Abstract
Interleukin-18(IL-18) plays a potential pathological role in rheumatoid arthritis (RA). The conclusions of the published reports on the relationship between single-nucleotide polymorphisms −607C/A (rs1946518) and −137G/C (rs187238) located in the IL-18 gene promoter and RA risk remain controversial. This meta-analysis was performed to evaluate the association between IL-18 gene promoter (−607A/C and −137C/G) polymorphisms and RA using (1) allele, (2) codominant, (3) dominant, and (4) recessive models. Literature search was conducted up to January, 2013, in PubMed, EMBASE, Spring-link, Web of Science, Wanfang (Chinese) and China National Knowledge Infrastructure (CNKI). A total of 10 studies from eight articles involving 2,662 cases and 2,168 controls for −607A/C polymorphism and 9 studies from six articles involving 1,331 cases and 1,468 controls for −137C/G polymorphism were considered in the meta-analysis. For the relationship of IL-18 −607A/C polymorphism with RA risk, significant association was observed in allele model (OR = 0.778, 95 % CI = 0.633–0.955) and dominant model (OR = 0.618, 95 % CI = 0.466–0.819). However, no significant association could be observed between −137C/G polymorphism and RA risk under all genetic models (allele model: OR = 0.940, 95 % CI = 0.777–1.138; codominant model: OR = 1.079, 95 % CI = 0.574–2.029; dominant model: OR = 0.913, 95 % CI = 0.779–1.069; recessive model: OR = 1.133, 95 % CI = 0.586–2.190). In the subgroup analysis by ethnicity, significant result was also found in Asian populations but not found in Caucasian populations for the relationship of IL-18 −607A/C polymorphism with RA risk; while no obvious association was found between IL-18 −137C/G polymorphism and RA risk. This meta-analysis indicates that IL-18 −607A/C polymorphism in promoter region may be associated with RA risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease characterized by multiple joints synovitis leading to joint destruction, disability and a poor quality of life. Both genetic and environmental factors play important roles in the pathogenesis of RA [1]. Large genome-wide association studies (GWAS) have identified more than 30 loci involved in RA pathogenesis [2]. Genetic factors including human leukocyte antigen gene (HLA) alleles such as HLA-DRB1*01, HLA-DRB1*13, HLA-DRB1*15, etc. [3], and non-HLA genes such as peptidylarginine deiminase type 4 gene (PADI4) [4], protein tyrosine phosphatase non-receptor 22 gene (PTPN22) [5], interleukin-23 receptor gene (IL-23R) [6], interleukin-1β gene (IL-1B) [7], macrophage migration inhibitory factor gene (MIF) [8], tumor necrosis factor alpha gene (TNF-α) [9] and CD40 [10] have been implicated in the pathogenesis of RA.
In RA, IL-18 is significantly elevated in sera, synovial fluid,and synovial tissues compared with those from osteoarthritic patients and healthy people [11], which is produced by synovial macrophages, synovial fibroblasts, endothelial cells, dendritic cells, articular chondrocytes, osteoblasts, and synovial fluid neutrophils [12]. IL-18 and other cytokines such as TNF-α, vascular endothelial growth factor (VEGF), IL-10 and IL-1 can activate nuclear factor-κB (NF-κB), promote expression of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS), and induce synthesis of prostaglandin E2 (PGE2) and nitric oxide (NO), which lead to synovial inflammation associated with arthrosis, swelling, hyperplasia, angiogenesis, and finally cartilage degeneration [13–16].
IL-18, known as IFN-gamma-inducing factor, is a cytokine of the IL-1 family. The human IL-18 gene is located on chromosome 11q22.2-q22.3, and is composed of six exons and five introns [12]. Many polymorphisms in the promoter region of the IL-18 gene have been identified. Recently, these polymorphisms have attracted widespread attention, especially the IL-18 gene promoter −607A/C and −137C/G polymorphisms. IL-18 polymorphisms in the promoter region has been reported to be associated with many kinds of diseases, such as asthma [17], type 1 diabetes [18], breast cancer [19], adult-onset Still’s disease [20], and virus infective disease [21]. Furthermore, some studies suggested that IL-18 gene promoter polymorphisms are associated with RA, but some studies failed to find any association. In this paper, to derive a more precise estimation of the relationship between IL-18 gene promoter −607A/C, −137C/G polymorphisms and RA, a meta-analysis was performed.
