Abstract
To clarify the anti-oxidant and anti-glycation properties of traditional edible algae, aqueous extract solutions (AESs) from eight dried algal products that were commercially available in the Miura Peninsula of Japan were prepared. AESs of the red alga, Pyropia sp. (nori), demonstrated strong Fe-reducing power with a high total phenolic compound content. On the other hand, the sporophyll of the brown alga Undaria pinnatifida (mekabu) exhibited high superoxide anion radical-scavenging capacity. Anti-glycation activity in bovine serum albumin (BSA)-fructose (Fru) and in BSA-methylglyoxal (MGO) was high for nori and the stem of Sargassum fusiforme (naga-hijiki). The results of the BSA-MGO model agreed with those of the phenolic content and Fe-reducing power. Anti-glycation activities of mekabu, Sargassum horneri (akamoku), naga-hijiki, the frond of U. pinnatifida (wakame), and Gelidium elegans (tengusa) in the BSA-Fru model were clearly increased by fermentation with Lactobacillus plantarum Miura-SU1 isolated from the Miura Peninsula. The results of the present study suggest that, once fermented with lactic acid bacteria (LAB), akamoku and other edible algae will have a potential role in preventing diabetes- and aging-related glycation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, anti-glycation properties have attracted attention as having food function (Yoon and Shim 2015; Alam et al. 2015; Sri Harsha et al. 2014). Glycation is a nonenzymatic reaction of reducing sugars with amino acids and/or proteins in processed food and in vivo (Raghav and Ahmad 2014; Scheijen et al. 2016). Advanced glycation end products (AGEs), such as carboxymethyl lysine and carboxyethyl lysine, are generated after various intermediates such as glyoxal, methylglyoxal (MGO), and 3-deoxyglucosone (Gaens et al. 2013; Vistoli et al. 2013). AGE formation is irreversible. AGEs are thought to induce diabetes and other diabetes- and aging-related illnesses such as retinopathy, cataracts, arteriosclerosis, and renal dysfunction (Kandarakis et al. 2014; Roca et al. 2014). Because there are oxidation reactions in several parts in the glycation reactions for AGE generation, anti-oxidants are considered inhibitory materials for medicines and treatment diets that prevent AGE formation (Shankaraiah et al. 2013; Ahmad et al. 2014). In the case of food materials, various foodstuffs are reported to be anti-glycative materials with anti-oxidant properties (Premakumara et al. 2013).
Since ancient times, the inhabitants of coastal regions of Far Eastern countries, such as Korea and Japan, have discovered and collected edible algae from beach cast (Kuda and Ikemori 2009; Murata and Nakazoe 2001). The Ministry of the Environment, Government of Japan, defines a Satoumi as a coastal area where biological productivity and biodiversity have increased as a consequence of human activity (Berque and Matsuda 2013). The traditional eating habit of various algae is considered one of the features of the Satoumi region. In the Tokyo Bay, located in the capital and largest metropolis of Japan, some marine algae, such as Pyropia sp. (nori), Undaria pinnatifida (wakame), and Saccharina japonica (kombu), have been cultured and used for human consumption. In the Miura Peninsula, located on the west side of the entrance to the Tokyo Bay and characterized by typical Satoumi areas, some wild algae such as Sargassum fusiforme (hijiki), Sargassum horneri (akamoku), and Gelidium elegans (tengusa) are harvested and used as food. Most of these edible algae are distributed after drying.
Among these algae, hijiki and akamoku have been reported to have high phenolic content (phlorotannins) and anti-oxidant properties (Shao et al. 2014; Wu et al. 2016). Some lactic acid bacteria (LABs) including probiotics also have anti-oxidant and anti-inflammatory activities in vitro and in mice (Kanno et al. 2012; Kuda et al. 2014a). Some LABs can increase the anti-oxidant capacities of vegetables, milk, and soy milk during the fermentation (Kuda et al. 2010; Kanno et al. 2012; Kawahara et al. 2015). Moreover, during fermentation, some LABs have been reported to increase the superoxide anion radical-scavenging capacity of a red alga, Gloiopeltis furcata (funori) (Kuda et al. 2015). However, anti-glycation properties of these algae and the fermented algae are still not clear.
