Abstract
Liver cancer is the sixth most common cancer worldwide and 3rd most common cause of cancer-related death. Hepatocellular carcinoma (HCC) represents more than 90% of primary liver cancer and is a major public health problem. Due to the advanced stages of HCC at the time of diagnosis, utilizing the conventional treatment for solid tumors frequently ends with treatment failure, recurrence, or poor survival. HCC is highly refractory to chemotherapy and other systemic treatments, and locoregional therapies or selective internal radiation therapies are largely palliative. Considering how the pathogenesis of HCC often induces an immunosuppressed state which is further amplified by post-treatment recurrence and reactivation, immunostimulation provides a potential novel approach for the treatment of HCC. Immune response(s) of the body may be potentiated by immunomodulation of various effector cells such as B-cells, T-cells, Treg cells, natural killer cells, dendritic cells, cytotoxic T-lymphocytes, and other antigen-presenting cells; cellular components such as genes and microRNA; and molecules such as proteins, proteoglycans, surface receptors, chemokines, and cytokines. Targeting these effectors individually has helped in the development of newer therapeutic approaches; however, combinational therapies targeting multi-faceted biomarkers have yielded better results. Still, there is a need for further research to develop novel therapeutic strategies which may act as either complementary or an alternative treatment to the standard therapy protocols of HCC. This review focuses on potential cellular and molecular targets, as well as the role of virotherapy and combinational therapy in the treatment of HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Liver cancer is the sixth most common cancer diagnosis (749,000 new cases) worldwide and 3rd most common cause of cancer-related death. Hepatocellular carcinoma (HCC) represents more than 90% of primary liver cancer and is a major public health problem [1]. Chronic infection with hepatitis B virus (HBV) or hepatitis C virus (HCV) causes an estimated 75% of all HCC cases. The incidence of HCC due to HCV infection is predicted to increase in western countries until the 2020s [2, 3]. Chronic hepatitis B virus infection, triggering 80% of all liver cancer, is a major cause of chronic liver disease, cirrhosis, and hepatocellular carcinoma [4] (Fig. 1). Nearly 1.4 million deaths occur annually due to chronic liver disease [5], and HCC is the foremost cause of death in cirrhotic patients [6]. Early diagnosis of HCC has a good prognosis with a 5-year survival of more than 70% while late diagnosis has a poor prognosis with a 5-year survival rate of less than 16% [1].
Schematic representation for the pathogenesis of hepatocellular carcinoma. HBV is carcinogenic by insertional activation and genetic changes caused by increased HBx protein in cytoplasm. HBx protein also activates various transcriptional factors such as NF-kB, JNK, and MAPK which results in enhanced viral replication and translation, resulting in more HBx formation and this cascade goes on with chronic HBV infection. HCC in case of HCV is mediated by immune suppression of the host cell by viral and host protein interaction, activating various transcriptional pathways. DNA deoxyribonucleic acid, ERK extracellular signal-regulated kinase, HBV hepatitis B virus, HBx hepatitis B viral X protein, HCV hepatitis C virus, JNK c-Jun N-terminal kinases, JAK-STAT Janus kinase/signal transducers and activators of transcription, MAPK mitogen-activated protein kinase, NF-κB nuclear factor-kappa beta, ROS reactive oxygen species
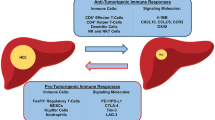
In a healthy liver, inflammation stimulates the process of growth and repair and restores the normal liver architecture. However, with chronic inflammation, the imbalance between regenerative and destructive processes results in chronic liver injury which progresses to fibrosis, cirrhosis, and ultimately the development of HCC (Fig. 2). The regulatory immune mechanism(s) in normal liver cells is mediated by hepatocytes and other non-parenchymal cells such as kupffer cells, dendritic cells (DCs), natural killer (NK) cells, natural killer T-cells, and liver-associated lymphocytes acting as local antigen-presenting cells (APCs). The major histocompatibility complex (MHC) class II expressing hepatocytes engage with naïve T-cells in the liver microenvironment protecting the liver from injury [7]. Spontaneous immune responses to different tumor-associated antigens are mediated by T-cells [8], humoral responses, [9] immune cell subsets, chemokines, and cytokines. Specific defects in host immune response or immune suppression contribute to the high rate of persistence of hepatitis-associated antigen(s) (hepatitis B virus) in patients [10]. The imbalance of different subsets of DCs in HBV- and HCV-positive HCC patients results in defective function of DCs and the defects in overall immune response [11, 12]. Immune suppression in the inflammatory liver cells by the neutralization of naïve lymphocytes, tolerization, inhibition of cytotoxic T-lymphocytes (CTL), T cell anergy, T-cell tolerance, and loss of CTL function and CTL apoptosis causes unopposed development of HCC [7]. Intratumoral immunosuppression in HCC can also be mediated by CD4+ type 1 T-regulatory (Tr1) cells involving interleukin (IL)-10. Furthermore, the Tr1-dependent immunosuppression is correlated with the infiltration of plasmacytoid (p) DCs resulting in increased IL-10 production through upregulation of the inducible co-stimulatory ligand [13]. Thus, defective or suppressed immune response of the host leads to progression and development of HCC. Hence, modulating the immune response to improve it by targeting the cellular and molecular effectors involved in the pathogenesis of HCC has been used in the treatment of HCC. This systematic review discusses a comprehensive description of various molecular and cellular targets for HCC immunotherapy.
Schematic and histological images showing pathological changes in various stages of liver disease. Chronic inflammation leads to oxidative stress, resistance to apoptosis, matrix deposition, disrupted architecture, parenchymal cell death, increased fibrogenesis and angiogenesis and aberrant hepatocyte regeneration (cirrhotic nodule). Steatohepatitis and fibrosis is characterized by fatty infiltration, increased portal inflammation, portal to portal (PP) and central to portal (CP) fibrosis. HCC is characterized by thickening of liver cell plates, acinar formation and increased cellularity. On higher magnification (not shown here), hemorrhage, necrosis, higher N/C ratio, round nuclei with coarse chromatin and thickened nuclear membrane; prominent nucleoli, portal vein thrombosis, vascular invasion and mitotic figures are found in hepatocellular carcinoma (HCC). Hematoxylin and Eosin stained images of human normal liver, steatosis, fibrosis, cirrhosis, and hepatocellular carcinoma were provided by one of the co-authors, Dr. Poonam Sharma, of this article, who is a board-certified pathologist
Post-treatment recurrence of HCC
Most solid tumors are managed by surgical resection, anti-cancer chemotherapy, anti-cancerous agents, and radiotherapy, but these end with a treatment failure or recurrence due to the late diagnosis of HCC. Liver transplant is another perioperative intervention for advanced cases of HCC; however, there are limitations due to the insufficient number of the matched donors as well as post-transplant allograft rejection [14]. Due to the advanced stage at which most patients are diagnosed, only a small percentage is eligible for potentially curative resection, local ablation, or liver transplantation [15]. Local ablative therapies are widely used in HCC for both curative and palliative treatment to induce tumor cell death by physical destruction, radiation, or elimination of vascular supply in combination with chemotherapy. Common ablative procedures are radiofrequency ablation (RFA), laser ablation, cryoablation, photodynamic therapy, high-intensity frequency ultrasound, and percutaneous ethanol or acetic acid injection. The tumor antigens released as a result of local ablative procedures are taken up by APCs and activate a tumor-specific immune response [16], which may prevent the recurrence and can treat the metastasis.
The increased activation and cytolytic activity of tumor-specific CD8+ T-cell response post-RFA [17,18,19,20] and expansion of alpha-fetoprotein (AFP)-specific CD4 T-cell response post-transarterial chemoembolization (TACE) lead to amplified tumor necrosis and improved outcomes [21]. Increased tumor-specific T-cell response has also been observed after local ablative therapy while combined with RFA and TACE treatment [22, 23], cryoablation, and high-intensity focused ultrasound (HiFU). HiFU can also cause a shift in the CD4:CD8 T-cell ratio in peripheral blood [24]. Although RFA and TACE can essentially cause a patient’s tumor to act like an endogenous vaccine, there is a correlation between the expression of programmed death-ligand 1/programmed death 1 (PD-L1/PD-1), and prognosis. PD-L1/PD-1 upregulation is associated with poor prognosis post-cryoablation [25]. Thus, PD-1 blockade may enhance the immune response post-RFA [26]. In cases where the surgical resection or local ablation is not possible, TACE is used as a palliative treatment [1]. However, the impaired drug metabolism due to cirrhosis and intrinsic resistance of tumor cells to commonly used cytotoxic chemotherapy in HCC limits the use of chemotherapeutic agents [27]. Anti-viral treatment may lower the viral load but cannot decrease the risk of HCC development [28]. The drug-resistant mutation of viruses is another concern during HCC therapy [29].
Recurrence that occurs in both surgically resected and non-resected tumors indicates that dormancy of disseminated tumor cells, dormancy of locoregional lesions, or dormancy of micrometastases may be other causes of recurrence besides blood circulation [30, 31]. The effect of surgery on mechanisms of cellular dormancy or tumor dormancy is not clear, but data suggest that partial hepatectomy can result in the growth of the remnant tumor and tumor metastasis. Epidermal growth factor (EGF), hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), transforming growth factors (TGF)-α, TGF-β, hypoxia-inducible factor-1 (HIF-1), and matrix metalloproteinases (MMPs) are responsible for liver regeneration and mediate the modulation of tumor angiogenesis responsible for tumor growth [32, 33]. If the concept of dormant tumor cell growth is correct, then preventing progression by activating an immune response could be an adjunct therapy (immunotherapy) along with surgery for treating HCC patients with stage IV metastasis not suitable for surgical resection. Immunomodulation in such patients can induce tumor regression or delay tumor progression yielding improved therapeutic efficacy [30]. Thus, to facilitate a more efficient immune response as well as develop a potential immunotherapy, it is important to understand the molecular and cellular targets involved in HCC.
Cellular targets in hepatocellular carcinoma
Immunosuppression in the host results in progression and development of HCC and immunostimulation of various cells and molecules involved in the pathogenesis of HCC has resulted in better treatment modalities such as immunotherapy, combinational therapy, and oncolytic viral therapy. Several clinical trials of immunotherapy for HCC in humans have been conducted (Table 1) involving lymphokine-activated killer (LAK) cells, cytokine injection with interferon-gamma (IFN-γ), IL-2, IL-4, and tumor necrosis factor alpha (TNF-α). These interventions demonstrated increased tumor immunogenicity as well as non-specifically activated subsets of immune cells, infusion of APCs, infusion of activated peripheral blood lymphocytes (PBL) or tumor-infiltrating lymphocytes (TIL), and yielded an autologous tumor-based vaccine [34]. These clinical trials have shown improved outcomes, suggesting the important role of immunotherapy in treating HCC. Alteration of the immunosuppressive tumor microenvironment may convert the non-immunogenic HCC into being more immunogenic. Activating a generalized and antigen-specific immune response allows the immune system to target its attack directly on the tumor. APCs such as B-cells, macrophages, and DCs secreting MHC-I and MHC-II, can directly activate the immune response [34].
Dendritic cells
DCs are potent immune stimulatory cells specialized for initiation and shaping the immune response [45]. DCs presenting the tumor antigen to the immune system can be used in immunotherapy of HCC. Mixing the immature DCs with tumor cell lysate or purified proteins will result in processing and presenting multiple peptides in MHC class I and II. Specific gene products can be expressed by engineering plasmid DNA or viruses with DCs. Fusion of tumor cells with DCs via polyethylene glycol or electrofusion and pulsing is the strategy for using DCs in immunotherapy. This procedure utilizes large concentrations of synthetic peptides of known epitopes or mild acid-eluted mixed peptides from tumor cells where they are planted onto the DC surface. Recently via PEG or electrofusion, DCs and tumor cells have been combined to facilitate the transfer of any or all tumor proteins to a cell expressing necessary immune-activating molecules. Many studies in the murine model have supported the use of tumor lysate-loaded DCs in the treatment of tumors or improvement in anti-tumor response; however, further studies are needed [46,47,48,49].
DCs mediate immunomodulation through CTLs; however, the activity of CTLs is completely inhibited due to the downregulated expression of major human leukocyte antigen (HLA) class I molecules by HCC cells. Transfected DC-stimulated T-lymphocytes produce CTLs, which may limit the growth of HepG2 tumors. DCs transfected with HCC total RNA induce specific immune responses against HCC in vitro and in vivo [50]. Thus, DCs may be used to induce immune response via immunotherapy, but the use of DCs may be too inefficient to significantly impact the advanced stages of disease [34]. However, Zhou et al. [51] demonstrated that upregulated expression of HLA-A2 and improved sensitivity to CTL response can be achieved by transduction of Hep3B cells with adeno-associated virus (rAAV) carrying human α-fetoprotein promoter (AFPp) and the interferon-γ (IFN-γ) gene (rAAV/AFPp-IFN-γ) (Fig. 3). Superantigen-staphylococcus enterotoxin-A gene-modified tumor vaccine HepG2-SEA primed CTLs (SEA-T) has a strong stimulatory effect on the production of HepG2-specific CTL and reinforces the immune response [52]. Hyperthermia may also improve the cellular immune response to human HCC subsequent to co-culture with tumor lysate-pulsed DCs [53].
