Abstract
Alpha-fetoprotein (AFP) is overexpressed in hepatocellular carcinoma (HCC) and could serve as a tumor-associated antigen (TAA) and potential target for adoptive immunotherapy. However, low frequency and severe functional impairment of AFP-specific T cells in vivo hamper adoptive infusion. TAA-specific T cell receptor (TCR) gene transfer could be an efficient and reliable alternation to generate AFP-specific cytotoxic T lymphocytes (CTLs). Autologous dendritic cells (DC) pulsed with AFP158-166 peptides were used to stimulate AFP-specific CTLs. TCR α/β chain genes of AFP-specific CTLs were cloned and linked by 2A peptide to form full-length TCR coding sequence synthesized into a lentiviral vector. Nonspecific activated T cells were engineered by lentivirus infection. Transgenetic CTLs were evaluated for transfection efficiency, expression of AFP158-166-specific TCR, interferon (IFN)-γ secretion, and specific cytotoxicity toward AFP+ HCC cells in vitro and in vivo. Flow cytometry revealed the AFP158-166-MHC-Pentamer positive transgenetic CTLs was 9.86 %. The number of IFN-γ secretion T cells and the specific cytotoxicity toward HpeG2 in vitro and in tumor-bearing NOD/SCID mice were significantly raised in transgenetic CTLs than that of AFP158-166-specific CTLs obtained by peptide-pulsed DCs or control group. TCR gene transfer is a promising strategy to generate AFP158-166-specific CTLs for the treatment of HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) represents the fifth most common cancer in men and the ninth in women with an increasing annual incidence of over 500,000 new cases worldwide. It was the third leading cause of death from cancer globally and the main cause of death among cirrhosis patients with a dismal 5-year survival rate of approximately 5–6 % [1]. Despite there were several therapy options available, only a minority of patients with early-stage tumors can benefit from curative treatment [2]. Therefore, effective systemic therapeutic strategies for locally advanced or metastatic HCC are urgently needed. Molecular-targeted therapy has recently shown efficacy in several human tumors and rejuvenated the field of cancer immunotherapy [3]. Although sorafenib, a broad tyrosine kinase inhibitor, was approved by Food and Drug Administration as the standard agent for systemic therapy of advanced HCC, it only provides limited survival benefits [4]. The prognosis of patients has not been significantly improved. Adoptive immunotherapy using tumor-specific receptor-engineered T cells has attracted more attention in last decade and provided novel concepts and promising directions in management of HCC for the future [5].
Alpha-fetoprotein (AFP) is highly expressed by fetal hepatocyte and yolk sac cells during embryonic development and repressed shortly after birth. Its re-expression occurs in 50 to 80 % of HCC patients during tumor progression. Serum AFP level had played an important role in HCC diagnosis and treatment responses monitor in past several decades [6]. AFP also served as a tumor-associated antigen and recognized by both murine and human T cells [7]. Although AFP-specific cytotoxic T lymphocytes (CTLs) are present in the natural T cell repertoire, low frequency and severe functional impairment in vivo hamper their application in immunotherapy of HCC. In this study, we genetically redirected T cells with high-affinity T cell receptor (TCR) by transgenic technologies to confer them robust AFP-specific cytotoxicity. We induced AFP158-166-specific T cells by peptides pulsed dendritic cells (DCs), cloned full-length coding sequence of AFP-specific TCR α and β chain, and constructed AFP158-166-specific TCR expression retroviral vector. Activated T cells were engineered with the AFP158-166-specific TCR gene and evaluated for their specific cytotoxicity both in vitro and in vivo.
Materials and methods
Healthy donors
Healthy volunteers were enrolled after providing a written informed consent in accordance with the Declaration of Helsinki. Blood samples from healthy volunteers were obtained under the Institutional Review Board of Tianjin Medical University. HLA-A*02:01 positive healthy donors were selected by flow cytometry (FCM) analysis using fluorescein isothiocyanate (FITC)-labeled anti-HLA-A2 antibody and polymerase chain reaction-sequence specific primers (PCR-SSP).