In this study, to clarify and apply the anti-glycation effect of traditional edible algae, we determined the inhibitory effect of aqueous extract solutions of dried algae products obtained from the Miura Satoumi region on glycation in bovine serum albumin-fructose (BSA-Fru) and BSA-methylglyoxal (BSA-MGO) models. Furthermore, to examine the additive or synergistic effect of the food materials and LAB or lactic acid fermentation, the effect of the Lactobacillus plantarum isolated from the Miura Satoumi region on the anti-glycation capacities of the selected algae samples was also investigated.
Materials and methods
Chemicals
Folin-Ciocalteu’s phenol reagent, the stable 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical phenazine methosulfate (PMS), 3-(2-pyridyl)-5,6-di(p-sulfophenyl)1,2,4-triazine disodium salt (ferrozine), β-nicotinamide adenine dinucleotide (NADH), nitroblue tetrazolium salt (NBT), and MGO were from Sigma-Aldrich (USA). Phloroglucinol dihydrate (PG), potassium ferricyanide, trichloroacetic acid (TCA), bovine serum albumin (BSA), and d-fructose (Fru) were from Wako Chemicals (Japan), while 1,10-phenanthroline was from Nacalai Tesque (Kyoto, Japan). The other reagents were of analytical grade.
Preparation of aqueous extract solutions from dried algae solutions
A total of eight dried products (Table 1) from three species of Phaeophyta, Undaria pinnatifida (wakame, Up and mekabu, Up’), Sargassum horneri (akamoku, Sh), and Sargassum fusiforme (hijiki, Sf1 and naga-hijiki, Sf2), as well as two species of Rhodophyta, Gelidium elegans (tengusa, G1 and G2) and Pyropia sp. (nori, P), were purchased from retail shops in the Miura Peninsula region. Tengusa G1 and G2 were obtained from different manufacturers.
The dried samples were milled using a blender (Oster 16 Speed Blender; Osaka Chemical Co., Japan) and sieved through 1-mm2 mesh. The algae powder (5 g) was added to 200 mL of distilled water and heated at 105 °C for 15 min using an autoclave. After cooling with tap water, the algae suspension was centrifuged at 3000×g for 10 min at 4 °C. The collected supernatant was used in the algal aqueous extract solutions (AESs) and stored at −20 °C. In our previous study, the AES of some brown algal products contained substances rich in minerals, phenolic compounds, and water-soluble polysaccharides, such as alginate, fucoidan, and laminaran (Kuda et al., 2005; 2009).
Miura Satoumi LAB strains
Two Miura Satoumi LAB strains, Lactobacillus plantarum Miura-SU1 (accession no. LC125266) and Lactococcus lactis subsp. lactis Miura-SU2 (accession no. LC125267), were used in this study. Both strains were isolated from algal beach casts in the Miura Satoumi region (Kuda et al. 2016) and stored using ceramic beads (Microbank, Iwaki Co., Ltd., Tokyo, Japan) at −80 °C. A bead of the each strain was inoculated into 5 mL of de Man, Rogosa, and Sharpe (MRS) broth (Oxoid, UK). After 48 h incubation at 30 °C, these cultures were used for algal fermentation.
Total phenolic content
Total phenolic content as polyphenol content level was determined as described previously (Kuda et al. 2006) with slight modifications. Briefly, 0.03 mL of a diluted sample solution and 0.06 mL of 10 % Folin-Ciocalteu solution were placed in a 96-well microplate. After 3 min, 0.12 mL of 10 % sodium carbonate was added. The mixture was allowed to stand for 60 min at ambient temperature, and the absorbance was measured at 750 nm using a grating microplate reader (SH-1000 Lab; Corona Electric, Japan). The phenolic content is expressed as PG equivalents PGEq mL−1.
The experiments to determine phenolic content, anti-oxidant properties, and anti-glycation properties were conducted in triplicate.
