Abstract
Rheumatoid arthritis (RA) is a chronic immunological disease, the invasive monocytes/macrophages and lymphocytes present in synovial cells and synovial tissue produce many cytokines and inflammatory mediators by paracrine signaling and plays a role in the pathological progress in RA patients. Interleukin-18 (IL-18) is a representative proinflammatory factor and displays multiple biological functions. This study was designed to investigate the expression of IL-18 and its receptor (IL-18R) and IL-18 binding protein (IL-18BP) in serum, synovial fluid, and synovial tissue of patients with RA, and to identify the pathological role of IL-18 in RA. Serum, synovial fluid, and synovial tissue were obtained from RA patients. Samples from patients with osteoarthritis and healthy people were obtained as controls. Levels of IL-18, IL-18BP, and PGE2 in serum and synovial fluid were measured by enzyme-linked immunosorbent assay. The biological activity of IL-18 in serum and synovial fluid was detected on the basis of IFN-γ secretion from IL-18-responding human myelomonocytic KG-1 cells. NO in serum and synovial fluid was detected by Griess reaction. Expression of IL-18, IL-18BP, IL-18R, iNOS, and COX-2 mRNA and protein in synovial tissues was determined by quantitative reverse transcriptase polymerase chain reaction and Western blot. This study shows the expression levels of IL-18, IL-18R, iNOS, COX-2, and the biological activity of IL-18 in both serum and synovial fluid and tissue of patients with RA were significantly increased compared with the corresponding samples from the two control groups. In addition, expression of IL-18BP in patients with RA was decreased compared with samples from the two control groups. In conclusion, the overexpression of IL-18 and IL-18R may play an important role in the pathogenesis of RA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a chronic immunological disease involving polyarticular synovitis that leads to the formation of rheumatoid pannus and subsequent erosion of articular cartilage and bone. Accumulating evidence suggests that the invasive monocytes/macrophages and lymphocytes present in synovial cells and synovial tissue produce many cytokines and inflammatory mediators by paracrine signaling and plays a role in the pathological progress in RA patients [1–4]. Recent studies have demonstrated monocyte Th1/Th2 imbalances in RA patients’ synovial fluid. The secretion of cytokines such as IFN-γ and interleukin-12 (IL-12) from inflammatory Th1 cells was increased; conversely, anti-inflammatory Th2 cell and their cytokines were relatively decreased, including IL-4 and IL-10 [5]. This is considered to be important in the pathogenesis of RA patients. IL-18, also known as IFN-gamma-inducing factor, is a cytokine of the IL-1 family [6] that was cloned from liver in toxic shock rats by Okamara [7] in 1995. IL-18 displays multiple biological functions and is a representative Th1 cytokine. It induces cells to synthesize IFN-γ, enhances cell toxicity mediated by FasL, and induces the expression and synthesis of TNF-α, IL-1β, and other chemotactic factors [8, 9]. IL-18 has a direct effect on arthritic chondrocytes and synovial tissue, and plays a pivotal role in articular cartilage degeneration [10]. A recent study also showed that IL-18 plays a unique role in inducing the secretion of angiogenic SDF-1/CXCL12, MCP-1/CCL2, and VEGF in RA synovial tissue fibroblasts via distinct signaling intermediates [11]. Activated and increased IL-18 in RA patients induces cytokine production by natural killer cells, macrophages, and neutrophils, promotes angiogenesis and reverses endothelial cell apoptosis, and retards fibroblast apoptosis and modulates function in varied tissue cell lineages including keratinocytes, osteoclasts, and chondrocytes [12]. As a proinflammatory factor, IL-18 stimulates NO expression and induces synovial cartilage cells to express iNOS, COX-2, and IL-6, resulting in a great deal of NO and PGE2 production, inflammatory injury, and articular recessive degeneration [13, 14]. However, some dispute remains regarding the function of IL-18 in RA pathogenesis. Some research has reported that the peripheral blood mononuclear cells (PBMCs) from RA patients reveal decreased IFN-γ production in response to IL-12 and IL-18 when compared with healthy subjects’ PBMCs in vitro [15]. In order to clarify the function of IL-18 during the RA process, we investigated levels of IL-18, IL-18BP, IL-18 receptor (IL-18R), iNOS, and COX-2 in serum, synovial fluid, and synovial tissue in samples from RA patients, osteoarthritis (OA) patients, and healthy controls. This study discusses the function of IL-18 in RA.