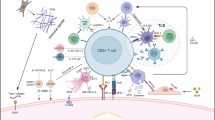
Schematic representation of innate immune response of liver to viral infection and potential targets for immunotherapy in HCC (1) Natural killer (NK) cells kills virus infected cells by producing cytokines or by activating T-cells and macrophages (M). (2) Blocking signal transducer and activator of transcription (STAT) 3 with inhibitors augment NK cell function by reducing TGF-β and interleukin (IL)-10. (3) Activated NK cells through licensing enhance cytotoxic activity of NK cells. (4) Kupffer cells secrete anti-viral cytokines (INF-γ) in response to infection. (5) Dendritic cells (DCs) combined with tumor cell lysate increase DC immune response. (6) Transfected DCs and staphylococcus enterotoxin-A (SEA-T) antigen stimulate the cytotoxic T-lymphocytes (CTLs) and increase the immune response. RAGE receptor for advanced glycation end products, TREM 1 triggering receptor expressed on myeloid cell-1, TLRs toll-like receptors
Natural Killer cells
Natural killer (NK) cells play an important role in liver immunity and account for 25–50% of the total number of liver lymphocytes. Further, the positive correlation of the number of NK cells in blood and tumor cells; the presence of abnormal NK cells in blood and liver; and their functional impairment in HBV and HCV infection has been documented [54]. NK cells play an important role in eradicating HBV from infected hepatocytes and inhibit the development of hepatitis B-related liver diseases like hepatitis, fibrosis, cirrhosis, and HCC. Disrupted cellular cytotoxicity and cytokine production by NK cells during HBV infection indicate that restoration of NK cell function may be a potential strategy for activating the immune response. Hence, immunotherapy directed against HBV using activators of NK cells can be a potential area for further research [55]. Licensing may enhance the function of NK cells causing increased cytotoxic activity, resulting in increased overall and recurrence-free survival [56]. Similarly, blocking signal transducer and activator of transcription 3 (STAT3) in hepatocellular carcinoma cells enhances NK cell activation via reduced expression of TGF-β and IL-10 and induced expression of type I interferon (IFN), resulting in increased anti-tumor activity [57]. NK cells in human HCC in vivo can be augmented by hIL-15 gene-modified human NK cells, and these modified NK cells can be used for adoptive immunotherapy against HCC [58] (Fig. 3). Recently, Kamiya et al. [59] reported enhanced anti-tumor activity of expanded NK cells in vitro and in immunodeficient mice with a chimeric NKG2D–CD3ζ–DAP10 receptor. Interestingly it was also reported that HCC cells treated with sorafenib remain sensitive to NK cells suggesting an additive advantage of utilizing NK cells in the presence of this multikinase inhibitor. Furthermore, IL-2-enhanced NK cells were found to decrease the pulmonary metastasis of HCC in mice [60]. Thus, improving the NK cell function via NK cell transfer or in conjugation with other therapies such as chemo-immunotherapy, gene therapy, and cytokine therapy may be effective therapeutic strategy in HCC treatment.
Lymphocytes: T-cells and T-regulatory cells
Suppression of the host immune response results in the development and progression of HCC. Decreased number and dysregulation of the T-lymphocyte has been reported in cirrhosis and HCC. Further, T-lymphocytes, both CD4+ T helper cells, and CD8+ cytotoxic T-cells are significant players in immune response and are effective in inhibiting and killing tumor cells [61]. Furthermore, T-cells are also important for B-cell maturation and their reduced number has been associated with low survival rates in HCC. Similarly, the role of Tregs, Th17 cells, and NKT cells in the pathogenesis of HCC has been discussed [62]. Thus, these cells can be targeted to enhance the immune response in HCC and may be potential targets for immunotherapy (Fig. 3).
Sorafenib, which targets multiple protein kinases, is the only effective systemic molecular-targeted treatment so far for advanced HCC, showing modest improvement in survival. However, the prolongation of survival associated with sorafenib therapy is under 3 months [63], and the median survival for patients with advanced stage, unresectable HCC is less than 1 year [15]. In vitro, it may augment effector CD4+ T-cell function by Treg suppressor function elimination in peripheral blood mononuclear cells (PBMC) obtained from HCC patients in low dose [64] and decreases Th2 and regulatory T-cells in peripheral blood of HCC patients [65]. Although sorafenib has anti-proliferative and anti-angiogenic effects [66] and prolongs survival, resistance to it results in low response rates. Sorafenib resistance is possibly due to genetic heterogeneity, but the exact mechanism is not yet known. However, the roles of three major pathways (JAK/STAT, RAF/MAPK, and PI3 K/AKT) have been implicated in sorafenib resistance acquisition [67, 68]. The activation of these pathways by upregulation of epidermal growth factor receptor, platelet-derived growth factor, vascular endothelial growth factor, as well as the mutation in these pathways, contributes to sorafenib resistance [69, 70].
Increasing the number of T-cells by T-cell transfer may be another method for increasing immune response. However, this may be ineffective due to the tumor microenvironment. Chuang et al. [71] reported that sorafenib in serial low dose injections enhances the efficacy of adoptive T-cells by modulating the tumor microenvironment. Adoptive cell transfer (ACT) involves the transfer of autologous TILs and/or donor lymphocytes to modulate the immune response. Many studies have suggested that ACT can successfully treat solid tumors. ACT may be combined with chemotherapy and radiotherapy for better outcomes. Genetically engineered T-cells receptors with potent anti-tumor activity can be used in ACT. Infusion of tumor-specific T-cells has the advantage that its ex vivo activation avoids the immune-suppressive influence of the tumor microenvironment. The advantages of ACT along with chemotherapy and radiotherapy have been proven and may be considered as a potential strategy for HCC treatment [72,73,74,75] (Fig. 3). Another study by Hu et al. [76] reported the enhanced synergistic tumor inhibitory effect with the combination of oncolytic adenovirus expressing Hsp70 with cytokine-induced killer (CIK) cells via infiltration of CD3+ T-cells in tumor stroma. This study further supports the synergistic effect of combined anti-tumor therapy. Similarly, the case report by Li et al. [77] supports the safety and feasibility of the CIK cells in liver transplant patients to kill the residual tumor cells.
Recently, it has been suggested that glucocorticoid-induced tumor necrosis factor receptor ligation and anti-CTLA-4 mAb can improve the anti-tumor immunity by abrogating tumor-infiltrating regulatory T-cell-mediated suppression in patients with primary and secondary liver cancer [78]. Tumor-induced CD4+ CD69+ Tregs may be another potential therapeutic targets for the immunotherapy [79]. Further, T-cell-mediated immune response involves the T-cell receptor (TCR), and it has been reported that hepatitis C-associated hepatocellular carcinoma tumors can be efficiently treated by TCR gene-modified T-cells [80]. These studies suggest that immune modulation in HCC by targeting the lymphocytes may be a potential strategy to improve the outcome of immunotherapy (Fig. 3).
Targeting the genes for the treatment of HCC
Genomic involvement in the pathogenesis of HCC has been well-documented. Gene products and an increase or decrease in expression act as diagnostic as well as prognostic factors in HCC. Gene therapy involves the induction of an immune response targeted at overexpressed protein thereby eliminating such markers. Gene therapy also aims to increase the level of proteins with reduced expression and replace non-functional or mutated genes. Several studies using gene therapy for the treatment of HCC have been conducted and have shown promising results. The injection of wild-type p53 in primary hepatocellular carcinoma showed tumor volume reduction and a significant decrease in serum alpha-fetoprotein (AFP). However, the mechanism of action of wild-type p53 needs to be investigated [81]. Similarly, treatment of HCC with allogeneic suicide gene-modified killer cells (aSGMKCs) has demonstrated a marked, rapid, and sustained regression of tumor [82, 83]. Inhibition of cellular growth and induction of apoptosis by recombinant adenovirus carrying the C-terminal fragment of human telomerase reverse transcriptase (rAdv–hTERTC27) and induction of antigen-specific T-cell proliferation along with activated cytotoxicity of T-cells by DCs transduced with rAdv–hTERTC27 in Hepa 1–6 HCC cells suggest an improved outcome of combining gene therapy with immunotherapy [84]. Cytotoxic T-cells recognize peptides with the help of TCR, leading to an immune response. But some antigen-presenting cells can also present proteins to CD8+ cells; hence, there is a need to target the immune response specifically to the tumor cells. TCR gene transfer is a promising strategy to generate stimulated AFP-specific CTLs for adoptive immunotherapy [85] and efficient treatment of HCV-associated HCC by TCR gene-modified antigen-reactive T-cells, providing another viable option of therapy [80].
When the MAGE-A family of genes is overexpressed, they become a possible target for immunotherapy. Along with MAGE-A, other genes found in HCC are members of tumor-specific “cancer-testes” gene families like NY-ESO and SSX-1. Hepatitis viral antigens and viral genes from HBV and HCV such as the HBx protein, which modulates p53 expression, can also be targeted. Non-malignant and malignant cells, as well as viral proteins can be affected by the immune system, but malignant cells should be the preferred target. Increased levels of oncogenes such as myc, fos, and ras (critical for the unregulated growth of the tumor), and mutated and/or dysregulated tumor suppressor genes such as p53 and pRb are other potential targets for immunotherapy [34]. Antigen-specific T-cell response against HCC cells in vitro is stimulated by DCs loaded with NY-ESO-1. Hence, NY-ESO-1 may be used as a potential target for immunotherapy in advanced HCC [86]. Fujiwara et al. [87] proposed that ROBO1 is expressed at high levels in hepatocellular carcinoma and plays a role in tumor metastasis and angiogenesis. It can act as a biomarker for immunotherapy, and they found that radio-immunotherapy with the radioisotope-labeled anti-ROBO1 monoclonal antibody (90Y-anti-ROBO1 MAb) is a promising treatment for ROBO1-positive hepatocellular carcinoma. Hepatocellular carcinoma-associated antigen-519/targeting protein for Xklp-2 (HCA519/TPX2) might be beneficial for T-cell-specific HCC immunotherapy as well [88].
Cellular surface receptors
Intra- and intercellular signaling is crucial for the synchronized development and vitality of multicellular organisms. This signaling cascade involves intricate signal transduction networks that run through protein receptors on the cell surface. These receptors regulate an assortment of biological functions such as cellular migration, differentiation, survival, death, or even proliferation. Impairment of the immune regulatory mechanism in HCC results in the loss of the immune system’s ability to discriminate tumor cells from unaffected hepatocytes. Studies have suggested the role of cell surface receptors in the treatment of carcinoma including HCC; however, a novel target to develop a potential drug for the treatment of HCC is yet to be determined [89, 90].
Folate receptor
Thirty percent of HCC patients are deemed ineligible to undergo harsh chemotherapies; therefore, it is imperative to cultivate unconventional therapeutic options that can target only malignant cells while avoiding toxicity in healthy organs and tissues [91]. Folate receptor (FR) is a cell surface biomarker that is overexpressed in a myriad of neoplasms, while its expression is uncommon in non-cancerous tissues [92]. Upregulation of FR has been observed in HCC; therefore, targeting this biomarker using covalent attachment to an immunotherapeutic agent could be a viable option for oncologists. This process could be utilized when the common immunotherapy target (PD-L1) is not present in the tumor. Adhesion of cancer therapy-based molecules to folic acid has demonstrated efficacy in cells that express folate receptor while at the same time avoiding uptake by healthy tissues [93]. However, the kidneys may undergo adverse effects under these biochemical conditions. Ideally, we hope to see researchers improve the potential of using folate’s capacity to transport attached molecules precisely to malignant HCC cells to transform tumors lacking immune activity into a robust immunogenic milieu. It has also been demonstrated that methods which increase IFN-α and IL-2 in the same cells in concert with immunotherapy targeting FR have a positive synergistic effect [93].
Toll-like receptors
Using toll-like receptors as an avenue to cease the development of HCC from HBV would not be a curative option for HCC but could be utilized as a preventative measure for patients infected with HBV. Heightened toll-like receptor (TLR) activity is linked to inflammatory cancers as well as viral detectors, and the increased TLR activity has a significant role in yielding a suitable immune response to antigens. The upregulation of TLR signaling has been detected in HCC suggesting that it may play a role in the inflammatory mechanisms involved with HBV progressing into HCC [94]. TLRs can sense and recognize HBV which in turn induces an immune response to combat the virus. TLR4 has been linked to the increased progression and amplified tumorigenesis of HCC; hence, exploiting TLR ligands may prove to be an effective method for treating HBV before it undergoes carcinogenesis [95]. Also, TLR2 (as a homodimer or heterodimer with TLR1/6) can detect several molecules associated with groups of pathogens (PAMPs) which successively activate NF κB via MyD88 [95]. Consequently, fostering TLR2 expression in patients with HBV could produce an amplified immune response against HBV which would hinder the virus from ultimately developing into HCC. TLR 2 initiates the innate immune response against HBV in acute hepatitis while suppressing it in chronic hepatitis, suggesting the TLR2-dependent status of the disease (Fig. 3). Thus, targeting TLR2 may be a potential strategy for modulating the immune response and immunotherapy [96]. Phosphorothioate-modified TLR9 agonist ODN M362 has anti-proliferative and anti-tumor activity. Attenuation of proliferation and increase in apoptosis with ODN M362 in HCC makes it a potential therapeutic agent as well [97].
Triggering receptor expressed on myeloid cells-1
Triggering receptor expressed on myeloid cells-1 (TREM-1) is a biomarker that has been found to restrain inflammatory responses in several conditions and its role in many cancerous indications is currently being elucidated [98]. Because HCC is known as cancer derived from excessive inflammation, the role of TREM-1 and its explicit influence on HCC cells have been recently investigated. In HCC cells, TREM-1 was found with statistical significance to enhance proliferation while preventing apoptosis. Pro-inflammatory cytokines IL-1β, TNF-α, and monocyte chemoattractant protein-1 (MCP-1) have been positively correlated with TREM-1 up- or downregulation, demonstrating that this receptor acts as an inflammatory switch in the liver [98] (Fig. 3). An experiment utilizing adoptive transfer of normal Kupffer cells in mice lacking TREM-1 attenuated liver injury and even modulated malignant progression [99]. TREM-1 is an essential trigger for the activation of Kupffer cells during liver pathogenesis, bringing to light the mechanisms of how excessive inflammation reinforces the development of HCC. Taken together, TREM-1 is a viable immunotherapeutic target which could be used to attenuate Kupffer cell activation leading to a decline of multiple pro-inflammatory cytokines which would have a positive immunological effect in liver cancer cells [99].