Materials and methods
Search strategy
We conducted a computer-based searches of PubMed, EMBASE, Spring-link, Web of Science, China National Knowledge Infrastructure (CNKI) and Wanfang (Chinese) to identify all studies examining the association of IL-18 promoter −607A/C (rs1946518) and −137C/G (rs187238) polymorphisms with RA susceptibility with the last report up to January 2013. The following keywords and subject terms were used for searching: “rheumatoid arthritis”, “RA”, “Interleukin-18”, “IL-18”, “genetic variant”, “genetic variation” and “polymorphism”. No language restrictions were applied.
Inclusion and exclusion criteria
The following inclusion criteria were used to select literatures for the meta-analysis: (1) investigation of the IL-18 promoter gene −607A/C and −137C/G polymorphisms and RA susceptibility, (2) only the case–control studies were considered, (3) the papers should clearly describe RA diagnoses and the sources of cases and controls, (3) the authors must offer the size of the sample, OR and their 95 % CI or the information that could help to infer the results in the papers. When a study reported the results on different subpopulation, we treated them as a separated study in the meta-analysis. The exclusion criteria were: (1) none-case–control studies, (2) studies that contained overlapping data, (3) review articles.
Data extraction
Two investigators reviewed and extracted information from all eligible publications independently, according to the inclusion and exclusion criteria listed above. An agreement was reached by discussion between the two reviewers whenever there was a conflict. The following items were collected from each study: first author’s surname, year of publication, study population (country), ethnicity, numbers of cases and controls for the studies, genotypic methods. The ethnicity was classified as Asian or Caucasian.
Statistical analysis
The Hard-Weinberg Equilibrium (HWE) was measured by Chi squared test for the control groups of each study. Studies with control groups that were not in HWE (P < 0.05) were excluded.
The strength of associations between IL-18 promoter −607A/C and −137C/G polymorphisms and RA risk were measured by ORs with 95 %CIs. Meta-analyses was performed using (1) allele model (−607A/C: A vs. C, and −137C/G: C vs. G), (2) codominant model (−607A/C: AA vs. CC, and −137C/G: CC vs. GG), (3) dominant model (−607A/C: AA + AC vs. CC, and −137C/G: CC + CG vs. GG), and (4) recessive model (−607A/C: AA vs. AC + CC, and −137C/G: CC vs. CG + GG). Heterogeneity among studies was assessed by the Chi squared-based Q-statistic test and I 2 Statistics, if P < 0.10 and I 2 > 50 %, was considered significant. When the effects were assumed to be homogenous, fixed-effects model was used (the Mantel–Haenszel method) [22]; otherwise, random-effects model (DerSimonian and Laird method) [23] was conducted. Meta-regression was performed with ethnicity, publication year, genotypic method and sample size to explore reasons for heterogeneity among the studies. Sensitivity analysis was performed to evaluate the influence of single studies on the overall estimate. Begg’s funnel plots [24] and Egger’s test [25] were used to assesse publication bias, and P < 0.05 was indicated significant.
All statistical tests performed in the study were two-tailed. All analyses were done using STATA 12.0 (STATA Corporation, College Station, TX, USA).
Results
Study characteristics
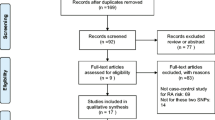
The combined search yielded 48 references. Study selection process was shown in Fig. 1. Two studies (Sivalingam’s [26] study which conducted in Chinese population and Rueda’s [27] study) with control groups of −607A/C that were not in HWE, so they were excluded. Finally, a total of 12 studies in nine articles were included with 3,041 patients and 2,646 controls in our meta-analysis [26–34]. For the −607A/C, a total of 10 studies from eight articles involved 2,662 cases and 2,168 controls. For the −137C/G, a total of 9 studies from six articles involved 1,331 cases and 1,468 controls. In our meta-analysis, 8 studies were conducted in Asian populations and 4 studies conducted in Caucasian populations. Of the articles, 6 were published in English and 4 in Chinese. In all studies, three genotypic methods were used, including Sequence-specific PCR (PCR-SSP), polymerase chain reaction-restriction fragment length polymorphism (PCR–RFLP) and Taqman. In Gracie’s [28] study, Taqman was used to detect −607A/C polymorphism and PCR-SSP was used to detect −137C/G polymorphism. The characteristics of those studies in the meta-analysis were listed in Table 1.