Materials and methods
Object
Twelve samples of synovial tissue were taken from RA patients who had an arthral replacement or synovial excision operation in The First Affiliated Hospital of Zhejiang University and that gave informed consent. All patients were diagnosed according to the standards defined by the American College of Rheumatology in 1987. Control samples came from eight OA patients, and seven patients who underwent operations for injury. Serum, synovial fluid, and synovial tissue were accumulated and preserved at −80°C for further use.
ELISA for IL-18, IL-18BP, PGE2, and NO in serum and synovial fluid
Interleukin-18, IL-18BP, and PGE2 levels in serum and synovial fluid were assayed by a specific enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. The detection of total nitrite was then determined as a colored azo-dye product of the Griess reaction that absorbs visible light at 540–570 nm.
Assay for bioactivity of IL-18
The bioactivity of IL-18 in serum and synovial fluid was detected by ELISA (R&D Systems, Minneapolis, MN, USA), based on IFN-γ secretion from IL-18-responding human myelomonocytic KG-1 cells. The protocol was in accordance with the method reported by Yamamura [16]. IL-18 activity in the samples was determined from the difference in the levels of IFN-γ between those cultures treated with and without anti-IL-18 monoclonal antibody (mAb; R&D Systems, 2 μg/ml).
Quantitative real-time polymerase chain reaction assay
To assay mRNA expression of IL-18, IL-18R, IL-18BP, iNOS, and COX-2 in synovial tissue, total RNA was isolated from 50 mg of tissue using TRIzol reagent (Invitrogen, CA, USA) according to the manufacturer’s protocol. All procedures were carried out using RNase-free reagents. RNA (1 μg) was reverse transcribed into cDNA using SuperScript II reverse transcriptase (Invitrogen) in a 20-μl reaction volume. Quantitative reverse transcriptase polymerase chain reaction (QRT-PCR) was performed using LightCycler technology (Roche Molecular Biochemicals, Mannheim, Germany) with SYBR Green I detection. In all assays, cDNA was amplified using a standardized program (a 10-min denaturing step; 50 cycles of 5 min at 95°C, 15 s at 65°C, and 15 s at 72°C; melting point analysis in 0.1°C steps; and a final cooling step). Each LightCycler capillary was loaded with 1.5 μl DNA Master Mix, 1.8 μl MgCl2 (25 mM), 10.1 μl H2O, and 0.4 μl of each primer (10 μM). The final amount of cDNA per reaction corresponded to 5.0 ng of RNA used for reverse transcription. Relative quantification of target gene expression was performed using a mathematical model, as recommended by Roche Molecular Biochemicals. The results are expressed as the ratios of the genes assayed to β-actin. The primer sequences are given in Table 1. The reaction specificity was confirmed by 1.5% agarose gel electrophoresis of products after real-time PCR and melting curve analysis.
Western blotting for IL-18, IL-18R, IL-18BP, iNOS, and COX-2
Synovial tissues were collected and lysed in a lysis buffer [20 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 1% NP-40, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM leupeptin, 1 mM phenyl methylsulfonyl fluoride] for 30 min at 4°C. The insoluble material was then removed by centrifugation at 8,000×g for 10 min at 4°C. The concentration of protein in each cell lysate was determined using a BCA protein assay kit (Pierce, IL, USA) with bovine serum albumin (BSA) as the standard. An identical amount of protein (40 μg) from each sample was loaded onto a 10% SDS-PAGE gel and electrophoresed at 200 V. Proteins were then transferred to nitrocellulose membranes (0.45 μm, Millipore, MA, USA) that were blocked with 5% BSA (Sigma, LA, USA) in TBS (25 mM Tris–HCl, 150 mM sodium chloride, pH 7.2) for 1 h at room temperature. Blots were incubated with anti-IL-18, anti-IL-18Rα, anti-IL-18BPα, anti-iNOS, or anti-COX-2 primary antibody (R&D Systems) or anti-β-actin specific primary antibody (Santa Cruz, CA, USA ) at 1:800 dilution at room temperature for 2 h. Blots were washed three times and then incubated with horseradish peroxidase-conjugated secondary antibody (1:2,000 dilution) for 1 h at room temperature. All blots were developed using enhanced chemiluminescence reagents (SuperSignal Dura Kit, Pierce) according to the manufacturer’s instructions. The signals were captured on X-ray film.