Chemokine receptors
In addition to stellate cells and immune cells, inflamed hepatocytes, and hepatoma cells also express various chemokines attracting immune cells. CXCR4 as a receptor for the chemotactic cytokine stromal-derived factor-1-alpha (SDF-1α/CXCL12) controls the cell migration to inflammatory foci as well as the migration of metastatic cells. CXCR4/SDF-1α axis also plays a role in tumor initiation and progression, angiogenesis, and tumor cell survival [100, 101]. Cepeda et al. [100] described the membrane as well as subcellular presence and the intracellular trafficking pathways of CXCR4 mediating the immune response through TGF-β signaling; hence, neutralization of the CXCR4 axis may reduce the tumor progression and metastasis. Further, it has been reported that there is a correlation between higher levels of MMP-10 and enhanced expression of CXCR4 [102]. Hence, blocking MMP-10 along with CXCR4 may have a pronounced effect on the suppression of tumor progression and metastasis. Targeting the tumor-infiltrating macrophage via inhibition of CCL2/CCR2 axis in HCC resulted in immunosuppression status reversal and activation of anti-tumorous CD8+ T-cell response, suggest that blocking CCR2 may be a potential therapeutic option [103]. Similarly, the suppression of CX3CL1 by inhibitors resulting in decreased expression of immune cells such as CD56+ NK and CD8+ T-cells suggest another possible therapeutic approach for immunomodulation [104] (Fig. 4).
Target sites for immunotherapy and mechanism of immunotherapeutic agents. GPC-3 Glypican, Ab antibody, EGF epidermal growth factor, HGF hepatocyte growth factor, VEGF vascular endothelial growth factor, TGFβ transforming growth factors, HIF1α hypoxia-inducible factor, JAK/STAT Janus kinase/signal transducer and activator of transcription, PI3K phosphatidylinositol-3 kinase, CCL2 chemokine, CC motif, ligand 2; CCR2 chemokine, CC motif, receptor 2; caspase 3 cysteinyl, aspartyl-directed protease; HSPGs heparan sulfate proteoglycans, PI-88 heparanase inhibitor phosphomannopentaose sulfate, BMP bone morphogenetic protein, IGF insulin-like growth factor, ERK extracellular signal-regulated kinase, MEK MAPK/ERK kinase, PD1 programmed death 1, SDF-1/CXCL12 stromal cell-derived factor 1, CXCR4 chemokine (C-X-C Motif) receptor 4
C–C motif chemokine ligand 2 (CCL2) is involved in recruiting CCR2+ immune cells as a mechanism to promote inflammation. This protein has been found to be prominently upregulated in HCC tumors and targeting CCL2–CCR2 may have a translational value in treating HCC [105]. The clinical utility of blocking CCL2–CCR2 heterodimers was tested in knockout mice with an antibody directed at CCL2 [106]. Inhibiting CCL2 with anti-CCL2 therapy triggered an immunotherapeutic-like response suppressing inflammation in the hepatocytes of these mice via the reduction of CD11highGr1+ inflammatory myeloid cells, reducing the expression levels of TNF-α and IL-6, downregulation of STAT2, c-MYC, and NF-kB expression, upregulation of IFN-γ production, and augmentation of NK cell infiltration in the liver. These events result in a substantial reduction in tumorigenesis, tumor burden, and overall liver damage in eight weeks of therapy suggesting it as a potential therapeutic strategy [106] (Fig. 4). CCR5 possesses an important role in the progression of HCC, and like receptor for advanced glycation end products (RAGE), an antagonist monoclonal antibody-based therapy directed at CCR5 has the potential in the prevention and treatment of HCC [107]. The recruitment of macrophages to hepatocytes was significantly reduced in mice lacking CCR5, which in turn reduced inflammation in the liver. Knocking out CCR5 in murine models yielded a noteworthy reduction in tumor size and rate of incidence [107]. The roles of CCL15/CCR1, CCL17, and CCL20/CCR6 in the migration, invasion, progression, and prognosis of HCC suggest that these chemokines may be useful in therapeutic targets [108,109,110,111,112].
Receptor for advanced glycation end product
RAGE is primarily involved in the tissue where inflammatory destruction has taken place, acting as an auxiliary to inflammatory responses when dispatched by the immune system [113]. This mechanism is typically observed when RAGE proteins encounter damage-associated molecular pattern proteins (DAMPs). Augmented RAGE expression has been detected in many solid tumor indications and recent investigations have shed light on its critical role in carcinogenesis and metastasis; however, the level of significance remains undetermined. RAGE has been shown to be involved in regulating oval cell activation and tumor progression in liver cancer caused by inflammation which would make blocking the RAGE receptors a potential immunotherapeutic avenue for HCC patients [113, 114] (Fig. 3).
In sum, there are several feasible immunotherapeutic targets on the cell surface of HCC tumors; however, it should be noted that tumors have evolved multiple immune-based escape mechanisms. These pathways involve cells like Tregs and myeloid-derived cells which possess the capacity to suppress immune responses [114]. It has been shown that these suppressor molecules mask tumor-specific immune responses in patients with HCC. Due to these escape mechanisms built into the tumors, novel immune-based therapeutic avenues including dendritic cell-based therapies have been investigated and yielded encouraging data in HCC patients [114]. Presumably, these methods should be delivered in concert with a drug directly targeting suppressor cells in HCC to potentially improve treatment efficacy.
Molecular Targets for treatment of HCC
Proteins and proteoglycans
Alpha-fetoproteins
Most of the studies on immunotherapy have used either cytokine-based or antigen-based approach [115, 116] and have proposed that immunotherapy can induce a tumor-specific immune response but lacks the clinical efficacy. Sensitivity, specificity, and self-regulation of the immune system to find and eradicate tumor cells from the body can be achieved by immune-based therapy. The activation of HCC-specific immune response can be achieved by targeting the AFP, a tumor-associated self-antigen and other such antigens and the HCC-specific genes [34]. Alpha-fetoprotein, a serum marker for HCC diagnosis, is found in high levels in serum, which is highly indicative of the presence of HCC [117] with a sensitivity of 25–65% and specificity of 79–95% [118] and AFP can be targeted to generate tumor antigen-specific immune responses to HCC. Strategies used to induce an immune response to this self-antigen include AFP vaccination, peptide-pulsed DC, plasmid DNA, AFP-derived peptides, antigen-engineered DC, and AFP-based prevention strategies in animal models, in vitro T-cell cultures, and clinical trials [34, 119,120,121]. Alpha-fetoprotein is a tumor marker for HCC, and it makes it a choice for HCC tumor cell target. AFP has been suggested as a target for antibody-based therapy, but as the AFP peptides are processed by the cell and can be presented to both CD8+ and CD4+ cells, the results of such studies are inconclusive [34].
Glypican 3
Glypican 3 (GPC3), a member of the glypican family of heparan sulfate (HS) proteoglycans, attached to the cell membrane is tumor specific and important for cell proliferation [122]. GPC3 is a carcinoembryonic antigen, and patients with GPC3-positive HCC have poor 5-year survival rates, linking it to poor prognosis [123]. GPC3 is found overexpressed in nearly 80% of HCC [124, 125], can be used as a serum marker, and also differentiate between high-grade dysplastic nodules from an early HCC by immunostaining [126]. Hence, it is a potential new target antigen for the immunotherapy. Anti-GPC3 antibody and peptide-based vaccines for the treatment of HCC are in the pre-clinical and clinical phase of development [127, 128]. The potential anti-tumor activity of recombinant fully humanized monoclonal antibody GC33 that binds to human GPC3 through antibody-dependent cellular toxicity has been reported. Treatment with humanized GPC3 antibody enhances the susceptibility of HCC to chemotherapy [129]. Pre-clinical studies have suggested the combination of anti-GPC3 antibody and sorafenib, to be more effective in inhibiting tumor progression than sorafenib alone [7].
Human heavy-chain variable domain antibody HN3 inhibits the proliferation of GPC3-positive cells and HCC xenograft tumor growth via cell-cycle arrest at G1 phase through Yes-associated protein signaling [128]. Combining NIR-PIT (near infrared photoimmunotherapy) using IR700-HN3 with fluorescence-guided surgery may result in a superior therapeutic outcome [130]. Gao et al. [131, 132] reported monoclonal antibody HS20 that inhibits the cell proliferation through Wnt/β-catenin signaling and HGF-mediated migration and motility by targeting the heparin sulfate chains of GPC3. Sun et al. [133] reported the role of GPC3 suppression in inhibiting the cell proliferation by arresting cell-cycle progression at G1 phase through TGF-β signaling pathway activation suggesting GPC3 as a target for HCC immunotherapy. Recently, Zaghloul et al. [134] suggested blocking of HSPG/SULF-2/IGF-II axis as a mechanism of action of anti-GPC3 antibody, leading to decreased gene expression of IGF-II and caspase-3 and the activity of sulfatase-2. The potential role of T-cells expressing GPC3-targeted chimeric antigen receptor in eliminating GPC3-positive cells in HCC has been suggested by Gao et al. [135]. Anti-GPC3 antibody such as YP7 and MDX-1414 has been reviewed in the literature [136]. Li et al. [137], using mouse model, reported that specific and effective cellular anti-tumor immunity against GPC3 can be elicited by GPC3 DNA vaccine, and Luo et al. [138] proposed that GPC3 expression in the mouse ovarian cancer increases F4/80+ CD86+ macrophage (M1) proportion and induces GPC3-specific CD8+ T-cell immune response through these macrophages, prolonging survival. These studies have demonstrated the mechanism of action and the chemopreventive effect of anti-GPC3 therapy by targeting the GPC3 for HCC therapy (Fig. 4). However, the therapeutic potency and hepatoprotective effect of anti-GPC3 antibody, its stability, its role in the modulation of GPC3 functions, and the specificity for the cancer-associated antigen involving signaling pathways remain to be elucidated.
Heparan sulfate
The basis of heparan sulfate (HS)-based immunotherapy is the emerging important role of heparan sulfate proteoglycans (HSPGs) in tumorigenesis, progression, and metastasis of the tumor through HS chain of HSPGs [139]. Heparanase inhibitor phosphormannopentaose sulfate (PI-88) exhibited anti-tumor, anti-metastasis, and anti-recurrence activity in HCC in pre-clinical trials [140], and it has been reported that upregulation of heparanase by overexpression of early growth response 1 enhances the sensitivity of HCC cells to PI-88 resulting in inhibitory cell proliferation and migration effect after hepatectomy [141]. Liu et al. [142] suggested that PI-88 is safe and well tolerated at 160 mg/d, and confer the most significant survival advantage. WSS25 is another HS-mimetic which inhibits the growth of xenograft HCC cells by decreasing the angiogenesis via bone morphogenetic protein (BMP)/Smad/Id1 signaling blockade [143] (Fig. 4).
Cytokines
The development of hepatocellular carcinoma is a highly complex multi-step pathologic process involving numerous cell, chemokine, and cytokine signaling. The role of Th1 cytokines including IL-1α, IL-1β, IL-2, IL-12p35, IL-12p40, IL-15, Th2 cytokines such as IL-4, IL-8, IL-10, and IL-5, and non-ILs including TNF-α and IFN-γ in the pathogenesis of HCC has been discussed [144]. Further, the pathogenic role of imbalance between pro-inflammatory and anti-inflammatory cytokines in the development of HCC suggests that targeting the cytokines may modulate the course of disease [144]. The role of cytokines in modulating the signaling pathways involved in the pathogenesis of HCC and the use of cytokine levels in the assessment of recurrence, prognosis, and post-treatment outcome suggest cytokines as potential targets in the immunotherapy of HCC [145, 146]. The immunomodulatory and anti-tumor activity of TNFα, type I or type II interferons, IL-2, IL-12, IL-15, IL-18, IL-21, IL-23, IL-27 has been discussed in literature [147]. The role of activated cytokine-induced killer (CIK) cells (CD3+/CD56+ and CD3+/CD56− T-cells and CD3−/CD56+ natural killer cells) as adjunct immunotherapy with curative treatment in prolonging the recurrence-free survival of patients after curative therapy for HCC suggests the role of cytokines in the treatment of HCC [77, 148,149,150]. Improved 1- and 2-year overall survival, overall response rate, disease control rate, and a better quality of life with the combination of DC-CIK immunotherapy and TACE or TACE plus local ablation therapy for patients with HCC indicate the role of cytokines in HCC immunotherapy [151]. The use of recombinant IL-10 therapy in the long term decreases the disease activity, but was associated with increased HCV viral burden via alterations in immunologic viral surveillance [152].
MicroRNA
MicroRNAs (miRNAs) are conserved endogenous small non-coding RNAs that regulate gene expression by interacting with the 3′-untranslated region (3′-UTR) of protein-coding mRNA. By recruiting the RNA-induced silencing factor complex (RISC), miRNAs binding generally leads to translational suppression and/or degradation of the target transcript [153,154,155]. Recently miRNAs have emerged as key factors involved in several biological processes, including differentiation, cell proliferation, metastasis, and tumorigenesis [156]. MiRNA biogenesis itself involves multiple steps, and each of these steps can be affected or altered to impact the amount of produced mature miRNA. Epigenetic mechanisms (e.g., histone deacetylation and DNA methylation) can result in miRNA silencing [157]. For instance, Furuta et al. [158] showed that methylation of miR-124 and miR-203 genes in HCC cell lines silenced their expression. Besides the possible alterations in the miRNA processing, miRNA polymorphism in the form of single nucleotide polymorphism (SNP) can also be associated with an increased risk of HCC [157]. Several evidences have shown that miRNAs may act as oncogenes or tumor suppressors by directly or indirectly controlling the expression of key genes involved in cancer-associated pathways [159]. One gene can be targeted by several miRNAs, and one miRNA may regulate the expression of many genes. Aberrant expressions of miRNAs have been widely reported in human cancers with both upregulation and downregulation detected in neoplastic cells compared with their normal counterparts [160,161,162].
