueous solutions of dried algal products from the Boso Peninsula, Japan. Food Biosci 19:85–91
Kuda T, Yazaki T, Ono M, Takahashi H, Kimura B (2013) In vitro cholesterol-lowering properties of Lactobacillus plantarum AN6 isolated from aji-narezushi. Lett Appl Microbiol 57:187–192
Kuda T, Kataoka M, Nemoto M, Kawahara M, Takahashi H, Kimura B (2016) Isolation of lactic acid bacteria from plants of the coastal Satoumi regions for use as starter cultures in fermented milk and soymilk production. LWT—Food Sci Technol 68:202–207
Sasaki T, Koshi E, Take H, Michihata T, Maruya M, Enomoto T (2017) Characterisation of odorants in roasted stem tea using gas chromatography–mass spectrometry and gas chromatography-olfactometry analysis. Food Chem 220:177–183
Wang W, Yagiz Y, Buran TJ, Nunes CN, Gu L (2011) Phytochemicals from berries and grapes inhibited the formation of advanced glycation end-products by scavenging reactive carbonyls. Food Res Int 44:2666–2673
Yokota Y, Shikano A, Kuda T, Takei M, Takahashi H, Kimura B (2018) Lactobacillus plantarum AN1 cells increase caecal L. reuteri in an ICR mouse model of dextran sodium sulphate-induced inflammatory bowel disease. Int Immunpharm 56:119–127
Campana R, Martinelli V, Scoglio S, Colombo E, Benedetti S, Baffone W (2017) Influence of Aphanizomenon flos-aquae and two of its extracts on growth ability and antimicrobial properties of Lactobacillus acidophilus DDS-1. LWT—Food Sci Technol 81:291–298
McDonough AA, Veiras LC, Guevara CA, Ralph DL (2017) Cardiovascular benefits associated with higher dietary K+ versus lower dietary Na+: evidence from population and mechanistic studies. Am J Physiol Endocrinol Metab 312:E348–E356
Kamel KS, Schreiber M, Halperin ML (2018) Renal potassium physiology: integration of the renal response to dietary potassium depletion. Kidney Int 93:41–53
Oulhote Y, Mergler D, Barbeau B, Bellinger DC, Bouffard T, Brodeur M et al (2014) Neurobehavioral function in school-age children exposed to manganese in drinking water. Environ Health Perspect 122:12
Tallino S, Duffy M, Ralle M, Cortés MP, Latorre M, Burkhead JL (2015) Nutrigenomics analysis reveals that copper deficiency and dietary sucrose up-regulate inflammation, fibrosis and lipogenic pathways in a mature rat model of nonalcoholic fatty liver disease. J Nutr Biochem 26:996–1006
Yumol JL, Wakefield CB, Sacco SE, Sullivan PJ, Comelli EM (2018) Bone development in growing female mice fed calcium and vitamin D at lower levels than is present in the AIN-93G reference diet. Bone Rep 8:229–238
Ishizaki-Koizumi S, Sonaka I, Fujitani S, Nishiguchi S (2002) Mechanisms of the protective effect of L-alanine to D-galactosamine-induced hepatocellular injury: Comparative studies of L-alanine and pyruvate. Biochem Biophys Res Commun 291:738–743
Brosnan JT, Brosnan ME (2013) Glutamate: a truly functional amino acid. Amino Acid 45:413–418
Poolman B, Driessen AJ, Konings WN (1988) Regulation of arginine-ornithine exchange and the arginine deiminase pathway in Streptococcus lactis. J Bacteriol 169:5597–5604
Cynober L (1994) Can arginine and omithine support gut functions? Gut 35(1):S42-S45
Goh ET, Stokes CS, Sidhu SS, Vilstrup H, Gluud LL, Morgan YY (2018) L-Ornithine L-aspartate for prevention and treatment of hepatic encephalopathy in people with cirrhosis. Cochrane Database Syst Rev 5:CD012410
Grosser N, Oberle S, Berndt G, Erdmann K, Hemmerle A, Schröder H (2004) Antioxidant action of L-alanine: heme oxygenase-1 and ferritin as possible mediators. Biochem Biophys Res Commun 314:351–355
Kawano T, Kagenishi T, Kadono T, Bouteau F, Hiramatsu T, Lin C et al (2015) Production and removal of superoxide anion radical by artificial metalloenzymes and redox-active metals. Commun Integr Biol 8:e1000710
Pisoschi AM, Pop A (2015) The role of antioxidants in the chemistry of oxidative stress: a review. Eur J Med Chem 97:55–74
Kim YH, Keeton JT, Smith SB, Maxim JE, Yang HS, Savell JW (2009) Evaluation of antioxidant capacity and colour stability of calcium lactate enhancement on fresh beef under highly oxidising conditions. Food Chem 115:272–278
Groussard C, Morel I, Chevanne M, Monnier M, Josianne C, Delamarche A (2000) Free radical scavenging and antioxidant effects of lactate ion: an in vitro study. J Appl Physiol 89:169–175
Lobo A, Patil A, Phatak N (2010) Chandra, Free radicals, antioxidants and functional foods: impact on human health. Pharmacol Rev 4:118–126
Pugh N, Ross SA, ElSohly HN, ElSohly MA, Pasco DS (2001) Isolation of three high molecular weight polysaccharide preparations with potent immunostimulatory activity from Spirulina platensis, Aphanizomenon flos-aquae and Chlorella pyrenoidosa. Planta Med 67:737–742
Aaron NH, Zasuke LA, Patterson KM, Drapeau C, Jensen GS (2007) Natural killer cell activation and modulation of chemokine receptor profile in vitro by an extract from the Cyanophyta Aphanizomenon flos-aquae. J Med Food 10:435–441
Mysliwa KB, Solymosi K (2017) Phycobilins and phycobiliproteins used in food industry and medicine. J Mini-Rev Med Chem 17:1173–1193
Kondo S, Kuda T, Nemoto M, Usami Y, Takahashi H, Kimura B (2016) Protective effects of rice bran fermented by Saccharomyces cerevisiae Misaki-1 and Lactobacillus plantarum Sanriki-SU8 in dextran sodium sulphate-induced inflammatory bowel disease model mice. Food Biosci 16:44–49
Acknowledgements
This work was partially supported by the Japan Health & Research Institute, Tokyo, Japan; Suzuki Nori Co., Choshi, Japan; and Dr’s Choice, Tokyo, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors do not declare any conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Taniguchi, M., Kuda, T., Shibayama, J. et al. In vitro antioxidant, anti-glycation and immunomodulation activities of fermented blue-green algae Aphanizomenon flos-aquae. Mol Biol Rep 46, 1775–1786 (2019). https://doi.org/10.1007/s11033-019-04628-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-04628-7