Data and statistical analysis
The data presented are mean ± SD. Significance of the differences between the experimental conditions was determined by a paired two-sample Student’s t-test.
Results
Levels of IL-18, IL-18BP, PGE2, and NO in serum and synovial fluid
Interleukin-18, IL-18BP, PGE2, and NO concentrations in serum and synovial fluid were measured in RA patients, OA patients, and healthy controls (Table 2). IL-18, PGE2, and NO levels in the RA group were significantly higher than those in the OA group and healthy controls, and IL-18BP levels were lower than those in the OA group and healthy controls, both in serum and synovial fluid (P < 0.01). Further, IL-18, PGE2, and NO levels in synovial fluid of RA patients were significantly higher than those in the serum of RA patients. Additionally, IL-18, PGE2, and NO levels in serum and synovial fluid of OA patients were higher than those in healthy controls (P < 0.01).
Bioactivity of IL-18 in serum and synovial fluid
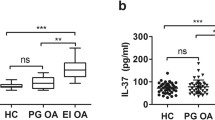
The bioactivity of IL-18 was determined by IFN-γ levels in supernatants of human myelomonocytic KG-1 cells induced by IL-18. Concentrations of IFN-γ in culture supernatants were measured by ELISA. Both in serum and synovial fluid, samples from RA patients were able to induce IFN-γ production by KG-1 cells more strongly than were those from OA patients and healthy controls (P < 0.01), as shown in Fig. 1. The mean level of IL-18 bioactivity in RA synovial fluid was estimated by taking the difference in IFN-γ levels between cultures with and without anti-IL-18 antibodies. Further, the bioactivity of IL-18 in serum and synovial fluid of OA patients was higher than in healthy controls (P < 0.01; Fig. 1).
Bioactivity of IL-18 in serum and synovial fluid. The serum and synovial fluid were collected for the assay. The bioactivity of IL-18 was detected based on IFN-γ secretion from IL-18-responding human myelomonocytic KG-1 cells. Concentrations of IFN-γ in culture supernatants were measured by ELISA. IL-18 bioactivity in each sample was determined by the difference in IFN-γ levels between cultures with and without anti-IL-18 antibody. Asterisk: compared with OA and healthy groups, P < 0.01; filled triangle: compared with healthy group, P < 0.01, by Student’s t-test
IL-18, IL-18BP, IL-18R, iNOS, and COX-2 mRNA expression in synovial tissue
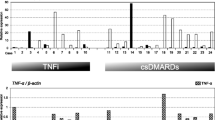
We performed QRT-PCR to determine the expression of IL-18, IL-18BP, IL-18R, iNOS, and COX-2 mRNA in synovial tissue among RA patients, OA patients, and healthy controls. We found that there were significant increases in mRNA expression of iNOS, COX-2, IL-18, and IL-18R in synovial tissue of RA patients, while IL-18BP expression was decreased in RA patients, as compared with those of OA patients and healthy controls. The mRNA expression of iNOS, COX-2, IL-18, and IL-18R in synovial tissue of OA patients was higher than those in healthy controls, while in OA patients, IL-18BP decreased (Fig. 2).