Several miRNAs are dysregulated in HCC and this results in changes in the expression profile of target genes involved in HCC onset and progression (Table 2). These specific miRNAs may serve as potential biomarkers for diagnosis, monitoring, prognosis, and therapeutic targets in patients with HCC. Cellular miRNAs can be released into the circulation and can be detected in most body fluids. Circulating miRNAs can, therefore, correlate with disease activity and progression. Several recent studies reported that miRNAs are stably detectable in plasma and serum [162, 163]. The most commonly used methods to detect miRNA are RT-qPCR, microarray, and next-generation sequencing (NGS). The high stability of miRNAs in circulation makes them very useful especially for early detection of HCC, even in pre-symptomatic diseases [164]. For instance, the circulating miR-21 [165, 166], miR-221 [166], and miR-223 [160] were found to be upregulated in the serum of HCC patients associated with HBV or HCV. At the same time, serum levels of miR-1, miR-25, miR-92a, miR-206, miR-375, and let-7f were also significantly elevated in HCC patients [167]. Serum miR-15b and miR-130b levels were also found to be upregulated in HCC with high sensitivity of 98.3 and 87.7%, respectively [153, 168]. Therefore, circulating miR-15b and miR-130b are potential biomarkers for HCC in early stage where patients may have low AFP levels despite the presence of the tumor [153]. In one study, the combination of miR-16 with chemical biomarkers (AFP), Lens culinaris agglutinin-reactive AFP (AFP-L3), and descarboxyprothrombin (DCP) allowed the detection of HCC cases with a high specificity even when the tumor is <3 cm in size [169].
The testing and interpretation of qualitative miRNA in a clinical setting is complex, firstly because of the variability in the technical procedure, from the method of sampling (open biopsy, cytology), time to freeze the tissue and procedure of freezing, RNA isolation, and method of detection. Secondly, HCC patients with a single underlying etiology (alcohol or viral) may yield different results than multiple etiologies (alcohol and viral). Thirdly, the stage of the disease should also be considered, although miRNA dysregulations occur from an early stage [157, 170]; it is not clear how miRNA expression changes during disease progression. Finally, in a clinical setting, the use of appropriate controls for miRNA is critical as HCC is often accompanied by viral hepatitis, cirrhosis, or other underlying liver conditions [171]. It is important to ensure that patients and control tissue used for normalization are matched not only by age and sex, but also by the etiology and severity of the underlying liver disease [153].
The use of miRNA-targeted treatment is in early stage but holds promises for HCC therapy by potentially affecting several target genes with only one miRNA. In HCC, miRNA associated with tumor suppressors are downregulated and the one associated with oncogenes are upregulated during tumor development and metastasis. Targeted approaches for miRNA replacement therapy have been developed using miR-26a [172], miR-122 [173], and miR-124 [174] in a HCC mouse model. For instance, restoration of tumor-suppressive miR-122 makes HCC cells more sensitive to Sorafenib treatment by downregulating drug resistance genes [153, 175]. In contrast, suppression of oncogenic miR-221 in HCC resulted in prolonged survival with reduction in the number and size of tumors in animals [176]. Furthermore, no toxicity was observed when miRNA-targeted therapy was used to treat HCC in a mice model [177]. MiRNA has been also shown to affect the sensitivity of tumors to anti-cancer drugs. Overexpression of miR-21 [178] and miR-181b [177, 179] induced resistance to interferon-α/5-fluorouracil (5FU) combination therapy and doxorubicin treatment in HCC. Interestingly, HCC cells transfected with anti-miR-21 were significantly sensitive to chemotherapy with combined IFN-α and 5-FU [153, 178].
The recent advances in gene therapy create more opportunities for miRNA-based gene therapy applications. Such therapy could even be used in conjunction with chemotherapy regimen. The main challenge to these treatments is the delivery of the synthetic effector molecule to their target genes in a specific tissue [157]. Gene therapy using viral-delivered miRNAs provides new therapeutic approach to deliver specific miRNAs via viral vector to tumor cells. However, this method raises safety concerns in clinical use as these viruses integrate their genetic material into host genome. No virus-delivered miRNA-based gene therapy has been tested in clinical trials yet [157]. MiRNA antagomirs are a class of chemically engineered oligonucleotides that cause transient suppression of target gene expression. To prevent rapid degradation, miRNAs are conjugated to improve stability of effector molecules to the target cells. Stable nucleic acid lipid particle (SNALP) formulations composed of a lipid bilayer and a PEG-lipid derivative have been used as a delivery system for miRNA. When miRNAs incorporated SNALPs, they protect them from degradation, prevent immunostimulation, and facilitate their uptake in endosomes [157, 180]. Miravirsen (SPC3649) is a miR-122 antagonist drug that shows extensive anti-viral effects among all HCV genotypes and a high barrier to drug resistance. This treatment was well tolerated among patients with chronic hepatitis C that led to the suppression of HCV viremia [181, 182]. However, resistance has been reported due to mutations in miRNA binding site in HCV 5′-UTR [183]. The field of miRNA-targeted therapy is quite recent and more research still needs to be done to explore the potential benefits in clinical practice.
Multiple target approach
Combinations of the therapeutic agents for HCC are more effective than the therapy with a single therapeutic agent due to the synergistic effect (Table 3). Combination therapies may consist of gene therapy with immunotherapy, gene therapy with oncolytic virotherapy, oncolytic virotherapy with gene and chemotherapy, and gene therapy with oncolytic virochemoradiotherapy.
Oncolytic virus therapy
The selective infection, within tumor replication, and the destruction/eradication of tumor cells via oncolysis make oncolytic viruses (OV) an important strategy in HCC therapy. Oncolytic vesicular stomatitis virus (VSV) and Newcastle disease virus (NDV) prolonged the survival in orthotopic HCC by tumor-specific cell lysis in the rat model [195,196,197], but due to the spontaneous malignant transformation in the immune-competent setting in patients, it is difficult to transfer the efficacy of the treatment by OVs to clinics. The microenvironment of the liver contributes to the inhibitory effect on OV therapy and limits the successful delivery and propagation of the virus to tumor tissue. The innate immune defense by NK cells against the virus, the production of anti-viral cytokines (IFN-γ and TNF-α) from non-parenchymal cells (kupffer cells, stellate cells), and the sinusoidal endothelial cells that provide a platform for non-specific uptake and a barrier for viral spread combine to limit the success of OV therapy. Additionally, the presence of chronic inflammation, altered hepatic blood flow, constitutive cytokine expression, and extracellular matrix deposition pose the further limitation to OV therapy [198].
The development of novel strategies with enhanced viral replication, propagation, and virus-mediated anti-tumor response in the complex setting of hepatocellular carcinomas is currently needed for more effective and efficient OV therapy. To be an efficient OV, the virus must reach and infect the intended tissue, should replicate and spread efficiently in the tissue, and the inserted transgene must be chosen rationally to provide maximum benefit for the treatment of the cancer [199]. Arming the therapeutic gene, antibody, and chemotherapeutic agent with OVs to enhance the anti-tumor activity synergistically is an emerging prevalent strategy to improve the oncovirotherapy of cancer. Armed OVs combine antibody therapy, gene therapy, immunotherapy, and oncovirotherapy, making OVs a potential and promising agent for HCC treatment [66, 200, 201] (Table 4).
Despite the improved outcome, low intra-tumor titers of OVs may affect the treatment and hamper the tumor lysis due to insufficient tumor targets and strong anti-viral immune response of the host. Hence, there is a need to improve the intra-tumor viral titers. Almstätter et al. [220] proposed the concept of combining OVs with magnetic nanoparticles for effective delivery, leading to higher titers of OVs in tumor tissue. Enhanced tumor necrosis and synergistically prolonged survival have been reported by combined transarterial viroembolization of VSV and degradable starch microspheres to produce the same effect of apoptosis, anti-angiogenesis, and induction of anti-tumor immunity in HCC [221]. Similarly, folate-conjugated chitosan nanoparticles (FA-CS-NPs) with mouse interferon-γ-inducible protein-10 (IP-10) plasmid showed enhanced anti-tumor activity with inhibition of tumor growth and prolonged the survival time, suggesting the potential role of nanoparticles in effective delivery [222].
Another strategy for effective virotherapy may be the suppression of the host immune response to facilitate the replication and propagation of OVs. Suppression of immune response limited to the area of viral replication is the best strategy, alleviating the concern of general immune response suppression, and can be achieved by incorporating anti-inflammatory protein-encoding genes directly into the OVs [223]. Production of chemokine-binding proteins (CKBPs) by viruses to counteract anti-viral immune response to promote their own growth and to enhance the potency may be another field of study in using OVs. High affinity and broad range vCKBPs, M3 gene from murine gammaherpesvirus-68, and recombinant VSV vectors encoding for the equine herpes virus-1 glycoprotein G are examples. Stimulation of the host’s immune response against tumor cells using OVs is another strategy to treat HCC, mainly in tumors where viral replication is not possible due to unfavorable conditions. OVs can break the tolerance of liver cells and enhance the immunogenicity of the tumorous microenvironment of the liver [198]. Interleukin (IL)-12 has potent immunostimulatory activity and anti-angiogenic properties. He et al. [224] reported the enhancement of tumor growth suppression, apoptosis induction, massive accumulation of immune cells (CD8+ T leukocytes, macrophages, and DCs), and reduction in angiogenesis in the tumor tissue with IL-12 combined with recombinant adenovirus expressing HBx (Ad-HBx-mIL-12), suggesting the potential benefit of combining virotherapy with immunotherapy therapy.
Other strategies for virotherapy with immunotherapy of HCC that require further research work include adoptive transferring of immune cells together with OVs, incorporation of “T-cell engagers” into OVs, and systemic use of oncolytic NDV followed by DCs pulsed with viral encolyste intradermal vaccination. Subsequent administration of two different recombinant OVs for priming the immune response through tumor-associated antigen expression by the first and boosting secondary response by the second leads to robust tumor-specific immunity. OVs cannot be administered systemically due to virus inactivation by blood components; hence, there is a need for a carrier that can save the virus from inactivation. PEGylation may serve this purpose [198].
Targeting the immune checkpoints
Effective and successful host immune response via effective CTL activity is needed to protect the liver from inflammatory injury, along with a balance between the positive and negative signals from the T-cell receptors and co-regulatory ligands. Any defect in the host immune response will cause liver cell injury. Evasion of the host immune response by cancer cells may be due to the protective microenvironment of the tumorous liver [225] or by the complex interaction between the tumor cells and the liver cells such as fibroblasts, regulatory immune cells, endothelial cells, and pericytes, IL-10, TGF-β, Fas, and other membrane-bound molecules [226]. Immune checkpoint inhibitor therapy can be a potential target for further research work. PD-L1, a T-cell co-stimulatory molecule of B7 family, is found overexpressed in HCC [227] and is related to tumor aggressiveness and poor prognosis [228, 229]. Stimulated B-cells, T-cells, and myeloid cells express PD-1 a receptor for PD-L1. PD-L1 ligation of PD-1 is immunosuppressive; hence, inhibition of this interaction may be a strategy for immunotherapy and may enhance HCV and HBV clearance along with preventing HCC recurrence [230, 231]. Blocking the ligands CD80 and CD86 and CTLA4 by anti-CTLA-4 antibody results in the promotion of T-cell activation and may be used to enhance the immune response [232, 233]. Suppression of both T and NK cells [234], along with induction of CD4+ regulatory T-cells in HCC patients, is done by a subset of immune suppressor cell called myeloid-derived suppressor cells (MDSC), which are generally higher in frequency in HCC; and it has been demonstrated that there is a correlation between outcome and MDSC frequencies. Poor prognosis in patients undergoing surgical resection has also been correlated with tumor-infiltrating CD4+ regulatory T-cells [235, 236].
VEGF, a promoter of vasculogenesis and angiogenesis, can act as an immunosuppressor by promoting immunosuppressive cell infiltration and enhances the immune checkpoint molecule expression. VEGF inhibition results in increased tumor hypoxia by decreased angiogenesis, hepatic vascular regression, and HIF-2α stabilization. Sorafenib is a pan-VEGF receptor inhibitor and thus by its anti-angiogenic action can increase tumor hypoxia. Increased hypoxia after sorafenib treatment promotes immunosuppression [237, 238]. Immunosuppression in HCC after sorafenib treatment is promoted by increasing tumor hypoxia through VEGF inhibition. This leads to increased intratumoral expression of the immune checkpoint inhibitor PD-L1 and accumulation of T-reg cells and M2-type macrophages. Hypoxia-induced upregulation of stromal cell-derived (SCD)-1 alpha mediates recruitment of these cells; hence, inhibition of SCD-1 alpha receptor (CXCR4) may prevent the immunosuppression. Though anti-PD-L1 antibody has anti-tumor potential, its potency is enhanced with inhibition of CXCR4. Hence, anti-VEGF therapy combined with immunotherapy may be a potential therapy for HCC [237, 238]. The augmentation of anti-tumor effect of peptide vaccine by increasing immune response of vaccine-induced CTLs via PD-1/PD-L1 blockade further supports the need to develop GPC3 peptide vaccine and αPD-1 antibody combination therapy [239] (Fig. 4).