Meta-analysis results and heterogeneity analysis
The results of this meta-analysis were shown in Table 2. For IL-18 −607A/C polymorphism with RA risk, significant association was found in allele model (OR = 0.778, 95 % CI = 0.633–0.955) and dominant model (OR = 0.618, 95 % CI = 0.466–0.819). However, no significant association could be observed between −137C/G and RA risk under all genetic models (allele model: OR = 0.940, 95 % CI = 0.777–1.138; codominant model: OR = 1.079, 95 % CI = 0.574–2.029; dominant model: OR = 0.913, 95 % CI = 0.779–1.069; recessive model: OR = 1.133, 95 % CI = 0.586–2.190). In the subgroup analysis based on ethnicity, significant result was also found in Asian population under allele model (OR = 0.717, 95 % CI = 0.527–0.977) and dominant model (OR = 0.541, 95 % CI = 0.417–0.702), but not found in Caucasian population for the relationship of IL-18 −607A/C polymorphism with RA risk; while no obvious association was found in Asian populations or Caucasian populations between IL-18 −137C/G polymorphism with RA risk.
There was significant heterogeneity in most comparison models. To explore sources of heterogeneity, we did meta-regression analyse of ethnicity, publication year, genotypic method and sample size, but we failed to identify the sources of heterogeneity.
Sensitivity analysis
Sensitivity analyses were conducted to assess the influence of each individual study on the pooled OR by deleting any single study each time. When Sugiura’s [31] study was excluded, the pooled result was significant for AA versus CC model of −607A/C in Asian subgroup (OR = 0.406, 95 % CI = 0.172–0.958). In the dominant model for −607A/C, when two of Sivalingam’s [26] studies and Sugiura’s [31] study were included, the pooled result was also significant in total group (OR = 0.667, 95 % CI = 0.483–0.920) and in Asian subgroup (OR = 0.628, 95 % CI = 0.396–0.995). The result of this meta-analysis is not stable just in codominant model for −607A/C polymorphism, it doesn’t affect the conclusion.
Evaluation of publication bias
Begg’s funnel plot and Egger’s test were performed to assess publication bias of the studies. We found no asymmetry of the funnel plot, and Egger’s test suggested that no publication bias was detected in any comparison model (P > 0.05) except the dominant model for −607A/C when all 10 studies were included. But two of Sivalingam’s [26] studies and Sugiura’s [31] study were excluded, no publication bias was observed. Data were shown in Table 2.
Discussion
The imbalance of Th1/Th2 is suggested to be one important mechanism in the induction and development of RA. IL-18 promotes upregulation of Th1 cells and inhibits Th2 cells in RA patient resulting in a greater imbalance of Th1/Th2 and aggravating patient illnesses [11]. IL-18 protein expression is regulated by the IL-18 promoter gene [35]. Three single nucleotide polymorphisms (SNPs) were detected at positions −137C/G, −607A/C and −656G/T within the IL-18 promoter region. Two of these, −137C/G and −607A/C have been extensively studied, which are located at the binding sites for CREB transcriptional factors (cAMP response-element binding proteins) and the H4TF-1 nuclear factor respectively. Mutation at these two sites could influence IL-18 expression and change the production of the cytokine [36]. In the IL-18 gene promoter transcription activity assay, after stimulation with PMA/ionomicin, low promoter activity was observed for A and C alleles at positions −607 and −137, respectively. In contrast, higher promoter activity was observed for C and G alleles at similar positions [36]. Theoretical higher frequencies of A alleles at position −607 and/or higher frequencies of C alleles at position −137 would confer some protective effect against the development of RA.