Detection of mRNA expression of iNOS, COX-2, IL-18, IL-18BP, and IL-18R in synovial tissue by real-time quantitative RT-PCR. Total RNA was isolated from synovial tissue using TRIzol reagent. Quantitative RT-PCR was performed by LightCycler technology with SYBR Green I detection. The reaction specificity was confirmed by 1.5% agarose gel electrophoresis of products after real-time PCR and melting curve analysis. a PCR products were separated on a 1.5% agarose gel with ethidium bromide in TBE buffer. b Expression of iNOS, COX-2, IL-18, IL-18BPα, and IL-18Rα mRNA in synovial tissue was detected by LightCycler technology with SYBR Green I detection. The results are expressed as the ratios of iNOS, COX-2, IL-18, IL-18BPα, and IL-18Rα to β-actin
IL-18, IL-18R, IL-18BP, iNOS, and COX-2 protein expression in synovial tissue
In order to illustrate protein levels in RA, we performed Western blots to detect the expression of IL-18, IL-18R, COX-2, and iNOS in synovial tissue (Fig. 3). We found IL-18, IL-18R, COX-2, and iNOS levels were elevated, while IL-18BP levels were decreased, as compared with the synovial tissue of OA patients and healthy controls. We also found IL-18, IL-18R, COX-2, and iNOS levels in OA patients were higher than those in healthy controls, while IL-18BP levers were slightly lower (Fig. 3).
Western blot analysis of protein expression of iNOS, COX-2, IL-18, IL-18BP, and IL-18R in synovial tissue. Synovial tissue was collected and lysed in a lysis buffer. Tissue protein (40 μg/sample) was subjected to Western blot analysis using a primary antibody specific to iNOS, COX-2, IL-18, IL-18BPα, or IL-18Rα, followed by incubation with HRP-conjugated secondary antibody. Actin staining was used as the loading control. The reaction was visualized by ECL, and signals were captured on an X-ray film
Discussion
Recent data has been presented to indicate a critical role for IL-18 in RA. T cells and macrophages invading the synovium or in the synovial fluid are the chief cellular targets of IL-18 in RA [17]. IL-18 was produced by macrophage-like cells after IL-1β-converting enzyme (ICE) induction [9]. Its biochemical effects on anticancer, anti-inflammatory, and immune regulation are important for human medicine [18]. As a Th1 relative cytokine, IL-18 highly correlates with immune diseases.
Olee [13] reported that IL-18 had an effect on chondrocytes and inhibited cartilage cell hyperplasia induced by TGF-β, thereby increasing NO expression and stimulating synovial cartilage cells to express iNOS, COX-2, and IL-6. The presence of these mediators resulted in a great deal of NO and PGE2 production, which furthered the inflammatory injury and articular recessive degeneration. Leung’s studies [19] revealed that IL-18 promoted RA’s occurrence induced by collagen, and that its mechanism was different than, but cooperated with, IL-12. Moreover, Wei [20] established the rat model of collagen-induced arthritis and showed that the inflammatory degree was obviously lower in IL-18 gene deficient rats compared with wild rats. Cho et al. [21] reported that IL-18 enhanced the production of VEGF, one of the angiogenic factors in RA, and that AP-1 was the major signal molecule involved in VEGF production by IL-18. Together, these data support the supposition that IL-18 plays an important role in the pathogenesis of RA.
Our study demonstrates an increase in both the protein concentration and biological activity of IL-18 in serum and synovial fluid, and in the expression of IL-18 in synovial tissue, compared to the OA patients and the control group. These results coincide with research by Munakata [22] and Möller [23]. We also found that levels of IL-18 in synovial fluid were higher than in serum, which supports the report of Gracie [10], who found that synovial fibroblasts and invasive monocytes/macrophages in synovial tissue were the main source of synovial IL-18. We detected that the IL-18R concentration in serum and synovial fluid and the expression of IL-18R in synovial tissue were much higher than in OA patients and the control group. IL-18 exerts its biological effects via its receptor complex. The IL-18R complex is a heterodimer containing an α chain responsible for extracellular binding of the IL-18 α chain and a non-binding signal-transducing β chain, both members of the IL-1R family [24]. On binding of IL-18 to IL-18Rα, IL-18Rβ is recruited and induces signaling pathways. Kawashima and Miossec [25] revealed that in a culture system including T cells, IL-18R mRNA was constitutively expressed by RA synovium cells, and IL-12 could induce the expression from PBMCs or RA synovium cells. In contrast, RA synoviocytes did not express IL-18Rβ mRNA, even after stimulation with IL-1 or IL-12. Thus, involvement of IL-18 in the pathogenesis and joint destruction of RA appears to be through T cell or macrophage activation. Some reports showed a massive production of INF-γ by T cells as a result of upregulation of IL-18R expression on naive T cells, Th1 cells and B cells by IL-12 [10, 26]. In addition to IL-18, we tested the expression of iNOS and COX-2 mRNA and protein in synovial tissue and the concentrations of NO and PGE2 in serum and synovial fluid. The results demonstrate that levels in RA patients were significantly higher than those in other groups. Our results are in accordance with Olee [13] and Gracie et al. [10], whose report showed NO is up-regulated in RA SM in vitro by IL-18.