Cell lines
HepG2 (AFP+, HLA-A*02:01), SW480, and MCF7 (AFP-, HLA-A*02:01) were cultured in DMEM supplemented with 10 % FBS, 50 U/mL penicillin, and 50 μg/mL streptomycin and maintained at 37 °C with 5 % CO2. T2 (AFP-, HLA-A*02:01) was cultured in RPMI 1640 with 10 % FBS and antibiotics (Invitrogen, Frederick, MD).
Mice
Studies were conducted using 6-week-old female NOD/SCID mice. Mice were maintained at the Animal Center of Tianjin Medical University (Tianjin, China). Animal experiments were carried out according to protocols approved by the Ethics Review Committee for Animal Experimentation.
Generation of monocyte derived DCs as stimulator cells
Peripheral blood mononuclear cells (PBMCs) were obtained from HLA-A*02:01 healthy donors and isolated by density gradient centrifugation. Monocytes were enriched from PBMCs with anti-CD14 dynabeads according to the manufacturer’s protocol (Miltenyi Biotec, Bergisch Gladbach, Germany). For generation of immature DCs, monocytes were differentiated in complete culture medium [RPMI 1640 with 10 % FBS, 2 mM L-glutamine, 1 mM sodium pyruvate, 1 mM nonessential amino acids, 55 μM β-mercaptoethanol, 50 U/mL penicillin, 50 μg/mL streptomycin (Invitrogen, Frederick, MD), 500 U/mL IL-4, and 800 U/mL GM-CSF] for 5 days. DCs maturation was induced by addition of 20 ng/mL TNF-α (PeproTech, Rocky Hill, NJ) for 2 days.
Generation and isolation of AFP158-166-specific CTL clone
Mature DCs were incubated with HLA-A*02:01-restricted AFP158-166 (FMNKFIYEI) peptide (40 mg/mL) for 4 h in FBS-free RPMI 1640. CTLs were sorted from PBMCs by anti-CD8 dynabeads according to the manufacturer’s protocol (Miltenyi Biotec, Bergisch Gladbach, Germany) and seeded into 96-well plates at a concentration of 5 × 105 cells per well in complete RPMI 1640. AFP158-166 peptide-pulsed autologous DCs were used as antigen-presenting cells to stimulate AFP158-166-specific CTLs at a DC/lymphocyte ratio of 1:10 in the presence of IL-2 (10 U/mL) (PeproTech, Rocky Hill, NJ). CTLs were restimulated at days 8 and 15 with AFP158-166 peptide-pulsed autologous DCs. Cell density in the culture was always maintained at 106/mL. CTLs were also nonspecific activated by anti-CD3/CD28 antibodies (eBioscience, San Diego, CA, USA) and specific activated by HER2/neu (KIFGSLAFL) peptides pulsed DCs as control group. Activated CTLs were stained by R-PE-labeled HLA-A*02:01-restricted AFP158-166 (FMNKFIYEI) peptide MHC Pantamer (Proimmune, Oxford, UK) and FITC-labeled anti-CD8 antibody. The CD8 and Pentamer doubt positive population was sorted and collected by fluorescence activated cell sorter (FACS) (Becton Dickinson, Franklin Lakes, NJ).
Cloning of full-length TCR α and β chain genes from the AFP158-166-specific CTL clone and lentiviral vector construction
Total RNA was extracted from the HLA-A*02:01-restricted AFP158-166-specific CTL clone using RNeasy micro kits (Qiagen, Hilden, Germany) and reverse-transcribed into cDNA using high capacity cDNA reverse transcription kits (Applied Biosystems, Carlsbad, CA). TCR α/β chains fragments from AFP158-166-specific CTL population were then identified and amplified by PCR using different 5′-variable region and 3′-constant region primers for TCR α/β chains, respectively. Full-length TCR was constituted by TCR α/β chains and sequence of 2A self-cleaving peptide as a linker using overlapping-PCR. The PCR products were purified and T/A-cloned into pMD19-T Vector (Takara Bio, Otsu, Japan). They were sequenced and subcloned into a third generation self-inactivating lentiviral vector CCS-TCRa-2A-TCRb-Lv201 with eGFP as a sorting marker (GeneCopoeia, Rockville, MD). HEK-293 T cells were transfected with CCS-TCRa-2A-TCRb-Lv201 and Lenti-Pac HIV mix contained packaging plasmids to produce vesicular stomatitis virus (VSV)-G pseudotyped lentiviral particles (GeneCopoeia, Rockville, MD). Viral supernatant was collected 48 and 72 h posttransfection, filtered (0.45 μm), and concentrated by ultracentrifugation. The viral pellet was resuspended in phosphate-buffered saline (PBS) and stored at −80 °C.