DPPH radical-scavenging capacity
DPPH radical-scavenging capacity was determined as described previously (Kuda and Yano 2014) with slight modification. Briefly, sample diluted solution (0.1 mL) and ethanol (0.1 mL) were put into a 96-well microplate, and absorbance at 517 nm (Abs1) was measured using a microplate reader (SH-1000 Lab). Next, 1 mmol L−1 DPPH radical was added and incubated at 37 °C for 30 min and the absorbance (Abs2) was measured again. The DPPH radical-scavenging capacity was calculated using the following formula:
Superoxide anion radical-scavenging activity
Superoxide anion radical-scavenging activity was measured using a nonenzymatic method (Kuda et al. 2014a). The sample solution (0.01 mL) was treated with 0.05 mL of 250 mmol L−1 phosphate buffer (pH 7.2), 2 mmol L−1 NADH (0.025 mL), and 0.5 mmol L−1 NBT (0.025 mL), while absorbance at 560 nm was measured as a blank value. After a 5 min incubation at ambient temperature with 0.025 mL of 0.03 mmol L−1 PMS, the absorbance was measured again. The radical-scavenging capacity was calculated using the above formula.
Ferrous reducing power
The reducing power was determined as described in our previous report (Kuda and Yano 2009) with slight modification. Briefly, each 0.05 mL of the sample solution, 0.025 mL of 0.1 mol L−1 phosphate buffer (pH 7.2), and 0.025 mL of 10 g L−1 potassium ferricyanide were placed in a 96-well microplate. After incubation at 37 °C for 60 min, 0.025 mL of 10 % TCA and 0.1 mL of distilled water were added and the absorbance was measured at 700 nm (Abs1). Next, 0.025 mL of 0.1 % FeCl3 was added to the mixture and the absorbance was measured again (Abs2). Ferrous reducing power was calculated using the following formula:
Anti-glycation properties of the AESs in BSA-Fru glycation model
The anti-glycation assay in the BSA-Fru model was determined using the method of Wang et al. (2011) with slight modification. This model evaluates all stages of protein glycation. Fru (1.5 mol L−1, 0.5 mL) was mixed with 0.5 mL of AES and 0.5 mL of sodium phosphate buffer (50 mmol, pH 7.4, with 0.02 % sodium azide) in screw-capped test tubes and kept at 37 °C for 2 h. BSA (30 mg mL−1, 0.5 mL) was added to each test tube, and the mixtures were incubated at 37 °C for 5 days. Fluorescent AGEs were monitored on a multiple microplate reader (SH-9000; Corona Electric) using 340 and 420 nm as the excitation and emission wavelengths. Percentage of the AGE inhibition was calculated by the following equation:
FI 0d and FI 5d represent fluorescent intensity after the reaction for 0 and 5 days, respectively.
Anti-glycation properties of the AESs in BSA-MGO glycation model
The anti-glycation assay in the BSA-MGO model was performed also using the method of Wang et al. (2011) with slight modification. This model evaluates the middle stage of protein glycation. MGO (60 mmol L−1, 0.5 mL) was mixed with 0.5 mL of AES and 0.5 mL of the sodium phosphate buffer and kept at 37 °C for 2 h. BSA (30 mg mL−1, 0.5 mL) was added to each test tube and incubated at 37 °C for 5 days. Fluorescent AGEs were monitored on the multiple microplate reader using 340 and 380 nm as the excitation and emission wavelengths. The percentage of AGE inhibition was calculated using the same equation as that in the BSA-Fru model.
Algal fermentation properties of Miura Satoumi LABs
The pre-cultured strains (0.03 mL) were inoculated in 3 mL of the AESs and incubated at 30 °C for 2 days. If turbidity could be observed by the naked eye, the pH was determined using a pH meter (Twin pH; Horiba; Japan). The anti-glycation assays in the BSA-Fru and BSA-MGO models were measured as described above.
Statistical analysis
Data of the anti-oxidant and anti-glycation activities and phenolic content of the algal AESs are presented as means and standard errors. Data pertaining to the anti-glycation capacities before versus after fermentation were subjected to Student’s t test.