Thus, we propose the role of IL-18 in RA is as follows: Pro-IL-18 is cleaved by ICE (caspase 1) to yield an active IL-18 glycoprotein. IL-18, in marked synergy with IL-12 and IL-15, promotes IFN-γ, TNF-α and GM-CSF production [12]. IL-18 expression is up-regulated in turn in FLS, chondrocytes, synovial cells, and infiltrated monocytes/macrophages by IL-1β and TNF-α in synovial fluid. This is thus a positive feedback loop that could explain the cytokine predominance in RA [27]. A part of the excess expression of IL-18 is released into the blood circulation, and produces biological effects by combining with its receptor. IL-18 induces overexpression of IFN-γ by T cells, which might result in the gene expression of iNOS and COX-2 by chondrocytes and FLS directly or indirectly. As a result, there is overproduction of NO and PGE2. All these factors together induce abnormal hyperplasia of synovial tissue and cartilage degeneration. Moreover, the consistency of IL-18 in serum and synovial tissue of RA patients promotes upregulation of Th1 cells and inhibits Th2 cells in blood and synovial fluid, which results in a greater imbalance of Th1/Th2 and aggravates patient illnesses. Chemoattraction of T cells by IL-18 was demonstrated both in vitro and in vivo. A recent study demonstrated that IL-18 induced the expression of chemotactic factors in RA such as CXC, chemotactic factors receptor-5 (CXCR-5), and macrophage inflammatory protein-1. This resulted in a great deal of inflammatory cells locally invading, and the development of inflammation [28]. IL-18 alone or in combination with IL-12 induces T cell adhesion to inflamed sites to regulate early inflammatory events [29]. IL-18 increased the proportion of T cells in polarized morphology in vitro and stimulated their subsequent invasion into collagen gels in an IL-18 concentration-gradient-dependent manner. Furthermore, RA synovial CD4+ but not CD8+ T cells also migrate to IL-18. Injection of IL-18 into the footpad of DBA/1 mice led to local accumulation of inflammatory cells. IL-18 can contribute to the development of an acquired immune response and the maintenance of chronic immune stimulation in diseases such as RA by promoting chemotaxis of activated T cells [17].