Up to date, the association between IL-18 promoter polymorphisms (−607A/C and −137C/G) and RA risk had been studied. However, Some studies indicated an association between IL-18 gene polymorphisms and RA risk, but some studies failed to find any association. In this paper, we updated previous studies of the association between IL-18 promoter polymorphisms (−607A/C and −137C/G) and RA risk. Previously, Pan et al. [37] and Chen et al. [38] have done a meta-analysis: the results showed no association between the IL-18 promoter polymorphisms (−607A/C and −137C/G) and RA risk in Caucasian populations or Asian populations, but Chinese population carrying A allele of −607C/A significant associated with decreased risk for RA was observed by Chen et al. [38]. In this meta-analysis, we included more studies and found that individuals carrying A allele or AA/AC genotype of −607A/C polymorphism was significantly associated with decreased risk for RA in allele model (OR = 0.778, 95 % CI = 0.633–0.955) and dominant model (OR = 0.618, 95 % CI = 0.466−0.819). But for −137C/G polymorphism, we failed to find any association with RA risk under all genetic models. In addition, subgroup analysis by ethnicity, we also observed significant association between the IL-18 −607A/C polymorphisms and RA risk in Asian populations, but not in Caucasian populations. However, we found no association between the IL-18 −137C/G polymorphism and RA risk in Asian populations or Caucasian populations.
The results of this meta-analysis should be explained with caution because of some limitations. Firstly, the sample size of the included studies was not large enough. For −607A/C polymorphisms in the subgroup analysis, the number of studies about Caucasian populations was only three studies; for −137C/G polymorphisms, only 1,331 cases and 1,468 controls were included. Secondly, the heterogeneity among studies was observed in our meta-analysis. We did meta-regression analyse of ethnicity, publication year, genotypic method and sample size, but failed to identify the sources of heterogeneity. It might be due to environmental backgrounds and other reasons. Thirdly, the outcomes were based on unadjusted estimate effects, and the effect of gene–gene and gene-environment interactions were not addressed in this meta-analysis. Fourthly, although the Begg’s funnel plot and Egger’s test showed no publication bias, bias of selection may have occurred because only studies in English or Chinese were selected.
In conclusion, this meta-analysis provide evidence that IL-18 −607A/C polymorphisms is associated with RA risk. Large-sample studies of different ethnic groups with carefully matched cases and controls are needed to clarify the role of the two promoter gene polymorphisms and RA risk in the future.
References
Criswell LA, Saag KG, Mikuls TR, Cerhan JR, Merlino LA, Lum RF et al (2006) Smoking interacts with genetic risk factors in the development of rheumatoid arthritis among older caucasian women. Ann Rheum Dis 65(9):1163–1167
de Vries R (2011) Genetics of rheumatoid arthritis: time for a change! Curr Opin Rheumatol 23(3):227–232
Kurkó J, Besenyei T, Laki J, Glant TT, Mikecz K, Szekanecz Z (2013) Genetics of rheumatoid arthritis—a comprehensive review. Clin Rev Allergy Immunol 1:1–10
Iwamoto T, Ikari K, Nakamura T, Kuwahara M, Toyama Y, Tomatsu T et al (2006) Association between PADI4 and rheumatoid arthritis: a meta-analysis. Rheumatology 45:804–807