Generation of AFP158-166-specific TCR-engineered T cells
PBMCs were isolated from healthy donors by density gradient centrifugation and activated with anti-CD3/CD28 antibodies for 2 days and 100 U/mL IL-2 for 1 day. Activated PBMCs were transduced with AFP158-166-specific TCR coding lentivirus in the presence of 8 μg/mL Polybrene (Sigma) and 100 U/mL IL-2 overnight. Seventy-two hours after transduction, inversion fluorescence microscope and FCM were used to evaluate the transfection efficiency by the expression of eGFP. TCR expression was evaluated by FCM using HLA-A*02:01-restricted AFP158-166 (FMNKFIYEI) peptide MHC Pantamer. The CD8 and eGFP doubt positive population was sorted and collected by FACS as AFP158-166-specific TCR-engineered CTLs, and then suspended in fresh medium containing 300 U/mL IL-2 and allowed to expand in vitro.
Human interferon (IFN)-γ enzyme-linked immunospot (ELISPOT) assay
IFN-γ production was compared between DC-induced AFP-specific CTLs and transgenetic CTLs by ELISPOT assay using Human IFN-γ ELISPOT Kit (R&D Systems, Minneapolis, MN). Then, 5 × 104 cells/well were plated in triplicate for each treatment into flat-bottom 96-well plates coated with human IFN-γ monoclonal antibodies and incubated for the indicated times prior to in situ detection of spot-forming units (SFU). CTLs treated with phytohemagglutinin (PHA) were used as positive control and CTLs without any treatment were used as negative control. Scanning and counting of SFUs in the plates were accomplished using an automatic immunospot analyzer. Data were obtained by calculating the means of triplicate wells. ELISPOT data were expressed as the total IFN-γ spots/106 PBMCs.
Lactate dehydrogenase (LDH) cytotoxicity assay
LDH assay was used to evaluate the specific cytotoxic effects of in vitro generated AFP158-166-specific TCR transgenetic CTLs on HCC cells. HepG2, SW480, MCF7, T2, T2 pulsed with AFP158-166 (FMNKFIYEI) peptides, and T2 pulsed with HER2/neu (KIFGSLAFL) peptides were used as target cells and seeded into 96-well culture plates at a density of 2 × 104 cells/well at 37 °C with 5 % CO2 for 24 h. Unactivated CTLs, nonspecific activated CTLs, DC-induced AFP-specific CTLs, and transgenetic CTLs were used as effector cells and added into target cells at different effector to target ratios (E/T) (10:1; 20:1; 40:1) to conduct LDH assay. After incubation for 6 h, cell supernatant was collected to test the amount of LDH in the cultured medium according to the manufacturer’s instructions (Promega, Madison, WI). The percentage lysis was calculated using the formula: 100 % × [(experimental release − effector spontaneous release − target effector spontaneous release) / (target maximum release − target spontaneous release)].
Adoptive cell transfer
HepG2 cells (2 × 106 in 0.2 mL PBS) were subcutaneously inoculated into the right flanks of mice. Tumors were monitored by caliper measurement every 2 days and tumor volume (V) was calculated as follows: (V = ab2 / 2) where a and b are the perpendicular smaller and large diameters, respectively. When the mean tumor volume reached 100 mm3, the mice were randomized into four groups with eight mice in each group to receive adoptive cell transfer. Nonspecific activated CTLs, DC-induced AFP-specific CTLs, and transgenetic CTLs (1 × 108) were suspended in 0.2 mL PBS and intravenously injected into a lateral tail vein of the different groups of NOD/SCID mice at days 0, 7, and 14. The mice of control group underwent intravenously injected with 0.2 mL PBS. Two mice of each group were sacrificed by cervical dislocation and tumors were excised at day 21 for pathological analysis. Other mice were sacrificed before the mean diameter of the tumor reached 20 mm according to institutional guidelines as a termination of the research.