Hormonal therapy
Males have two times higher risk of developing fibrosis and HCC compared to females. Among females, risk increases with age and post-menopausal women have higher risk of developing fibrosis and HCC than pre-menopausal age. The difference in the severity of fibrosis and HCC with gender and age indicate the probable role of sex hormone in the disease pathogenesis and may be due to the protective effects of estrogen against fibrogenesis and inhibition of the stellate cell activation with estrogen. Menopausal state in women with decreased estrogen level is associated with mitochondrial dysfunction, cellular senescence, decreased immune response to injury, and increased oxidative stress [240,241,242]. Collectively, these changes may increase the susceptibility to liver fibrosis and HCC. Since decreased estrogen is associated with increased susceptibility to HCC, estrogen therapy might be beneficial in decreasing the progression of disease. The protective effect of estrogen through IL-6 restrictions, STAT3 inactivation, tumor-associated macrophage inhibition, and decreased inflammation has been documented in patients with HCV and HBV. Furthermore, decreased probability of liver fibrosis has been shown with hormone replacement therapy in post-menopausal women [240,241,242]. This suggests that estrogen therapy might be used to decrease the progression of disease in the early disease stage. But, diethylnitrosamine-induced development of liver cancer has also been documented. Thus, the effect of estrogen therapy on estrogen receptor, its anti-inflammatory effect, interactions between microRNAs and estrogen in HCC, extent of benefits and overall safety, and the dose and time of estrogen administration warrant further investigations [240,241,242].
Conclusion
HCC represents more than 90% of the primary liver cancers and is an emerging cause of mortality. The inadequacy of present-day treatment modalities like surgical resection, chemotherapy, radiotherapy and local ablation such as RAF and TACE raises concern and indicates the need for more effective treatments. Host immune response suppression results in the unopposed development of HCC; thus, activation of the host immune system may serve as a potential treatment strategy yielding improved outcomes. Combination therapy, gene therapy, and immunotherapy have shown promising results in treating the tumor directly and in preventing recurrence and reactivation. Immunotherapy of HCC by targeting AFP, Glypican-3, DCs and NK cells, NY-ESO-1, MAGE-A family, SSX-1, and ROBO1 are still in the pre-clinical or clinical trial phase and require further studies to develop effective therapeutic immunotherapy for HCC. Immune checkpoint inhibition and adoptive cell transfer therapy are other treatment modalities in the developing phase. The existing therapies for the treatment of HCC have shown promising results. However, immunogenicity and the presence of an immunological barrier, viral toxicity, side effects from chemotherapy, and refractoriness to chemotherapy, drug resistance, and problems in translating the research findings into clinical utility remain major concerns.
References
Greten TF, Duffy AG, Korangy F (2013) Hepatocellular carcinoma from an immunologic perspective. Clin Cancer Res 19(24):6678–6685
El-Serag HB (2007) Epidemiology of hepatocellular carcinoma in USA. Hepatol Res 37(Suppl 2):S88–S94
El-Serag HB, Rudolph KL (2007) Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 132(7):2557–2576
Zeng Z (2014) Human genes involved in hepatitis B virus infection. World J Gastroenterol 20(24):7696–7706
Zanetti AR (1999) Global surveillance and control of hepatitis C. Report of a WHO Consultation organized in collaboration with the Viral Hepatitis Prevention Board, Antwerp, Belgium. J Viral Hepat 6(1):35–47
Parkin DM, Bray F, Ferlay J, Pisani P (2001) Estimating the world cancer burden: Globocan 2000. Int J Cancer 94(2):153–156
Miamen AG, Dong H, Roberts LR (2012) Immunotherapeutic approaches to hepatocellular carcinoma treatment. Liver Cancer 1(3–4):226–237
Mizukoshi E, Nakamoto Y, Arai K, Yamashita T, Sakai A, Sakai Y et al (2011) Comparative analysis of various tumor-associated antigen-specific t-cell responses in patients with hepatocellular carcinoma. Hepatology 53(4):1206–1216
Korangy F, Ormandy LA, Bleck JS, Klempnauer J, Wilkens L, Manns MP et al (2004) Spontaneous tumor-specific humoral and cellular immune responses to NY-ESO-1 in hepatocellular carcinoma. Clin Cancer Res 10(13):4332–4341
Primack A, Vogel CL, Barker LF (1973) Immunological studies in Ugandan patients with hepatocellular carcinoma. BMJ 1(5844):16–19
Beckebaum S, Cicinnati VR, Dworacki G, Muller-Berghaus J, Stolz D, Harnaha J et al (2002) Reduction in the circulating pDC1/pDC2 ratio and impaired function of ex vivo-generated DC1 in chronic hepatitis B infection. Clin Immunol 104(2):138–150
Ninomiya T, Akbar SM, Masumoto T, Horiike N, Onji M (1999) Dendritic cells with immature phenotype and defective function in the peripheral blood from patients with hepatocellular carcinoma. J Hepatol 31(2):323–331
Pedroza-Gonzalez A, Zhou G, Vargas-Mendez E, Boor PP, Mancham S, Verhoef C et al (2015) Tumor-infiltrating plasmacytoid dendritic cells promote immunosuppression by Tr1 cells in human liver tumors. Oncoimmunology 4(6):e1008355
Rai V, Dietz NE, Agrawal DK (2016) Immunological basis for treatment of graft versus host disease after liver transplant. Expert Rev Clin Immunol 12(5):583–593
El-Serag HB, Marrero JA, Rudolph L, Reddy KR (2008) Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology 134(6):1752–1763
den Brok M, Sutmuller RPM, Nierkens S, Bennink EJ, Frielink C, Toonen LWJ et al (2006) Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumour immunity. Br J Cancer 95(7):896–905
Zerbini A, Pilli M, Penna A, Pelosi G, Schianchi C, Molinari A et al (2006) Radiofrequency thermal ablation of hepatocellular carcinoma liver nodules can activate and enhance tumor-specific T-cell responses. Can Res 66(2):1139–1146
Hansler J, Wissniowski TuT, Schuppan D, Witte A, Bernatik T, Hahn EG et al (2006) Activation and dramatically increased cytolytic activity of tumor specific T lymphocytes after radio-frequency ablation in patients with hepatocellular carcinoma and colorectal liver metastases. World J Gastroenterol 12(23):3716–3721
Zerbini A, Pilli M, Laccabue D, Pelosi G, Molinari A, Negri E et al (2010) Radiofrequency thermal ablation for hepatocellular carcinoma stimulates autologous NK-cell response. Gastroenterology 138(5):1931–1942
Mizukoshi E, Yamashita T, Arai K, Sunagozaka H, Ueda T, Arihara F et al (2013) Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology 57(4):1448–1457
Ayaru L, Pereira SP, Alisa A, Pathan AA, Williams R, Davidson B et al (2007) Unmasking of alpha-fetoprotein-specific CD4(+) T cell responses in hepatocellular carcinoma patients undergoing embolization. J Immunol 178(3):1914–1922
Hiroishi K, Eguchi J, Baba T, Shimazaki T, Ishii S, Hiraide A et al (2008) Strong CD8+ T-cell responses against tumor-associated antigens prolong the recurrence-free interval after tumor treatment in patients with hepatocellular carcinoma. J Gastroenterol 45(4):451–458
Nobuoka D, Motomura Y, Shirakawa H, Yoshikawa T, Kuronuma T, Takahashi M et al (2012) Radiofrequency ablation for hepatocellular carcinoma induces glypican-3 peptide-specific cytotoxic T lymphocytes. Int J Oncol 40(1):63–70
Wu F, Wang ZB, Lu P, Xu ZL, Chen WZ, Zhu H et al (2004) Activated anti-tumor immunity in cancer patients after high intensity focused ultrasound ablation. Ultrasound Med Biol 30(9):1217–1222
Zeng Z, Shi F, Zhou L, Zhang MN, Chen Y, Chang XJ et al (2011) Upregulation of circulating PD-L1/PD-1 is associated with poor post-cryoablation prognosis in patients with HBV-related hepatocellular carcinoma. PLoS ONE 6(9):e23621
Shi L, Chen L, Wu C, Zhu Y, Xu B, Zheng X et al (2016) PD-1 Blockade Boosts Radiofrequency Ablation-Elicited Adaptive Immune Responses against Tumor. Clin Cancer Res 22(5):1173–1184
Asghar U, Meyer T (2012) Are there opportunities for chemotherapy in the treatment of hepatocellular cancer? J Hepatol 56(3):686–695
Cabibbo G, Craxi A (2010) Epidemiology, risk factors and surveillance of hepatocellular carcinoma. Eur Rev Med Pharmacol Sci 14(4):352–355
Bartosch B (2010) Hepatitis B and C viruses and hepatocellular carcinoma. Viruses 2(8):1504–1509
Tomov B, Popov D, Tomova R, Vladov N, Den Otter W, Krastev Z (2013) Therapeutic response of untreatable hepatocellular carcinoma after application of the immune modulators IL-2, BCG and melatonin. Anticancer Res 33(10):4531–4535
Aguirre-Ghiso JA (2007) Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer 7(11):834–846
Christophi C, Harun N, Fifis T (2008) Liver regeneration and tumor stimulation-a review of cytokine and angiogenic factors. J Gastrointest Surg 12(5):966–980
Chen JA, Shi M, Li JQ, Qian CN (2010) Angiogenesis: multiple masks in hepatocellular carcinoma and liver regeneration. Hepatol Int 4(3):537–547
Butterfield LH (2004) Immunotherapeutic strategies for hepatocellular carcinoma. Gastroenterology 127(5 Suppl 1):S232–S241
Guidotti LG, Chisari FV (2006) Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol Mech Dis 1:23–61
Tan S-L, Katze MG (2001) How hepatitis C virus counteracts the interferon response: the jury is still out on NS5A. Virology 284(1):1–12
Reyes GR (2002) The nonstructural NS5A protein of hepatitis C virus: an expanding, multifunctional role in enhancing hepatitis C virus pathogenesis. J Biomed Sci 9(3):187–197
Macdonald A, Harris M (2004) Hepatitis C virus NS5A: tales of a promiscuous protein. J Gen Virol 85(9):2485–2502
Fournier C, Fo Helle, Vr Descamps, Morel V, François C, Dedeurwaerder S et al (2013) Natural selection of adaptive mutations in non-structural genes increases trans-encapsidation of hepatitis C virus replicons lacking envelope protein genes. J Gen Virol 94(Pt 5):996–1008
Gong G, Waris G, Tanveer R, Siddiqui A (2001) Human hepatitis C virus NS5A protein alters intracellular calcium levels, induces oxidative stress, and activates STAT-3 and NF-kappa B. Proc Natl Acad Sci USA 98(17):9599–9604
Majumder M, Ghosh AK, Steele R, Ray R, Ray RB (2001) Hepatitis C virus NS5A physically associates with p53 and regulates p21/waf1 gene expression in a p53-dependent manner. J Virol 75(3):1401–1407
Polyak SJ, Khabar KSA, Paschal DM, Ezelle HJ, Duverlie G, Barber GN et al (2001) Hepatitis C virus nonstructural 5A protein induces interleukin-8, leading to partial inhibition of the interferon-induced antiviral response. J Virol 75(13):6095–6106
Foy E, Li K, Wang C, Sumpter R, Ikeda M, Lemon SM et al (2003) Regulation of interferon regulatory factor-3 by the hepatitis C virus serine protease. Science 300(5622):1145–1148
Park KJ, Choi SH, Choi DH, Park JM, Yie SW, Lee SY et al (2003) Hepatitis C virus NS5A protein modulates c-Jun N-terminal kinase through interaction with tumor necrosis factor receptor-associated factor 2. J Biol Chem 278(33):30711–30718
Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392(6673):245–252
Homma S, Toda G, Gong J, Kufe D, Ohno T (2001) Preventive antitumor activity against hepatocellular carcinoma (HCC) induced by immunization with fusions of dendritic cells and HCC cells in mice. J Gastroenterol 36(11):764–771
Tatsumi T, Takehara T, Kanto T, Miyagi T, Kuzushita N, Sugimoto Y et al (2001) Administration of interleukin-12 enhances the therapeutic efficacy of dendritic cell-based tumor vaccines in mouse hepatocellular carcinoma. Cancer Res 61(20):7563–7567
Lee WC, Wang HC, Jeng LB, Chiang YJ, Lia CR, Huang PF et al (2001) Effective treatment of small murine hepatocellular carcinoma by dendritic cells. Hepatology 34(5):896–905
Su S, Zhou H, Xue M, Liu JY, Ding L, Cao M et al (2013) Anti-tumor efficacy of a hepatocellular carcinoma vaccine based on dendritic cells combined with tumor-derived autophagosomes in murine models. Asian Pac J Cancer Prev 14(5):3109–3116
Xie BH, Yang JY, Li HP, Zhang B, Chen W, Zhou B et al (2014) Dendritic cells transfected with hepatocellular carcinoma (HCC) total RNA induce specific immune responses against HCC in vitro and in vivo. Clin Transl Oncol 16(8):753–760