Results and discussion
Total phenolic content
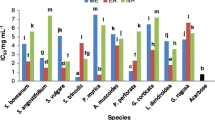
The total phenolic content in the AESs of the eight dried algae products is shown in Fig. 1a. The phenolic concentrations in the AES sample of nori Pyropia sp. (P) were high, at approximately 3.5 μmol PGEq mL−1 of AES corresponding to 140 μmol PGEq g−1 of the dried products. AES from akamoku S. horneri (Sh), hijiki, naga-hijiki S. fusiforme (Sf1, Sf2), and mekabu from the sporophyll of the U. pinnatifida (Up’) had phenolic contents of that ranged approximately between 0.9 and 1.2 μmol PGEq mL−1. The phenolic compound contents of the other AESs were <0.4 μmol PGEq mL−1. The high total phenolic contents in the AES of nori were consistent with those of our previous reports (Kuda et al. 2005; 2016b). On the other hand, the phenolic contents of the AESs of mekabu (Up’) and wakame, from the frond of U. pinnatifida (Up), were different. There are some reports on higher polyphenol content in mekabu (Kuda et al. 2015), although the contents of various compounds, including polysaccharides and polyphenols, in mekabu were thought to change drastically during the annual stages (Holdt and Kraan 2011).
Total phenolic compound content (a), 1,1-diphenyl-2-picrylhydrazyl (DPPH) and superoxide anion radical-scavenging capacity (b and d), and Fe-reducing power (c) of aqueous solutions of dried algae products. See Table 1. Values are the mean ± standard error of the mean (n = 3)
DPPH radical-scavenging capacity
DPPH has been used extensively as a free radical to evaluate the reduction of substances in various foods including edible algae (Hermund et al. 2015). The percentage of DPPH-scavenging activities is shown in Fig. 1b. Among the eight algal AES samples, the scavenging activity was high in AESs of nori (P). Although this order appeared to agree with the results of the phenolic content (Fig. 1a), the scavenging activity of nori (P) was not high compared with that of some Ecklonia-derived products reported previously (Kuda et al. 2007).
Ferrous reducing power
As shown in Fig. 1c, the highest amount of the reducing power was obtained in AES of nori (P), followed by akamoku (Sh), hijiki (Sf1, Sf2), and mekabu Up’, which supported the phenolic content and DPPH radical-scavenging results. Most nonenzymatic anti-oxidant activities such as the scavenging of free radicals and the inhibition of peroxidation are mediated by redox reactions (Zhuet al 2002). Compounds with reducing power are electron donors that can reduce the oxidized intermediates of lipid peroxidation processes and thereby act as primary and secondary anti-oxidants (Yen and Chen, 1995).
Superoxide anion radical-scavenging capacity
The percentage of superoxide anion radical-scavenging activities at a concentration of 1.56 μL mL−1 AES is shown in Fig. 1d. The scavenging capacity of mekabu (Up’) was high, followed by those of akamoku (Sh) and naga-hijiki (Sf2). In our previous report, the correlation between superoxide anion radical-scavenging activity and phenolic compound content was moderate (Kuda and Ikemori 2009; Kuda et al. 2016b). There are several reports on the superoxide anion radical-scavenging capacity of fucoidan, one of the water-soluble polysaccharides in brown algae (Kuda et al. 2005). The superoxide anion radical-scavenging activity of the AESs was caused not only by the phenolic compounds but also by other water-soluble compounds such as polysaccharides.
In most organisms, superoxide anion radicals are converted to hydrogen peroxide by superoxide dismutase. In the absence of transition metal ions, hydrogen peroxide is stable. However, hydroxyl radicals can be formed by the reaction of superoxide with hydrogen peroxide in the presence of metal ions, usually ferrous or copper (Macdonald and Galley 2003). Hydroxyl free radicals are much more reactive (toxic) than superoxide anions. The superoxide anion-scavenging activity of AESs of the edible algae is shown in Fig. 1d.