Research has shown that a IL-18 mAb, a dissoluble IL-18 receptor (sIL-18R), and IL-18 binding protein (IL-18BP) have been applied in the therapy of some immunological diseases and RA, and can control disease development [16, 30–33]. Here, we detected the levels of IL-18BP in synovial fluid, serum, and IL-18BP expression in synovial tissue. We found that IL-18BP decreased in serum, synovial fluid, and synovial tissue along with the increases of IL-18 and IFN-γ. An in vitro study [34] indicated that production of IL-18BP did not increase without stimulation of IL-12 in the culture supernatants of PBMCs and synovial tissue from RA patients. As a constitutively secreted protein, IL-18BP is able to bind IL-18 with high affinity, providing a potential mechanism whereby IL-18 activity could be regulated. IL-18BP inhibits IL-18-induced IFN-γ and IL-8 production and NF-κB activation in vitro, as well as LPS-induced IFN-γ production in vivo [35, 36]. However, Möller et al. [37] found IFN-γ markedly up-regulated IL-18BP mRNA levels in long-term cultured FLS. Conditioned media from IFN-γ-stimulated FLS cultures reduced IL-12/IL-18-dependent IFN-γ production by PBMCs. Combined with our results, it is possible that a negative feedback mechanism of IFN-γ-induced IL-18BP inhibiting IL-18 could repress established inflammation. IL-18BP isoforms are capable of binding to and neutralizing IL-18 [38], and can inhibit IL-18 activity in vitro [39]. All these results make it feasible that expression of IL-18BP in RA patients could inhibit IL-18 to reverse pathological progression. Therapeutic administration of IL-18BP in patients may modulate the cytokine balance, thereby ameliorating established arthritis, as observed in an RA animal model [40]. Our previous research showed that combination treatment with IL-18BP and IL-4 has significant modifying effects on the Th1/Th2 imbalance by increasing serum concentrations of IL-4 and IL-10 but greatly decreasing serum concentrations of IFN-γ, TNF-α, and IL-18 in murine CIA that is produced by local overexpression of IL-18BP and IL-4. This indicates that co-treatment with IL-18BP and IL-4 is a promising potential therapy for RA [41].
In conclusion, our research indicates that IL-18 might play an important role during RA progression. Overexpression of IL-18, IL-18R, PGE2, and NO in serum, synovial fluid, and synovial tissue, and an imbalance between serum/synovial fluid and synovial tissue, might be responsible for RA. Moreover, IL-18 might be a target for binding with IL-18BP, a neutralizing protein of IL-18, that could control the progression of RA.
References
Kinne RW, Bräuer R, Stuhlmüller B, Palombo-Kinne E, Burmester GR (2000) Macrophages in rheumatoid arthritis. Arthritis Res 2:189–202
Moss ST, Hamilton JA (2000) Proliferation of a subpopulation of human peripheral blood monocytes in the presence of colony stimulating factors may contribute to the inflammatory process in diseases such as rheumatoid arthritis. Immunobiology 202:18–25
Sato K, Takayanagi H (2005) Regulation of osteoclastogenesis by activated T cells. Nippon Rinsho 63:1529–1532
Komano Y, Nanki T, Hayashida K, Taniguchi K, Miyasaka N (2006) Identification of a human peripheral blood monocyte subset that differentiates into osteoclasts. Arthritis Res Ther 8:R152
Yudoh K, Matsuno H, Nakazawa F, Yonezawa T, Kimura T (2000) Reduced expression of the regulatory CD4+ T cell subset is related to Th1/Th2 balance and disease severity in rheumatoid arthritis. Arthritis Rheum 43:617–627
Nakamura K, Okamura H, Wada M, Nagata K, Tamura T (1989) Endotoxin-induced serum factor that stimulates gamma interferon production. Infect Immun 57:590–595
Okamara H, Tsutsui H, Komatsu T (1995) Cloning of a new cytokine that induces IFN-γ production by T cells. Nature 378:88–91