Zhou J, Ma P, Li J, Cui X, Song W (2016) Improvement of the cytotoxic T lymphocyte response against hepatocellular carcinoma by transduction of cancer cells with an adeno-associated virus carrying the interferon-gamma gene. Mol Med Rep 13(4):3197–3205
Lu SY, Sui YF, Li ZS, Ye J, Dong HL, Qu P et al (2004) Superantigen-SEA gene modified tumor vaccine for hepatocellular carcinoma: an in vitro study. World J Gastroenterol 10(1):53–57
Schueller G, Stift A, Friedl J, Dubsky P, Bachleitner-Hofmann T, Benkoe T et al (2003) Hyperthermia improves cellular immune response to human hepatocellular carcinoma subsequent to co-culture with tumor lysate pulsed dendritic cells. Int J Oncol 22(6):1397–1402
Sun C, Sun HY, Xiao WH, Zhang C, Tian ZG (2015) Natural killer cell dysfunction in hepatocellular carcinoma and NK cell-based immunotherapy. Acta Pharmacol Sin 36(10):1191–1199
Shabani Z, Bagheri M, Zare-Bidaki M, Hassanshahi G, Arababadi MK, Nejad MM et al (2014) NK cells in hepatitis B virus infection: a potent target for immunotherapy. Arch Virol 159(7):1555–1565
Cariani E, Missale G (2013) KIR/HLA immunogenetic background influences the evolution of hepatocellular carcinoma. Oncoimmunology 2(12):e26622
Sun X, Sui Q, Zhang C, Tian Z, Zhang J (2013) Targeting Blockage of STAT3 in Hepatocellular Carcinoma Cells Augments NK Cell Functions via Reverse Hepatocellular Carcinoma-Induced Immune Suppression. Mol Cancer Ther 12(12):2885–2896
Jiang W, Zhang C, Tian Z, Zhang J (2014) hIL-15 gene-modified human natural killer cells (NKL-IL15) augments the anti-human hepatocellular carcinoma effect in vivo. Immunobiology 219(7):547–553
Kamiya T, Chang YH, Campana D (2016) Expanded and activated natural killer cells for immunotherapy of hepatocellular carcinoma. Cancer Immunol Res 4(7):574–581
Hong ZF, Zhao WX, Yin ZY, Xie CR, Xu YP, Chi XQ et al (2016) Natural killer cells inhibit pulmonary metastasis of hepatocellular carcinoma in nude mice. Oncol Lett 11(3):2019–2026
Attallah AM, Tabll AA, El-Sadany M, Ibrahim TA, El-Dosoky I (2003) Dysregulation of blood lymphocyte subsets and natural killer cells in schistosomal liver cirrhosis and hepatocellular carcinoma. Clin Exp Med 3(3):181–185
Sachdeva M, Chawla YK, Arora SK (2015) Immunology of hepatocellular carcinoma. World J Hepatol 7(17):2080–2090
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc J-F et al (2008) Sorafenib in advanced hepatocellular carcinoma. New Eng J Med 359(4):378–390
Cabrera R, Ararat M, Xu Y, Brusko T, Wasserfall C, Atkinson MA et al (2013) Immune modulation of effector CD4+ and regulatory T cell function by sorafenib in patients with hepatocellular carcinoma. Cancer Immunol Immunother 62(4):737–746
Nagai H, Mukozu T, Matsui D, Kanekawa T, Kanayama M, Wakui N et al (2012) Sorafenib prevents escape from host immunity in liver cirrhosis patients with advanced hepatocellular carcinoma. Clinical Dev Immunol 2012:607851
Ady JW, Heffner J, Mojica K, Johnsen C, Belin LJ, Love D et al (2014) Oncolytic immunotherapy using recombinant vaccinia virus GLV-1h68 kills sorafenib-resistant hepatocellular carcinoma efficiently. Surgery 156(2):263–269
Tai WT, Cheng AL, Shiau CW, Liu CY, Ko CH, Lin MW et al (2012) Dovitinib induces apoptosis and overcomes sorafenib resistance in hepatocellular carcinoma through SHP-1-mediated inhibition of STAT3. Mol Cancer Ther 11(2):452–463
Chen KF, Chen HL, Liu CY, Tai WT, Ichikawa K, Chen PJ et al (2012) Dovitinib sensitizes hepatocellular carcinoma cells to TRAIL and tigatuzumab, a novel anti-DR5 antibody, through SHP-1-dependent inhibition of STAT3. Biochem Pharmacol 83(6):769–777
Ezzoukhry Z, Louandre C, Trecherel E, Godin C, Chauffert B, Dupont S et al (2012) EGFR activation is a potential determinant of primary resistance of hepatocellular carcinoma cells to sorafenib. Int J Cancer 131(12):2961–2969
Maheswaran T, Rushbrook SM (2012) Epithelial-mesenchymal transition and the liver: role in hepatocellular carcinoma and liver fibrosis. J Gastroenterol Hepatol 27(3):418–420
Chuang HY, Chang YF, Liu RS, Hwang JJ (2014) Serial low doses of sorafenib enhance therapeutic efficacy of adoptive T cell therapy in a murine model by improving tumor microenvironment. PLoS ONE 9(10):e109992
Rosenberg SA (2011) Cell transfer immunotherapy for metastatic solid cancer-what clinicians need to know. Nat Rev Clin Oncol 8(10):577–585
Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G et al (2008) Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med 14(11):1264–1270
Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U et al (2008) Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol 26(32):5233–5239
Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ et al (2011) Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res 17(13):4550–4557
Hu H, Qiu Y, Guo M, Huang Y, Fang L, Peng Z et al (2015) Targeted Hsp70 expression combined with CIK-activated immune reconstruction synergistically exerts antitumor efficacy in patient-derived hepatocellular carcinoma xenograft mouse models. Oncotarget 6(2):1079–1089
Li R, Yan F, Liu L, Li H, Ren B, Hui Z et al (2016) Cytokine-induced killer cell therapy for the treatment of primary hepatocellular carcinoma subsequent to liver transplantation: a case report. Oncol Lett 11(3):1885–1888
Pedroza-Gonzalez A, Zhou G, Singh SP, Boor PP, Pan Q, Grunhagen D et al (2015) GITR engagement in combination with CTLA-4 blockade completely abrogates immunosuppression mediated by human liver tumor-derived regulatory T cells. Oncoimmunology 4(12):e1051297
Han Y, Yang Y, Chen Z, Jiang Z, Gu Y, Liu Y et al (2014) Human hepatocellular carcinoma-infiltrating CD4+ CD69+ Foxp3− regulatory T cell suppresses T cell response via membrane-bound TGF-β1. J Mol Med 92(5):539–550
Spear TT, Callender GG, Roszkowski JJ, Moxley KM, Simms PE, Foley KC et al (2016) TCR gene-modified T cells can efficiently treat established hepatitis C-associated hepatocellular carcinoma tumors. Cancer Immunol Immunother 65(3):293–304
Habib NA, Ding SF, El-Masry R, Mitry RR, Honda K, Michail NE et al (1996) Preliminary report: the short-term effects of direct p53 DNA injection in primary hepatocellular carcinomas. Cancer Detect Prev 20(2):103–107
Leboeuf Cl, Mailly L, Wu T, Bour G, Durand S, Brignon N et al (2014) In Vivo Proof of Concept of Adoptive Immunotherapy for Hepatocellular Carcinoma Using Allogeneic Suicide Gene-modified Killer Cells. Mol Ther 22(3):634–644
Wu T, Leboeuf C, Durand S, Su B, Deschamps M, Zhang X et al (2016) Suicide gene-modified killer cells as an allogeneic alternative to autologous cytokine-induced killer cell immunotherapy of hepatocellular carcinoma. Mol Med Rep 13(3):2645–2654
He L, Gong HX, Li XP, Wang YD, Li Y, Huang JJ et al (2013) Inhibition of hepatocellular carcinoma growth by adenovirus-mediated expression of human telomerase reverse transcriptase COOH-27 terminal polypeptide in mice. Oncol Lett 6(3):748–752
Sun L, Guo H, Jiang R, Lu L, Liu T, He X (2016) Engineered cytotoxic T lymphocytes with AFP-specific TCR gene for adoptive immunotherapy in hepatocellular carcinoma. Tumour Biol 37(1):799–806
Chen Y, Huang A, Gao M, Yan Y, Zhang W (2013) Potential therapeutic value of dendritic cells loaded with NY-ESO-1 protein for the immunotherapy of advanced hepatocellular carcinoma. Int J Mol Med 32(6):1366–1372
Fujiwara K, Koyama K, Suga K, Ikemura M, Saito Y, Hino A et al (2014) A 90 Y-labelled anti-ROBO1 monoclonal antibody exhibits antitumour activity against hepatocellular carcinoma xenografts during ROBO1-targeted radioimmunotherapy. EJNMMI Res 4(1):29
Aref AM, Hoa NT, Ge L, Agrawal A, Dacosta-Iyer M, Lambrecht N et al (2014) HCA519/TPX2: a potential T-cell tumor-associated antigen for human hepatocellular carcinoma. Onco Targets Ther 7:1061–1070
Hong YP, Li ZD, Prasoon P, Zhang Q (2015) Immunotherapy for hepatocellular carcinoma: from basic research to clinical use. World J Hepatol 7(7):980–992
Mahoney KM, Rennert PD, Freeman GJ (2015) Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov 14(8):561–584
Pinter M, Trauner M, Peck-Radosavljevic M, Sieghart W (2016) Cancer and liver cirrhosis: implications on prognosis and management. ESMO Open 1(2):e000042
Zwicke GL, Mansoori GA, Jeffery CJ (2012) Utilizing the folate receptor for active targeting of cancer nanotherapeutics. Nano Rev 3:1–17
Ling D, Xia H, Park W, Hackett MJ, Song C, Na K et al (2014) pH-sensitive nanoformulated triptolide as a targeted therapeutic strategy for hepatocellular carcinoma. ACS Nano 8(8):8027–8039
Bagheri V, Askari A, Arababadi MK, Kennedy D (2014) Can Toll-Like Receptor (TLR) 2 be considered as a new target for immunotherapy against hepatitis B infection? Hum Immunol 75(6):549–554
Lu X, Xu Q, Bu X, Ma X, Zhang F, Deng Q et al (2014) Relationship between expression of toll-like receptors 2/4 in dendritic cells and chronic hepatitis B virus infection. Int J Clin Exp Pathol 7(9):6048–6055
Bagheri V, Askari A, Arababadi MK, Kennedy D (2014) Can Toll-Like Receptor (TLR) 2 be considered as a new target for immunotherapy against hepatitis B infection? Hum Immunol 75(6):549–554
Zhang Y, Lin A, Zhang C, Tian Z, Zhang J (2014) Phosphorothioate-modified CpG oligodeoxynucleotide (CpG ODN) induces apoptosis of human hepatocellular carcinoma cells independent of TLR9. Cancer Immunol Immunother 63(4):357–367
Duan M, Wang ZC, Wang XY, Shi JY, Yang LX, Ding ZB et al (2015) TREM-1, an inflammatory modulator, is expressed in hepatocellular carcinoma cells and significantly promotes tumor progression. Ann Surg Oncol 22(9):3121–3129
Wu J, Li J, Salcedo R, Mivechi NF, Trinchieri G, Horuzsko A (2012) The proinflammatory myeloid cell receptor TREM-1 controls Kupffer cell activation and development of hepatocellular carcinoma. Cancer Res 72(16):3977–3986
Cepeda EB, Dediulia T, Fernando J, Bertran E, Egea G, Navarro E et al (2015) Mechanisms regulating cell membrane localization of the chemokine receptor CXCR4 in human hepatocarcinoma cells. Biochim Biophys Acta 1853:1205–1218
Hu F, Miao L, Zhao Y, Xiao YY, Xu Q (2015) A meta-analysis for C-X-C chemokine receptor type 4 as a prognostic marker and potential drug target in hepatocellular carcinoma. Drug Des Dev Ther 9:3625–3633
Garcia-Irigoyen O, Latasa MU, Carotti S, Uriarte I, Elizalde M, Urtasun R et al (2015) Matrix metalloproteinase 10 contributes to hepatocarcinogenesis in a novel crosstalk with the stromal derived factor 1/C-X-C chemokine receptor 4 axis. Hepatology 62(1):166–178
Li X, Yao W, Yuan Y, Chen P, Li B, Li J et al (2015) Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut 66(1):157–167
Kondo Y, Kimura O, Tanaka Y, Ninomiya M, Iwata T, Kogure T et al (2015) Differential expression of CX3CL1 in hepatitis B virus-replicating hepatoma cells can affect the migration activity of CX3CR1+ immune cells. J Virol 89(14):7016–7027
Li X, Yao W, Yuan Y, Chen P, Li B, Li J et al (2017) Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut 66(1):157–167
Teng KY, Han J, Zhang X, Hsu SH, He S, Wani N et al (2017) Blocking the CCL2-CCR2 axis using CCL2 neutralizing antibody is an effective therapy for hepatocellular cancer in a mouse model. Mol Cancer Ther 16(2):312–322
Barashi N, Weiss ID, Wald O, Wald H, Beider K, Abraham M et al (2013) Inflammation-induced hepatocellular carcinoma is dependent on CCR5 in mice. Hepatology 58(3):1021–1030
Gao Y, Zhou Z, Lu S, Huang X, Zhang C, Jiang R et al (2016) Chemokine CCL15 mediates migration of human bone marrow-derived mesenchymal stem cells toward hepatocellular carcinoma. Stem Cells 34(4):1112–1122
Li Y, Yu HP, Zhang P (2016) CCL15 overexpression predicts poor prognosis for hepatocellular carcinoma. Hepatol Int 10(3):488–492
Li Y, Wu J, Zhang P (2016) CCL15/CCR1 axis is involved in hepatocellular carcinoma cells migration and invasion. Tumour Biol 37(4):4501–4507
Zhu F, Li X, Chen S, Zeng Q, Zhao Y, Luo F (2016) Tumor-associated macrophage or chemokine ligand CCL17 positively regulates the tumorigenesis of hepatocellular carcinoma. Med Oncol 33(2):17
Chang X, Wang L, Zang M, Rong W, Wu Z, Liu L et al (2015) Relationship between CCL20/CCR6/Th17 axis and vascular invasion and metastasis in patients with primary hepatocellular carcinoma. Zhonghua Zhong Liu Za Zhi 37(1):5–10
Pusterla T, Nemeth J, Stein I, Wiechert L, Knigin D, Marhenke S et al (2013) Receptor for advanced glycation endproducts (RAGE) is a key regulator of oval cell activation and inflammation-associated liver carcinogenesis in mice. Hepatology 58(1):363–373