Anti-glycation property in the BSA-Fru model
We used BSA in this study to determine the anti-glycation property. Serum albumin is abundant in the serum, and it can be glycated at multiple sites (Anguizola et al. 2013). The BSA-Fru model system was used to simulate the protein glycation that occurs at an accelerated rate in vivo under nonphysiological conditions, accounting for some of the complications of hyperglycemia and diabetes (Wang et al. 2011).
In the BSA-Fru model (Fig. 2a), the AESs of nori (P) and naga-hiziki (Sf2), followed by akamoku (Sh), had high DPPH radical-scavenging capacity (Fig. 1b). However, no clear anti-glycation effect was observed in hijiki (Sf1), wakame (Up), and tengusa (G2). This finding is not consistent with the results of DPPH radical-scavenging capacity, ferrous reducing power, and the content of total phenolic compounds.
Effects of aqueous solutions of dried algae products on glycation of bovine serum albumin (BSA) with fructose (Fru; a) and methylglyoxal (MGO; b). See Table 1. Values are the mean ± standard error of the mean (n = 3)
Anti-glycation property in the BSA-MGO model
This model evaluates the middle stage of protein glycation (Wang et al. 2011). MGO is a well-known intermediator for AGE formation. MGO can react with serum albumin as well as other extracellular (e.g., BSA) and intracellular proteins within the tissues. Furthermore, some MGO-modified proteins can be toxic to cells (Chondrogianni et al. 2014). In the BSA-MGO model (Fig. 2b), the AES of nori (P) showed high anti-glycation capacity. The inhibitory effects of the other AESs were not as high.
Fermentation properties
We determined the growth of L. plantarum Miura-SU1 and L. lactis subsp. lactis Miura-SU2 in the eight AES samples. Among them, seven dried algae, except for tengusa (G1), could be fermented by both the Satoumi LABs (Table 1). The fermentation activity, estimated by the reduction in pH, of L. plantarum Miura-SU1 was stronger than that of L. lactis subsp. lactis Miura-SU2. Each AES of wakame (Up), mekabu (Up’), and akamoku (Sh) was easily fermented by L. plantarum Miura-SU1 from pH 6.1, 6.2, and 6.0 to pH 4.1, 4.1, and 4.3, respectively.
Anti-glycation properties of the fermented aqueous solutions and suspensions
The AES samples showed fermentation by the Satoumi LABs, particularly by L. plantarum Miura-SU1. Therefore, we determined the anti-glycation activities of the AESs fermented by L. plantarum Miura-SU1. In the BSA-MGO model, no effect of fermentation by L. plantarum Miura-SU1 was observed (data are not shown). As shown in Fig. 3, in the BSA-Fru model, the anti-glycation capacities of the AESs of akamoku (Sh), naga-hijiki (Sf2), wakame (Up), and tengusa (G2) were increased by the fermentation. Although the increase of anti-glycation activity in the BSA-Fru model of mekabu was previously reported (Kuda et al. 2016b), that of hijiki, akamoku, and tengusa represents new findings.
As shown in Figs. 1 and 2, the anti-glycation capacities in BSA-Fru model agreed with the superoxide anion radical-scavenging capacity, rather than the DPPH radical-scavenging capacity, Fe-reducing power or total phenolic compound content. We previously reported that the fermentation by several L. plantarum strains isolated from the Satoumi region induced the superoxide anion radical-scavenging and anti-inflammation capacities in MRS broth, Japanese white radish, milk, soybean milk, and also a dried alga funori G. furcata (Kuda et al. 2010; 2015; Kawahara et al. 2015).
In this study, we determined the anti-glycation properties of AESs of eight dried products of edible algae obtained from the Miura Peninsula. Among the samples, nori (P) demonstrated high total phenolic content and anti-glycation activity in the BSA-MGO model. On the other hand, three brown algal samples, akamoku (Sh), naga-hijiki (Sf2), and wakame (Up), were fermented by the LABs. Furthermore, these fermented AESs showed high anti-glycation activity in the BSA-Fru model. Although we have not directly investigated the absorption of algal polyphenols and other compounds from intestinal epithelial cells, it had been reported that small polyphenols such as apigenin and resveratrol could be absorbed rapidly by human entero-epithelial Caco-2 cells, likely via transporters (Teng et al. 2012).