Kohno K, Kataoka J, Ohtsuki T, Suemoto Y, Okamoto I, Usui M, Ikeda M, Kurimoto M (1997) IFN-gamma-inducing factor (IGIF) is a costimulatory factor on the activation of Th1 but not Th2 cells and exerts its effect independently of IL-12. J Immunol 158:1541–1550
Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming MA, Hayashi N, Higashino K, Okamura H, Nakanishi K, Kurimoto M, Tanimoto T, Flavell RA, Sato V, Harding MW, Livingston DJ, Su MS (1997) Activation of interferon-gamma-inducing factor mediated by interleukin-1 beta converting enzyme. Science 275:206–209
Gracie JA, Forsey RJ, Chan WL, Gilmour A, Leung BP, Greer MR, Kennedy K, Carter R, Wei XQ, Xu D, Field M, Foulis A, Liew FY, McInnes IB (1999) A proinflammatory role for IL-18 in rheumatoid arthritis. J Clin Invest 104:1393–1401
Amin MA, Mansfield PJ, Pakozdi A, Campbell PL, Ahmed S, Martinez RJ, Koch AE (2007) Interleukin-18 induces angiogenic factors in rheumatoid arthritis synovial tissue fibroblasts via distinct signaling pathways. Arthritis Rheum 56:1787–1797
Gracie JA, Robertson SE, McInnes IB (2003) Interleukin-18. J Leukoc Biol 73:213–224
Olee T, Hashimoto S, Quach J, Lotz M (1999) IL-18 is produced by articular chondrocytes and induces proinflammatory and catabolic responses. J Immunol 162:1096–1100
Hirth A, Skapenko A, Kinne RW, Emmrich F, Schulze-Koops H, Sack U (2002) Cytokine mRNA and protein expression in primary-culture and repeated-passage synovial fibroblasts from patients with rheumatoid arthritis. Arthritis Res 4:117–125
Kawashima M, Miossec P (2004) Decreased response to IL-12 and IL-18 of peripheral blood cells in rheumatoid arthritis. Arthritis Res Ther 6:R39–R45
Yamamura M, Kawashima M, Taniai M, Yamauchi H, Tanimoto T, Kurimoto M, Morita Y, Ohmoto Y, Makino H (2001) Interferon-gamma-inducing activity of interleukin-18 in the joint with rheumatoid arthritis. Arthritis Rheum 44:275–285
Dai SM, Shan ZZ, Xu H, Nishioka K (2007) Cellular targets of interleukin-18 in rheumatoid arthritis. Ann Rheum Dis 66:1411–1418
Ye XJ, Tang B, Ma Z, Kang AH, Myers LK, Cremer MA (2004) The role of interleukin-18 in collagen-induced arthritis in the BB rat. Clin Exp Immunol 136:440–447
Leung BP, McInnes IB, Esfandiari E, Wei XQ, Liew FY (2000) Combined effects of IL-12 and IL-18 on the induction of collagen-induced arthritis. J Immunol 164:6495–6502
Wei XQ, Leung BP, Arthur HM, McInnes IB, Liew FY (2001) Reduced incidence and severity of collagen-induced arthritis in mice lacking IL-18. J Immunol 166:517–521
Cho ML, Jung YO, Moon YM, Min SY, Yoon CH, Lee SH, Park SH, Cho CS, Jue DM, Kim HY (2006) Interleukin-18 induces the production of vascular endothelial growth factor (VEGF) in rheumatoid arthritis synovial fibroblasts via AP-1-dependent pathways. Immunol Lett 103:159–166
Munakata T, Uzuki M, Shimamura T, Sawai T (2001) Dynamics of interleukin (IL)-18 in serum, synovial fluid and synovial membrane in the patients with rheumatoid arthritis. Ryumachi 41:625–634
Möller B, Kukoc-Zivojnov N, Kessler U, Rehart S, Kaltwasser JP, Hoelzer D, Kalina U, Ottmann OG (2001) Expression of interleukin-18 and its monokine-directed function in rheumatoid arthritis. Rheumatology 40:302–309
Torigoe K, Ushio S, Okura T, Kobayashi S, Taniai M, Kunikata T, Murakami T, Sanou O, Kojima H, Fujii M, Ohta T, Ikeda M, Ikegami H, Kurimoto M (1997) Purification and characterization of the human interleukin-18 receptor. J Biol Chem 272:25737–25742
Kawashima M, Miossec P (2003) Heterogeneity of response of rheumatoid synovium cell subsets to interleukin-18 in relation to differential interleukin-18 receptor expression. Arthritis Rheum 48:631–637
Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H (2001) Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol 19:423–474