Buttner N, Schmidt N, Thimme R (2016) Perspectives of immunotherapy in hepatocellular carcinoma (HCC). Z Gastroenterol 54(12):1334–1342
Greten TF, Manns MP, Korangy F (2008) Immunotherapy of HCC. Rev Recent Clin Trials 3(1):31–39
Greten TF, Manns MP, Korangy F (2006) Immunotherapy of hepatocellular carcinoma. J Hepatol 45(6):868–878
Kew MC (1989) Tumour markers of hepatocellular carcinoma. J Gastroenterol Hepatol 4(4):373–384
Soresi M, Magliarisi C, Campagna P, Leto G, Bonfissuto G, Riili A et al (2002) Usefulness of alpha-fetoprotein in the diagnosis of hepatocellular carcinoma. Anticancer Res 23(2C):1747–1753
Vollmer CM Jr, Eilber FC, Butterfield LH, Ribas A, Dissette VB, Koh A et al (1999) Alpha-fetoprotein-specific genetic immunotherapy for hepatocellular carcinoma. Cancer Res 59(13):3064–3067
Grimm CF, Dr Ortmann, Mohr L, Michalak S, Krohne TU, Meckel S et al (2000) Mouse α-fetoprotein-specific DNA-based immunotherapy of hepatocellular carcinoma leads to tumor regression in mice. Gastroenterology 119(4):1104–1112
Pardee AD, Yano H, Weinstein AM, Ponce AA, Ethridge AD, Normolle DP et al (2015) Route of antigen delivery impacts the immunostimulatory activity of dendritic cell-based vaccines for hepatocellular carcinoma. J Immunother Cancer 3:32
Capurro MI, Xiang YY, Lobe C, Filmus J (2005) Glypican-3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Res 65(14):6245–6254
Shirakawa H, Suzuki H, Shimomura M, Kojima M, Gotohda N, Takahashi S et al (2003) Glypican-3 expression is correlated with poor prognosis in hepatocellular carcinoma. Cancer Sci 8:1403–1407
Capurro M, Wanless IR, Sherman M, Deboer G, Shi W, Miyoshi E et al (2003) Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology 125(1):89–97
Filmus J, Capurro M (2013) Glypican-3: a marker and a therapeutic target in hepatocellular carcinoma. FEBS J 280(10):2471–2476
Kojiro M, Wanless IR, Alves V, Badve S, Balabaud C, Bedossa P et al (2009) Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology 49(2):658–664
Sawada Y, Yoshikawa T, Fujii S, Mitsunaga S, Nobuoka D, Mizuno S et al (2013) Remarkable tumor lysis in a hepatocellular carcinoma patient immediately following glypican-3-derived peptide vaccination: an autopsy case. Hum Vaccin Immunother 9(6):1228–1233
Feng M, Gao W, Wang R, Chen W, Man Y-G, Figg WD et al (2013) Therapeutically targeting glypican-3 via a conformation-specific single-domain antibody in hepatocellular carcinoma. Proc Natl Acad Sci USA 110(12):E1083–E1091
Ishiguro T, Sugimoto M, Kinoshita Y, Miyazaki Y, Nakano K, Tsunoda H et al (2008) Anti-glypican3 antibody for treatment of human liver cancer. Cancer Res 68(23):9832–9838
Hanaoka H, Nagaya T, Sato K, Nakamura Y, Watanabe R, Harada T et al (2015) Glypican-3 targeted human heavy chain antibody as a drug carrier for hepatocellular carcinoma therapy. Mol Pharm 12(6):2151–2157
Gao W, Kim H, Feng M, Phung Y, Xavier CP, Rubin JS et al (2014) Inactivation of Wnt signaling by a human antibody that recognizes the heparan sulfate chains of glypican-3 for liver cancer therapy. Hepatology 60(2):576–587
Gao W, Kim H, Ho M (2015) Human monoclonal antibody targeting the heparan sulfate chains of glypican-3 inhibits HGF-mediated migration and motility of hepatocellular carcinoma cells. PLoS ONE 10(9):e0137664
Sun CK, Chua MS, He J, So SK (2011) Suppression of glypican 3 inhibits growth of hepatocellular carcinoma cells through up-regulation of TGF-beta2. Neoplasia 13(8):735–747
Zaghloul RA, El-Shishtawy MM, El Galil KH, Ebrahim MA, Metwaly AA, Al-Gayyar MM (2015) Evaluation of antiglypican-3 therapy as a promising target for amelioration of hepatic tissue damage in hepatocellular carcinoma. Eur J Pharmacol 746:353–362
Gao H, Li K, Tu H, Pan X, Jiang H, Shi B et al (2014) Development of T cells redirected to glypican-3 for the treatment of hepatocellular carcinoma. Clin Cancer Res 20(24):6418–6428
Feng M, Ho M (2014) Glypican-3 antibodies: a new therapeutic target for liver cancer. FEBS Lett 588(2):377–382
Li SQ, Lin J, Qi CY, Fu SJ, Xiao WK, Peng BG et al (2014) GPC3 DNA vaccine elicits potent cellular antitumor immunity against HCC in mice. Hepatogastroenterology 61(130):278–284
Luo C, Shibata K, Suzuki S, Kajiyama H, Senga T, Koya Y et al (2014) GPC3 expression in mouse ovarian cancer induces GPC3-specific T cell-mediated immune response through M1 macrophages and suppresses tumor growth. Oncol Rep 32(3):913–921
Sasisekharan R, Shriver Z, Venkataraman G, Narayanasami U (2002) Roles of heparan-sulphate glycosaminoglycans in cancer. Nat Rev Cancer 2(7):521–528
Liu CJ, Lee PH, Lin DY, Wu CC, Jeng LB, Lin PW et al (2009) Heparanase inhibitor PI-88 as adjuvant therapy for hepatocellular carcinoma after curative resection: a randomized phase II trial for safety and optimal dosage. J Hepatol 50(5):958–968
Liao BY, Wang Z, Hu J, Liu WF, Shen ZZ, Zhang X et al (2016) PI-88 inhibits postoperative recurrence of hepatocellular carcinoma via disrupting the surge of heparanase after liver resection. Tumour Biol 37(3):2987–2998
Liu CJ, Chang J, Lee PH, Lin DY, Wu CC, Jeng LB et al (2014) Adjuvant heparanase inhibitor PI-88 therapy for hepatocellular carcinoma recurrence. World J Gastroenterol 20(32):11384–11393
Qiu H, Yang B, Pei ZC, Zhang Z, Ding K (2010) WSS25 inhibits growth of xenografted hepatocellular cancer cells in nude mice by disrupting angiogenesis via blocking bone morphogenetic protein (BMP)/Smad/Id1 signaling. J Biol Chem 285(42):32638–32646
Budhu A, Wang XW (2006) The role of cytokines in hepatocellular carcinoma. J Leukoc Biol 80(6):1197–1213
Gelu-Simeon M, Samuel D (2013) Role of cytokine levels in assessment of prognosis and post-treatment outcome in hepatocellular carcinoma. Hepatol Int 7(3):788–791
Chen ZY, Wei W, Guo ZX, Peng LX, Shi M, Li SH et al (2014) Using multiple cytokines to predict hepatocellular carcinoma recurrence in two patient cohorts. Br J Cancer 110(3):733–740
Weiss JM, Subleski JJ, Wigginton JM, Wiltrout RH (2007) Immunotherapy of cancer by IL-12-based cytokine combinations. Expert Opin Biol Ther 7(11):1705–1721
Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, Gwak GY et al (2015) Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology 148(7):1383–1391
Wang H, Liu A, Bo W, Feng X, Hu Y, Tian L et al (2016) Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma patients after curative resection, a systematic review and meta-analysis. Dig Liver Dis 48(11):1275–1282
Ma Y, Xu YC, Tang L, Zhang Z, Wang J, Wang HX (2012) Cytokine-induced killer (CIK) cell therapy for patients with hepatocellular carcinoma: efficacy and safety. Exp Hematol Oncol 1(1):11
Su Y, Yang Y, Ma Y, Zhang Y, Rao W, Yang G et al (2016) The efficacy and safety of dendritic cells co-cultured with cytokine-induced killer cell therapy in combination with TACE-predominant minimally-invasive treatment for hepatocellular carcinoma: a meta-analysis. Clin Lab 62(4):599–608
Nelson DR, Tu Z, Soldevila-Pico C, Abdelmalek M, Zhu H, Xu YL et al (2003) Long-term interleukin 10 therapy in chronic hepatitis C patients has a proviral and anti-inflammatory effect. Hepatology 38(4):859–868
Hung CH, Chiu YC, Chen CH, Hu TH (2014) MicroRNAs in hepatocellular carcinoma: carcinogenesis, progression, and therapeutic target. Biomed Res Int 2014:486407
Guo H, Ingolia NT, Weissman JS, Bartel DP (2010) Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 466(7308):835–840
Gottwein E, Cullen BR (2008) Viral and cellular microRNAs as determinants of viral pathogenesis and immunity. Cell Host Microbe 3(6):375–387
Bushati N, Cohen SM (2007) microRNA functions. Annu Rev Cell Dev Biol 23:175–205
Borel F, Konstantinova P, Jansen PL (2012) Diagnostic and therapeutic potential of miRNA signatures in patients with hepatocellular carcinoma. J Hepatol 56(6):1371–1383
Furuta M, Kozaki KI, Tanaka S, Arii S, Imoto I, Inazawa J (2010) miR-124 and miR-203 are epigenetically silenced tumor-suppressive microRNAs in hepatocellular carcinoma. Carcinogenesis 31(5):766–776
Lujambio A, Lowe SW (2012) The microcosmos of cancer. Nature 482(7385):347–355
Tomimaru Y, Eguchi H, Nagano H, Wada H, Kobayashi S, Marubashi S et al (2012) Circulating microRNA-21 as a novel biomarker for hepatocellular carcinoma. J Hepatol 56(1):167–175
Croce CM, Calin GA (2005) miRNAs, cancer, and stem cell division. Cell 122(1):6–7
Calin GA, Croce CM (2006) MicroRNA signatures in human cancers. Nat Rev Cancer 6(11):857–866
Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K et al (2008) Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res 18(10):997–1006
Petrelli A, Perra A, Cora D, Sulas P, Menegon S, Manca C et al (2014) MicroRNA/gene profiling unveils early molecular changes and nuclear factor erythroid related factor 2 (NRF2) activation in a rat model recapitulating human hepatocellular carcinoma (HCC). Hepatology 59(1):228–241
Xu J, Wu C, Che X, Wang L, Yu D, Zhang T et al (2011) Circulating microRNAs, miR-21, miR-122, and miR-223, in patients with hepatocellular carcinoma or chronic hepatitis. Mol Carcinog 50(2):136–142
Li J, Wang Y, Yu W, Chen J, Luo J (2011) Expression of serum miR-221 in human hepatocellular carcinoma and its prognostic significance. Biochem Biophys Res Commun 406(1):70–73
Li LM, Hu ZB, Zhou ZX, Chen X, Liu FY, Zhang JF et al (2010) Serum microRNA profiles serve as novel biomarkers for HBV infection and diagnosis of HBV-positive hepatocarcinoma. Cancer Res 70(23):9798–9807
Liu AM, Yao TJ, Wang W, Wong KF, Lee NP, Fan ST et al (2012) Circulating miR-15b and miR-130b in serum as potential markers for detecting hepatocellular carcinoma: a retrospective cohort study. BMJ Open 2(2):e000825
Qu KZ, Zhang K, Li H, Afdhal NH, Albitar M (2011) Circulating microRNAs as biomarkers for hepatocellular carcinoma. J Clin Gastroenterol 45(4):355–360
Gao P, Wong CC, Tung EK, Lee JM, Wong CM, Ng IO (2011) Deregulation of microRNA expression occurs early and accumulates in early stages of HBV-associated multistep hepatocarcinogenesis. J Hepatol 54(6):1177–1184
Wang XW, Heegaard NH, Orum H (2012) MicroRNAs in liver disease. Gastroenterology 142(7):1431–1443
Zhu Y, Lu Y, Zhang Q, Liu JJ, Li TJ, Yang JR et al (2012) MicroRNA-26a/b and their host genes cooperate to inhibit the G1/S transition by activating the pRb protein. Nucleic Acids Res 40(10):4615–4625
Tsai WC, Hsu PW, Lai TC, Chau GY, Lin CW, Chen CM et al (2009) MicroRNA-122, a tumor suppressor microRNA that regulates intrahepatic metastasis of hepatocellular carcinoma. Hepatology 49(5):1571–1582
Lang Q, Ling C (2012) MiR-124 suppresses cell proliferation in hepatocellular carcinoma by targeting PIK3CA. Biochem Biophys Res Commun 426(2):247–252
Bai S, Nasser MW, Wang B, Hsu SH, Datta J, Kutay H et al (2009) MicroRNA-122 inhibits tumorigenic properties of hepatocellular carcinoma cells and sensitizes these cells to sorafenib. J Biol Chem 284(46):32015–32027
Callegari E, Elamin BK, Giannone F, Milazzo M, Altavilla G, Fornari F et al (2012) Liver tumorigenicity promoted by microRNA-221 in a mouse transgenic model. Hepatology 56(3):1025–1033
Morishita A, Masaki T (2015) miRNA in hepatocellular carcinoma. Hepatol Res 45(2):128–141
Tomimaru Y, Eguchi H, Nagano H, Wada H, Tomokuni A, Kobayashi S et al (2010) MicroRNA-21 induces resistance to the anti-tumour effect of interferon-alpha/5-fluorouracil in hepatocellular carcinoma cells. Br J Cancer 103(10):1617–1626
Wang B, Hsu S-H, Majumder S, Kutay H, Huang W, Jacob ST et al (2010) TGFβ-mediated upregulation of hepatic miR-181b promotes hepatocarcinogenesis by targeting TIMP3. Oncogene 29(12):1787–1797
Morrissey DV, Lockridge JA, Shaw L, Blanchard K, Jensen K, Breen W et al (2005) Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat Biotechnol 23(8):1002–1007
Hildebrandt-Eriksen ES, Aarup V, Persson R, Hansen HF, Munk ME, Orum H (2012) A locked nucleic acid oligonucleotide targeting microRNA 122 is well-tolerated in cynomolgus monkeys. Nucleic Acid Ther 22(3):152–161
Lanford RE, Hildebrandt-Eriksen ES, Petri A, Persson R, Lindow M, Munk ME et al (2010) Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science 327(5962):198–201
Li YP, Gottwein JM, Scheel TK, Jensen TB, Bukh J (2011) MicroRNA-122 antagonism against hepatitis C virus genotypes 1-6 and reduced efficacy by host RNA insertion or mutations in the HCV 5′ UTR. Proc Natl Acad Sci USA 108(12):4991–4996