The receptor for advanced glycation end products is a multiligand cell surface molecule of the immunoglobulin superfamily (Schmidt et al. 2000). It was originally described as a receptor for protein adducts formed by AGEs that accumulate in diseases such as diabetes and renal failure (Zill et al. 2001). Recently, the inhibitory effect of dietary prebiotic supplementation on advanced glycation was found to be correlated with intestinal microbiota (Kellow et al. 2014). It can be considered that, not only low-molecular-weight compounds, but also high-molecular-weight compounds in the algal AESs, as well as LAB, ameliorate the AGE-related damages with some intestinal microbiota. Thus, studies on anti-glycation properties in intact and fermented AESs of some edible algae using human enterocyte cell cultures and in vivo experiments are now in progress. Further, we consider that the purification and analysis of active compounds in the intact and fermented AESs are needed.
Conclusion
In conclusion, we determined the anti-oxidant and anti-glycation properties of the AESs of dried algae products commercially available in the Miura Peninsula. The AESs of nori (P) showed high Fe-reducing ability with high total phenolic compound content. On the other hand, the superoxide anion radical-scavenging capacity of mekabu Up’, akamoku (Sh), and nagahijiki (Sf2) was high. The anti-glycation activities shown by the BSA-Fru and BSA-MGO models were also high in nori (P). In the BSA-Fru model, which evaluates all stages of protein glycation, nori (P) and naga-hijiki (Sf2) suppressed the glycation process. On the other hand, the results of the BSA-MGO model, which evaluates the middle stage of protein glycation, agreed with the results of total phenolic content and Fe-reducing power. The anti-glycation activities of hijiki (Sf2), akamoku (Sh), mekabu (Up’), and tengusa (G2) in the BSA-Fru model were clearly increased by fermentation with L. plantarum Santiku-SU1. The results of the present study suggest that, once fermented with LAB, akamoku and other edible algae will have a potential role in preventing diabetes- and aging-related glycation.
References
Ahmad S, Khan MS, Akhter F, Khan MS, Khan A, Ashral JM, Pandey RP, Shahab U (2014) Glycoxidation of biological macromolecules: a critical approach to halt the menace of glycation. Glycobiol 24:979–990
Alam M, Ahmed I, Naseem I (2015) Inhibitory effect of quercetin in the formation of advance glycation end products of human serum albumin: an in vitro and molecular interaction study. Int J Biol Macromol 79:336–343
Anguizola J, Matsuda R, Barnaby OS, Hoy KS, Wa C, DeBolt E, Koke M, Hage DS (2013) Glycation of human serum albumin. Clin Chim Acta 425:64–76
Berque J, Matsuda O (2013) Coastal: 6 biodiversity management in Japanese satoumi. Mar Policy 39:191–200
Chondrogianni N, Petropoulos I, Grimm S, Georglia K, Catalgol B, Friguet B, Grune T, Gonos ES (2014) Protein damage, repair and proteolysis. Mol Aspects Med 35:1–71
Gaens KHJ, Stehouwer CDA, Schalkwijk CG (2013) Advanced glycation endproducts and its receptor for advanced glycation endproducts in obesity. Curr Opin Lipidol 24:4–11
Hermund DB, Yeşiltaş B, Honold P, Jónsdóttir R, Kristinsson HG, Jacobsen C (2015) Characterisation and antioxidant evaluation of Icelandic F. vesiculosus extracts in vitro and in fish-oil-enriched milk and mayonnaise. J Funct Foods 19:828–841
Holdt SL, Kraan S (2011) Bioactive compounds in seaweed: functional food applications and legislation. J Appl Phycol 23:543–597