Gracie JA (2004) Interleukin-18 as a potential target in inflammatory arthritis. Clin Exp Immunol 136:402–404
Morel JC, Park CC, Kumar P, Koch AE (2001) Interleukin-18 induces rheumatoid arthritis synovial fibroblast CXC chemokine production through NFkappaB activation. Lab Invest 81:1371–1383
Ariel A, Novick D, Rubinstein M, Dinarello CA, Lider O, Hershkoviz R (2002) IL-12 and IL-18 induce MAP kinase-dependent adhesion of T cells to extracellular matrix components. J Leukoc Biol 72:192–198
Holmes S, Abrahamson JA, Al-Mahdi N, Abdel-Meguid SS, Ho YS (2000) Characterization of the in vitro and in vivo activity of monoclonal antibodies to human IL-18. Hybridoma 19:363–367
Dinarello CA (2000) Targeting interleukin-18 with interleukin-18 binding protein. Ann Rheum Dis 59:i17–i20
Bresnihan B, Roux-Lombard P, Murphy E, Kane D, FitzGerald O, Dayer JM (2002) Serum interleukin 18 and interleukin 18 binding protein in rheumatoid arthritis. Ann Rheum Dis 61:726–729
McInnes IB, Liew FY, Gracie JA (2005) Interleukin-18: a therapeutic target in rheumatoid arthritis? Arthritis Res Ther 7:38–41
Kawashima M, Novick D, Rubinstein M, Miossec P (2004) Regulation of interleukin-18 binding protein production by blood and synovial cells from patients with rheumatoid arthritis. Arthritis Rheum 50:1800–1805
Novick D, Kim SH, Fantuzzi G, Reznikov LL, Dinarello CA, Rubinstein M (1999) Interleukin-18 binding protein: a novel modulator of the Th1 cytokine response. Immunity 10:127–136
Aizawa Y, Akita K, Taniai M, Torigoe K, Mori T, Nishida Y, Ushio S, Nukada Y, Tanimoto T, Ikegami H, Ikeda M, Kurimoto M (1999) Cloning and expression of interleukin-18 binding protein. FEBS Lett 445:338–342
Möller B, Paulukat J, Nold M, Behrens M, Kukoc-Zivojnov N, Kaltwasser JP, Pfeilschifter J, Mühl H (2003) Interferon-gamma induces expression of interleukin-18 binding protein in fibroblast-like synoviocytes. Rheumatology 42:442–445
Kim SH, Eisenstein M, Reznikov L, Fantuzzi G, Novick D, Rubinstein M, Dinarello CA (2000) Structural requirements of six naturally occurring isoforms of the IL-18 binding protein to inhibit IL-18. Proc Natl Acad Sci USA 97:1190–1195
Born TL, Morrison LA, Esteban DJ, VandenBos T, Thebeau LG, Chen N, Spriggs MK, Sims JE, Buller RM (2000) A poxvirus protein that binds to and inactivates IL-18, and inhibits NK cell response. J Immunol 164:3246–3254
Plater-Zyberk C, Joosten LA, Helsen MM, Sattonnet-Roche P, Siegfried C, Alouani S, van De Loo FA, Graber P, Aloni S, Cirillo R, Lubberts E, Dinarello CA, van Den Berg WB, Chvatchko Y (2001) Therapeutic effect of neutralizing endogenous IL-18 activity in the collagen-induced model of arthritis. J Clin Invest 108:1825–1832
Leng J, Yao H, Shen J, Wang K, Zhou G, Wang Z (2008) Co-expression of IL-18 binding protein and IL-4 regulates Th1/Th2 cytokine response in murine collagen-induced arthritis. Acta Biochim Biophys Sin (Shanghai) 40:116–124
Acknowledgments
This work was supported by grants from the Traditional Chinese Medicine Foundation (No. 2008CA047, No. 2004C086, No. 2006Y007), and the Medical and Health Science Foundation (No. 2004B069, No. 2006C166) of Zhejiang Province, China. This paper is proofread by a native English professional with science background at Elixigen Corporation.
Conflict of interest statement
The authors declare that they have no conflict of interest related to the publication of this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
X.-T. Shao and L. Feng have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Shao, XT., Feng, L., Gu, LJ. et al. Expression of interleukin-18, IL-18BP, and IL-18R in serum, synovial fluid, and synovial tissue in patients with rheumatoid arthritis. Clin Exp Med 9, 215–221 (2009). https://doi.org/10.1007/s10238-009-0036-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-009-0036-2