Jiang L, Cheng Q, Zhang BH, Zhang MZ (2015) Circulating microRNAs as biomarkers in hepatocellular carcinoma screening: a validation set from China. Medicine 94(10):e603
Sun J, Lu H, Wang X, Jin H (2013) MicroRNAs in hepatocellular carcinoma: regulation, function, and clinical implications. Sci World J 2013:924206
Ko KS, Peng H, Tang H, Cho ME, Peng J, Aller M-A et al (2012) Recent advances of miRNA involvement in hepatocellular carcinoma and cholangiocarcinoma. Open J Int Med 2(3):135–162
Zhang T, Liu W, Zeng XC, Jiang N, Fu BS, Guo Y et al (2016) Down-regulation of microRNA-338-3p promoted angiogenesis in hepatocellular carcinoma. Biomed Pharmacother 84:583–591
Zhu H, Wang G, Zhou X, Song X, Gao H, Ma C et al (2016) miR-1299 suppresses cell proliferation of hepatocellular carcinoma (HCC) by targeting CDK6. Biomed Pharmacother 83:792–797
Chen L, Zhou S, Qin J, Hu H, Ma H, Liu B et al (2013) Combination of SLC administration and Tregs depletion is an attractive strategy for targeting hepatocellular carcinoma. Mol Cancer 12(1):153
Guo WW, Liu L, Wu DH (2014) Dendritic cell-cytokine induced killer cell immunotherapy combined with transcatheter arterial chemoembolization for hepatocellular carcinoma: safety and efficacy. Nan Fang Yi Ke Da Xue Xue Bao 34(5):674–678
Chen R, Yu H, An YL, Yu-Jia Z, Teng GJ (2014) Genetic immunotherapy for hepatocellular carcinoma by endothelial progenitor cells armed with cytosine deaminase. J Biomed Nanotechnol 10(2):271–277
Nemunaitis J, Barve M, Orr D, Kuhn J, Magee M, Lamont J et al (2014) Summary of bi-shRNA/GM-CSF augmented autologous tumor cell immunotherapy (FANG) in advanced cancer of the liver. Oncology 87(1):21–29
Jozuka H, Jozuka E, Suzuki M, Takeuchi S, Takatsu Y (2003) Psycho-neuro-immunological treatment of hepatocellular carcinoma with major depression-a single case report. Curr Med Res Opin 19(1):59–63
Shimizu K, Kotera Y, Aruga A, Takeshita N, Katagiri S, Ariizumi S et al (2014) Postoperative dendritic cell vaccine plus activated T-cell transfer improves the survival of patients with invasive hepatocellular carcinoma. Hum Vaccin Immunother 10(4):970–976
Ebert O, Shinozaki K, Huang TG, Savontaus MJ, Garcia-Sastre A, Woo SLC (2003) Oncolytic vesicular stomatitis virus for treatment of orthotopic hepatocellular carcinoma in immune-competent rats. Cancer Res 63(13):3605–3611
Shinozaki K, Ebert O, Woo SLC (2005) Eradication of advanced hepatocellular carcinoma in rats via repeated hepatic arterial infusions of recombinant VSV. Hepatology 41(1):196–203
Shinozaki K, Ebert O, Kournioti C, Tai YS, Woo SLC (2004) Oncolysis of multifocal hepatocellular carcinoma in the rat liver by hepatic artery infusion of vesicular stomatitis virus. Mol Ther 9(3):368–376
Altomonte J, Ebert O (2014) Sorting out Pandora’s box: discerning the dynamic roles of liver microenvironment in oncolytic virus therapy for hepatocellular carcinoma. Front Oncol 4:85
Ady JW, Johnsen C, Mojica K, Heffner J, Love D, Pugalenthi A et al (2015) Oncolytic gene therapy with recombinant vaccinia strain GLV-2b372 efficiently kills hepatocellular carcinoma. Surgery 158(2):331–338
Wei D, Li Q, Wang XL, Wang Y, Xu J, Feng F et al (2015) Oncolytic Newcastle disease virus expressing chimeric antibody enhanced anti-tumor efficacy in orthotopic hepatoma-bearing mice. J Exp Clin Cancer Res 34:153
Ma B, Wang Y, Zhou X, Huang P, Zhang R, Liu T et al (2015) Synergistic suppression effect on tumor growth of hepatocellular carcinoma by combining oncolytic adenovirus carrying XAF1 with cisplatin. J Cancer Res Clin Oncol 141(3):419–429
Bai FL, Yu YH, Tian H, Ren GP, Wang H, Zhou B et al (2014) Genetically engineered Newcastle disease virus expressing interleukin-2 and TNF-related apoptosis-inducing ligand for cancer therapy. Cancer Biol Ther 15(9):1226–1238
Bai F, Niu Z, Tian H, Li S, Lv Z, Zhang T et al (2014) Genetically engineered Newcastle disease virus expressing interleukin 2 is a potential drug candidate for cancer immunotherapy. Immunol Lett 159(1–2):36–46
Qian CY, Wang KL, Fang FF, Gu W, Huang F, Wang FZ et al (2015) Triple-controlled oncolytic adenovirus expressing melittin to exert inhibitory efficacy on hepatocellular carcinoma. Int J Clin Exp Pathol 8(9):10403–10411
Wang Y, Liu T, Huang P, Zhao H, Zhang R, Ma B et al (2015) A novel Golgi protein (GOLPH2)-regulated oncolytic adenovirus exhibits potent antitumor efficacy in hepatocellular carcinoma. Oncotarget 6(15):13564–13578
Huang F, Ma B, Wang Y, Xiao R, Kong Y, Zhou X et al (2014) Targeting gene-virus-mediated manganese superoxide dismutase effectively suppresses tumor growth in hepatocellular carcinoma in vitro and in vivo. Cancer Biother Radiopharm 29(10):403–411
Mao CY, Hua HJ, Chen P, Yu DC, Cao J, Teng LS (2009) Combined use of chemotherapeutics and oncolytic adenovirus in treatment of AFP-expressing hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int 8(3):282–287
Wirth T, Kühnel F, Fleischmann-Mundt B, Woller N, Djojosubroto M, Rudolph KL et al (2005) Telomerase-dependent virotherapy overcomes resistance of hepatocellular carcinomas against chemotherapy and tumor necrosis factor-related apoptosis-inducing ligand by elimination of Mcl-1. Cancer Res 65(16):7393–7402
Boozari B, Mundt B, Woller N, Strüver N, Gürlevik E, Schache P et al (2010) Antitumoural immunity by virus-mediated immunogenic apoptosis inhibits metastatic growth of hepatocellular carcinoma. Gut 59(10):1416–1426
Callegari E, Elamin BK, D’Abundo L, Falzoni S, Donvito G, Moshiri F et al (2013) Anti-tumor activity of a miR-199-dependent oncolytic adenovirus. PLoS ONE 8(9):e73964
Sharon D, Schumann M, MacLeod S, McPherson R, Chaurasiya S, Shaw A et al (2013) 2-aminopurine enhances the oncolytic activity of an E1b-deleted adenovirus in hepatocellular carcinoma cells. PLoS ONE 8(6):e65222
He G, Lei W, Wang S, Xiao R, Guo K, Xia Y et al (2012) Overexpression of tumor suppressor TSLC1 by a survivin-regulated oncolytic adenovirus significantly inhibits hepatocellular carcinoma growth. J Cancer Res Clin Oncol 138(4):657–670
Zhang Y, Fang L, Zhang Q, Zheng Q, Tong J, Fu X et al (2013) An oncolytic adenovirus regulated by a radiation-inducible promoter selectively mediates hSulf-1 gene expression and mutually reinforces antitumor activity of I131-metuximab in hepatocellular carcinoma. Mol Oncol 7(3):346–358
Kung CH, Kuo SC, Chen TL, Weng WS (2015) Isolation of vaccinia JX594 from pustules following therapy for hepatocellular carcinoma. BMC Cancer 15:704
Cripe TP, Ngo MC, Geller JI, Louis CU, Currier MA, Racadio JM et al (2015) Phase 1 study of intratumoral Pexa-Vec (JX-594), an oncolytic and immunotherapeutic vaccinia virus, in pediatric cancer patients. Mol Ther 23(3):602–608
Altomonte J, Marozin S, De Toni EN, Rizzani A, Esposito I, Steiger K et al (2013) Antifibrotic properties of transarterial oncolytic VSV therapy for hepatocellular carcinoma in rats with thioacetamide-induced liver fibrosis. Mol Ther 21(11):2032–2042
Marozin S, Altomonte J, Munoz-Alvarez KA, Rizzani A, De Toni EN, Thasler WE et al (2015) STAT3 inhibition reduces toxicity of oncolytic VSV and provides a potentially synergistic combination therapy for hepatocellular carcinoma. Cancer Gene Ther 22(6):317–325
Sieben M, Herzer K, Zeidler M, Heinrichs V, Leuchs B, Schuler M et al (2008) Killing of p53-deficient hepatoma cells by parvovirus H-1 and chemotherapeutics requires promyelocytic leukemia protein. World J Gastroenterol 14(24):3819
Lampe J, Bossow S, Weiland T, Smirnow I, Lehmann R, Neubert W et al (2013) An armed oncolytic measles vaccine virus eliminates human hepatoma cells independently of apoptosis. Gene Ther 20(11):1033–1041
Almstatter I, Mykhaylyk O, Settles M, Altomonte J, Aichler M, Walch A et al (2015) Characterization of magnetic viral complexes for targeted delivery in oncology. Theranostics 5(7):667–685
Altomonte J, Braren R, Schulz S, Marozin S, Rummeny EJ, Schmid RM et al (2008) Synergistic antitumor effects of transarterial viroembolization for multifocal hepatocellular carcinoma in rats. Hepatology 48(6):1864–1873
Lai C, Yu X, Zhuo H, Zhou N, Xie Y, He J et al (2014) Anti-tumor immune response of folate-conjugated chitosan nanoparticles containing the IP-10 gene in mice with hepatocellular carcinoma. J Biomed Nanotechnol 10(12):3576–3589
Prestwich RJ, Errington F, Diaz RM, Pandha HS, Harrington KJ, Melcher AA et al (2009) The case of oncolytic viruses versus the immune system: waiting on the judgment of Solomon. Hum Gene Ther 20(10):1119–1132
He H, Fan P, Yin T, Chen Q, Shi H, Liu S et al (2012) Local delivery of recombinant adenovirus expressing hepatitis B virus X protein and interleukin-12 results in antitumor effects via inhibition of hepatoma cell growth and intervention of tumor microenvironment. Int J Mol Med 30(3):599–605
Romero P, Dunbar PR, Valmori D, Ml Pittet, Ogg GS, Rimoldi D et al (1998) Ex vivo staining of metastatic lymph nodes by class I major histocompatibility complex tetramers reveals high numbers of antigen-experienced tumor-specific cytolytic T lymphocytes. J Exp Med 188(9):1641–1650
Dong H, Chen L (2003) B7-H1 pathway and its role in the evasion of tumor immunity. J Mol Med 81(5):281–287
Dong H, Zhu G, Tamada K, Chen L (1999) B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 5(12):1365–1369
Chen J, Li G, Meng H, Fan Y, Song Y, Wang S et al (2012) Upregulation of B7-H1 expression is associated with macrophage infiltration in hepatocellular carcinomas. Cancer Immunol Immunother 61(1):101–108
Gao Q, Wang X-Y, Qiu S-J, Yamato I, Sho M, Nakajima Y et al (2009) Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res 15(3):971–979
Peng G, Li S, Wu W, Tan X, Chen Y, Chen Z (2008) PD-1 upregulation is associated with HBV-specific T cell dysfunction in chronic hepatitis B patients. Mol Immunol 45(4):963–970
Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR (2007) Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol 81(17):9249–9258
Melero I, Hervas-Stubbs S, Glennie M, Pardoll DM, Chen L (2007) Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer 7(2):95–106
Sangro B, Gomez-Martin C, de la Mata M, Iñarrairaegui M, Garralda E, Barrera P et al (2013) A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol 59(1):81–88
Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H et al (2009) Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology 50(3):799–807
Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B et al (2007) Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology 132(7):2328–2339
Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS et al (2007) Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol 25(18):2586–2593
Chen Y, Ramjiawan RR, Reiberger T, Ng MR, Hato T, Huang Y et al (2015) CXCR4 inhibition in tumor microenvironment facilitates anti-programmed death receptor-1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice. Hepatology 61(5):1591–1602
Hato T, Zhu AX, Duda DG (2016) Rationally combining anti-VEGF therapy with checkpoint inhibitors in hepatocellular carcinoma. Immunotherapy 8(3):299–313
Sawada Y, Yoshikawa T, Shimomura M, Iwama T, Endo I, Nakatsura T (2015) Programmed death-1 blockade enhances the antitumor effects of peptide vaccine-induced peptide-specific cytotoxic T lymphocytes. Int J Oncol 46(1):28–36
Yang JD, Abdelmalek MF, Pang H, Guy CD, Smith AD, Diehl AM, Suzuki A (2014) Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology 59(4):1406–1414
Brady CW (2015) Liver disease in menopause. World J Gastroenterol 21(25):7613–7620
Shi L, Feng Y, Lin H, Ma R, Cai X (2014) Role of estrogen in hepatocellular carcinoma: is inflammation the key? J Transl Med 12:93
Acknowledgements
This work was supported by research Grants R01 HL116042, R01 HL112597, and R01 HL120659 to DK Agrawal from the National Heart, Lung and Blood Institute, National Institutes of Health, USA. The content of this review article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with the financial interest or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Rights and permissions
About this article
Cite this article
Rai, V., Abdo, J., Alsuwaidan, A.N. et al. Cellular and molecular targets for the immunotherapy of hepatocellular carcinoma. Mol Cell Biochem 437, 13–36 (2018). https://doi.org/10.1007/s11010-017-3092-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-017-3092-z