Kandarakis SA, Piperi C, Topouzis F, Papavassiliou AG (2014) Emerging role of advanced glycation-end products (AGEs) in the pathobiology of eye diseases. Prog Retin Eye Res 42:85–102
Kanno T, Kuda T, An C, Takahashi H, Kimura B (2012) Radical scavenging capacities of saba-narezushi, Japanese fermented chub mackerel, and its lactic acid bacteria. LWT-Food Sci Technol 47:25–30
Kawahara M, Nemoto M, Nakata T, Kondo S, Takahashi H, Kimura B, Kuda T (2015) Anti-inflammatory properties of fermented soymilk with Lactococcus lactis subsp. lactis S-SU2 in murine macrophage RAW264.7 cells and DSS-induced IBD model mice. Int Immunopharmacol 26:295–303
Kellow NJ, Coughlan MT, Savige GS, Reid CM (2014) Effect of dietary prebiotic supplementation on advanced glycation, insulin resistance and inflammatory biomarkers in adults with pre-diabetes: a study protocol for a double-blind placebo-controlled randomised crossover clinical trial. BMC Endocr Disord 14:55
Kuda T, Ikemori T (2009) Minerals, polysaccharides and antioxidant properties of aqueous solutions obtained from macroalgal beach-casts in the Noto Peninsula, Ishikawa, Japan. Food Chem 112:575–581
Kuda T, Yano T (2009) Changes of radical-scavenging capacity and ferrous reducing power in chub mackerel Scomber japonicus and Pacific saury Cololabis saira during 4 °C storage and retorting. LWT-Food Sci Technol 42:1070–1075
Kuda T, Yano T (2014) Mineral composition of seawater bittern nigari products and their effects on changing of browning and antioxidant activity in the glucose/lysine Maillard reaction. Appl Biochem Biotechnol 172:2989–2997
Kuda T, Tsunekawa M, Goto H, Araki Y (2005) Antioxidant properties of four edible algae harvested in the Noto Peninsula, Japan. J Food Comp Anal 18:625–633
Kuda T, Hishi T, Maekawa S (2006) Antioxidant properties of dried product of ‘haba-nori’, an edible brown alga, Petalonia binghamiae (J. Agaradh) Vinogradova. Food Chem 98:545–550
Kuda T, Kunii T, Goto H, Suzuki T, Yano T (2007) Varieties of antioxidant and antibacterial properties of Ecklonia stolonifera and Ecklonia kurome products harvested and processed in the Noto peninsula, Japan. Food Chem 103:900–905
Kuda T, Kaneko N, Yano T, Mori M (2010) Induction of superoxide anion radical scavenging capacity in Japanese white radish juice and milk by Lactobacillus plantarum isolated from aji-narezushi and kaburazushi. Food Chem 120:517–522
Kuda T, Kawahara M, Nemoto M, Takahashi H, Kimura B (2014) In vitro antioxidant and anti-inflammation properties of lactic acid bacteria isolated from fish intestines and fermented fish from the Sanriku Satoumi region in Japan. Food Res Int 64:248–255
Kuda T, Nemoto M, Kawahara M, Oshio S, Takahashi H, Kimura B (2015) Induction of the superoxide anion radical scavenging capacity of dried ‘funori’ Gloiopeltis furcata by Lactobacillus plantarum S-SU1 fermentation. Food Funct 6:2535–2541
Kuda T, Eda M, Kataoka M, Nemoto M, Kawahara M, Oshio S, Takahashi H, Kimura B (2016a) Anti-glycation properties of the aqueous extract solutions of dried algae products and effect of lactic acid fermentation on the properties. Food Chem 192:1109–1115
Kuda T, Kataoka M, Nemoto M, Kawahara M, Takahashi H, Kimura B (2016b) Isolation of lactic acid bacteria from plants of the coastal Satoumi regions for use as starter cultures in fermented milk and soymilk production. LWT-Food Sci Technol 68:202–207
Macdonald J, Galley HF, Webster NR (2003) Oxidative stress and gene expression in sepsis. Br J Anaesth 90:221–232
Murata M, Nakazoe J (2001) Production and use of marine algae in Japan. Agr Res Quart 35:281–290