Ramirez M, Quintana G, Diaz-Gallo LM, Caminos J, Garces M, Cepeda L, Rondon F et al (2012) The PTPN22 C1858T variant as a risk factor for rheumatoid arthritis and systemic lupus erythematosus but not for systemic sclerosis in the Colombian population. Clin Exp Rheumatol 30(4):520–524
Zhai Y, Xu K, Huang F, Peng H, Feng CC, Zhu KK, Leng RX, Pan HF, Ye DQ (2012) Association of interleukin 23 receptor gene polymorphisms (rs10489629, rs7517847) with rheumatoid arthritis in European population: a meta-analysis. Mol Biol Rep 39(9):8987–8994
Harrison P, Pointon JJ, Chapman K, Roddam A, Wordsworth BP (2008) Interleukin-1 promoter region polymorphism role in rheumatoid arthritis: a meta-analysis of IL-1B -511A/G variant reveals association with rheumatoid arthritis. Rheuma- tology 47:1768–1770
Xie Q, Wang SC, Bian G, Zhan FL, Xie JK, Li J (2012) Association of MIF- 173G/C and MBL2 codon 54 gene polymorphisms with rheumatoid arthritis: a meta-analysis. Hum Immunol 73(9):966–971
Toonen EJ, Barrera P, Fransen J, de Brouwer AP, Eijsbouts AM, Miossec P et al (2012) Meta-analysis identified the TNFA −308G > A promoter polymorphism as a risk factor for disease severity in patients with rheumatoid arthritis. Arthritis Res Ther 14(6):R264
Orozco G, Eyre S, Hinks A, Ke X, Wilson AG, Bax DE et al (2010) Association of CD40 with rheumatoid arthritis confirmed in a large UK case-control study. Ann Rheum Dis 69:813–816
Shao XT, Feng L, Gu LJ, Wu LJ, Feng TT, Yang YM, Wu NP, Yao HP (2009) Expression of interleukin-18, IL-18BP, and IL-18R in serum, synovial fluid, and synovial tissue in patients withrheumatoid arthritis. Clin Exp Med 9(3):215–221
Volin Michael V, Koch Alisa E (2011) Interleukin-18: a mediator of inflammation and angiogenesis in rheumatoid arthritis. J Interf Cytokine Res 31(10):745–751
Sivalingam SP, Thumboo J, Vasoo S, Thio ST, Tse C, Fong KY (2007) In vivo pro- and anti-inflammatory cytokines in normal and patients with rheumatoid arthritis. Ann Acad Med Singapore 36(2):96–99
Lee EY, Seo M, Juhnn YS, Kim JY, Hong YJ, Lee YJ, Lee EB, Song YW (2011) Po-tential role and mechanism of IFN-gamma inducible protein-10 on receptor activa- tor of nuclear factor kappa-B ligand (RANKL) expression in rheumatoid arthritis. Arthritis Res Ther 27;13(3):R104
Wang B, Ma L, Tao X, Lipsky PE (2004) Triptolide, an active component of the Chinese herbal remedy tripterygium wilfordii hook F, inhibits production of nitric oxide by decreasing inducible nitric oxide synthase gene transcription. Arthritis Rheum 50(9):2303–2995
Yao H, Zhou J, Li D, Wu N, Bader A, Höxtermann S, Altmeyer P, Brockmeyer NH (2005) FK506 enhances triptolide-induced down-regulation of cyclooxygenase-2, inducible nitric oxide synthase as well as their products PGE2 and NO in TNF-alpha- stimulated synovial fibroblasts from rheumatoid arthritic patients. Eur J Med Res 29 10(3):110–116
Ma Y, Zhang B, Tang RK, Liu Y, Peng GG (2012) Interleukin-18 promoter poly- morphism and asthma risk: a meta-analysis. Mol Biol Rep 39(2):1371–1376
Kretowski A, Mironczuk K, Karpinska A, Bojaryn U, Kinalski M, Puchalski Z, Kinalska I (2002) Interleukin-18 promoter polymorphisms in type 1 diabetes. Diabetes 51(11):3347–3349
Khalili-Azad T, Razmkhah M, Ghiam AF, Doroudchi M, Talei AR, Mojtahedi Z, Ghaderi A (2009) Association of interleukin-18 gene promoter polymorphisms with breast cancer. Neoplasma 56(1):22–25
Sugiura T, Kawaguchi Y, Harigai M, Terajima-Ichida H, Kitamura Y, Furuya T et al (2002) Association between adult-onset Still’s disease and interleukin-18 gene polymorphisms. Genes Immun 3:394–399
Migita K, Sawakami-Kobayashi K, Maeda Y, Nakao K, Kondoh S, Sugiura M et al (2009) Interleukin-18 promoter polymorphisms and the disease progression of Hepatitis B virus-related liver disease. Transl Res 153(2):91–96
Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22:719–748
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50:1088–1101
Egger M, Davey SG, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. Br Med J 315:629–634
Sivalingam SP, Yoon KH, Koh DR, Fong KY (2003) Single-nucleotide poly- morphisms of the interleukin-18 gene promoter region in rheumatoid arthritis patients: protective effect of AA genotype. Tissue Antigens 62(6):498–504
Rueda B, González-Gay MA, Mataran L, López-Nevot MA, Martín J (2005) Inter- leukin-18-promoter polymorphisms are not relevant in rheumatoid arthritis. Tissue Antigens 65(6):544–548
Huang XZ, Zhuang JH, Ren YG, Zhou LJ, Zhou Q (2007) Association of inter- leukin-6 and interleukin-18 gene polymorphism with rheumatoid arthritis in Guang- dong Han population. Nan Fang Yi Ke Da Xue Xue Bao 27(11):1661–1664
Ying B, Shi Y, Pan X, Song X, Huang Z, Niu Q, Cai B, Wang L (2011) Association of polymorphisms in the human IL-10 and IL-18 genes with rheumatoid arthritis. Mol Biol Rep 38(1):379–385
Ping SHI, Lin-sheng XIAO, Ying FEI, Fang YU (2011) Polymorphisms of IL-18 gene in rheumatoid arthitis and its association with rheumatoid factor or anti-cyclic citrulliated peptide antibody in Han population of Guizhou of China. Clinical Focus 26(4):289–295
Sugiura T, Kawaguchi Y, Ikari K, Ichida H, Kawamoto M, Momohara S, Hara M, Yamanaka H (2011) Interleukin-18 promoter polymorphisms in Japanese patients with rheumatoid arthritis: protective effect of the T allele and T/T genotype at rs360722. Mod Rheumatol 21(4):359–364
Xin-qiang SONG, Zhao-hui YI, Xue-qun WANG, Rong-kun LIU, Si-he ZHANG (2012) Detection of IL-18 gene promoter −607C/C polymorphism in han population with rheumatoid arthritis in the South of Henan Provine. J Xinyang Norm Univ Nat Sci Ed 25(3):329–332
Gracie JA, Koyama N, Murdoch J, Field M, McGarry F, Crilly A et al (2005) Disease association of two distinct interleukin-18 promoter polymorphisms in Caucasian rheumatoid arthritis patients. Genes Immun 6:211–216
Pawlik A, Kurzawski M, Czerny B, Gawronska-Szklarz B, Drozdzik M, Herczyns- ka M (2006) Interleukin-18 promoter polymorphism in patients with rheumatoid arthritis. Tissue Antigens 67(5):415–418
Khripko OP, Sennikova NS, Lopatnikova JA, Khripko JI, Filipenko ML, Khrapov EA et al (2008) Association of single nucleotide polymorphisms in the IL-18 gene with production of IL-18 protein by mononuclear cells from healthy donors. Mediators Inflamm 2008:1–6
Giedraitis V, He B, Huang WX, Hillert J (2001) Cloning and mutation analysis of the human IL-18 promoter: a possible role of polymorphisms in expression regulation. J Neuroimmunol 112(1–2):146–152
Pan H-F, Leng R-X, Ye D-Q (2011) Lack of association of interleukin-18 gene promoter −607 A/C polymorphism with susceptibility to autoimmune diseases: a meta- analysis. Lupus 20:945–951
Chen S, Jiang F, Ren J, Liu J, Meng W (2012) Association of IL-18 polymorphisms with rheumatoid arthritis and systemic lupus erythematosus in Asianpopulations: a meta-analysis. BMC Med Genet 13:107
Acknowledgments
We thank all authors of primary studies included in our meta-analyses. There was no funding support
Conflict of interest
We declare that we have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cai, LP., Zhou, LJ., Lu, SY. et al. Association of IL-18 promoter gene polymorphisms with rheumatoid arthritis: a meta-analysis. Mol Biol Rep 41, 8211–8217 (2014). https://doi.org/10.1007/s11033-014-3723-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-014-3723-3