Premakumara GAS, Abeysekera WKSM, Ratnasooriya WD, Chandrasekharan NV, Bentota AP (2013) Antioxidant, anti-amylase and anti-glycation potential of brans of some Sri Lankan traditional and improved rice (Oryza sativa L.) varieties. J Cereal Sci 58:451–456
Raghav A, Ahmad J (2014) Glycated serum albumin: a potential disease marker and an intermediate index of diabetes control. Diabetes Metab Syndr 8:245–251
Roca F, Grossin N, Chassagne P, Puisieux F, Boulanger E (2014) Glycation: the angiogenic paradox in aging and age-related disorders and diseases. Age Res Rev 15:146–160
Scheijen JLJM, Clevers E, Engelan L, Dagnelie PC, Brouns F, Stehouwer CDA, Schalkwijk CG (2016) Analysis of advanced glycation endproducts in selected food items by ultra-performance liquid chromatography tandem mass spectrometry: presentation of a dietary AGE database. Food Chem 190:1145–1150
Schmidt AM, Hofmann M, Taguchi A, Yan SD, Stem DM (2000) RAGE: a multiligand receptor contributing to the cellular response in diabetic vasculopathy and inflammation. Semin Thromb Hemost 26:485–493
Shankaraiah G, Tiwari AK, Kumar TV, Kumar DA, Raju SSN, Babu KH, Varala R, Rao MVB, Babu KS (2013) New protein glycation inhibitory free radical scavenging compound from Duranta repens L. J Pharmacol Res 7:163–166
Shao P, Chen X, Sun P (2014) Chemical characterization, antioxidant and antitumor activity of sulfated polysaccharide from Sargassum horneri. Carbohydr Polym 105:260–269
Sri Harsha PSC, Lavelli V, Scarafoni A (2014) Protective ability of phenolics from white grape vinification by-products against structural damage of bovine serum albumin induced by glycation. Food Chem 156:220–226
Teng A, Yuan C, Zhang F, Huan M, Cao W, Li K, Yang J, Cao D, Zhou S, Mei Q (2012) Intestinal absorption and first-pass metabolism of polyphenol compounds in rat and their transport dynamics in Caco-2 cells. PLoS One 7:e29647
Vistoli G, De Maddis D, Zarkovic N, Cipak A, Carini M, Aldini G (2013) Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): an overview of their mechanisms of formation. Free Rad Res 47:3–27
Wang W, Yagiz Y, Buran TJ, Nunes CN, Gu L (2011) Phytochemicals from berries and grapes inhibited the formation of advanced glycation end-products by scavenging reactive carbonyls. Food Res Int 44:2666–2673
Wu M, Tong C, Wu Y, Liu S, Li W (2016) A novel thyroglobulin-binding lectin from the brown alga Hizikia fusiformis and its antioxidant activities. Food Chem 201:7–13
Yen GC, Chen HY (1995) Antioxidant activity of various tea extracts in relation to their antimutagenicity. J Agr Food Chem 43:27–32
Yoon SR, Shim SM (2015) Inhibitory effect of polyphenols in Houttuynia cordata on advanced glycation end-products (AGEs) by trapping methylglyoxal. LWT-Food Sci Technol 61:158–163
Zhu QT, Hackman RM, Ensuma JL, Holt L, Keen CL (2002) Antioxidative activities of oolong tea. J Agr Food Chem 50:6929–6934
Zill H, Günther R, Erbersdobler HF, Fölschb UR, Faista F (2001) RAGE expression and AGE-induced MAP kinase activation in Caco-2 cells. Biochem Biophys Res Commn 288:1108–1111
Acknowledgments
The present study was supported by The Towa Foundation for Food Science & Research and Warehouse TERRADA, Tokyo, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eda, M., Kuda, T., Kataoka, M. et al. Anti-glycation properties of the aqueous extract solutions of dried algae products harvested and made in the Miura Peninsula, Japan, and effect of lactic acid fermentation on the properties. J Appl Phycol 28, 3617–3624 (2016). https://doi.org/10.1007/s10811-016-0891-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-016-0891-7