Pathological analysis
The tumor mass were paraformaldehyde-fixed and embedded in paraffin. The 5-μm sections of tumor specimens were made and then stained with hematoxylin and eosin (H&E) to evaluate histopathologic changes. Lymphocyte infiltration of tumor specimens was analyze by immunohistochemical (IHC) staining using mouse monoclonal anti-human CD3ξ antibody (Santa Cruz Biotechnology, Santa Cruz, CA).
Statistical analyses
Data were expressed as mean ± SD. Differences between groups were examined for statistical significance using the Student’s t test. p value less than 0.01 denoted a statistically significant difference.
Results
Isolation of HLA-A*02:01 restricted AFP158-166-specific TCR
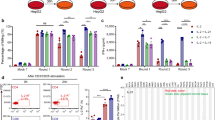
The AFP158-166-specific CTLs were generated from a HLA-A*02:01 healthy donor’s CTLs after in vitro stimulation with AFP158-166 peptide-pulsed autologous mature DCs. FCM showed that this T cell population was AFP158-166 peptide MHC Pantamer and CD8 double positive with a positive rate of 1.7 % (Fig. 1a). They were then sorted and collected by FACS for AFP158-166-specific TCR gene extracting. Isolated TCR α and β sequences from AFP158-166-specific CD8-positive T cell subset were linked by 2A sequence to form a full-length TCR gene and cloned into the lentiviral vector pEZ-Lv201 as CCS-TCRa-2A-TCRb-Lv201 (Fig. 1c).
Generation of AFP158-166-specific TCR transgenetic CTLs. a Generation and isolation of AFP-specific CTL clone. CTLs were sorted from PBMCs by anti-CD8 dynabeads and activated by anti-CD3/CD28 antibodies, HER2/neu (KIFGSLAFL) peptides pulsed DCs, and AFP158-166 (FMNKFIYEI) peptide-pulsed DCs. AFP158-166 MHC Pantamer and CD8 doubt positive population was sorted and collected by FACS as AFP158-166-specific CTL clone. b Nonspecific activated PBMCs were transfected with AFP-specific TCR coding lentivirus. The AFP158-166 MHC Pantamer and CD8 double positive population was AFP-specific TCR transgenetic CTLs. c Construction of retroviral vector encoding full-length AFP-specific TCR gene
Transfection efficiency of lentiviral and AFP158-166-specific TCR expression of transgenic CTLs
To investigate the clinical application potential of AFP158-166-specific CTLs, nonspecific activated T cells from HLA-A*02:01 health donors were transfected by lentivirus to test the expression of HLA-A*02:01 restricted AFP158-166-specific TCR in transgenic T cells. FCM revealed that the transfection efficiency was 85.4 %, and AFP158-166 peptide MHC Pantamer, CD8, and eGFP triple positive rate was 4.2 % (Fig. 1b). CD8 and eGFP double positive population was sorted by FACS for downstream experiments and the triple positive rate was 9.86 %.
Functional test of AFP158-166-specific TCR transgenic CTLs
IFN-γ positive SFU of different groups of T cells were counted using ELISPOT assay to compare their IFN-γ secretion activity. Representative ELISPOT images for DC-induced AFP-specific CTLs, AFP-specific TCR transgenic CTLs, and control groups are shown in Fig. 2b, demonstrating the TCR transgenic CTLs elicited a higher IFN-γ ELISPOT response. The mean (±SEM) number of SFU per million was 1421.5 ± 112.6 in TCR transgenic CTLs versus 785.4 ± 52.5 in DC-induced AFP-specific CTLs and 585.6 ± 36.3 in the positive control group (p < 0.01). These results indicated that the TCR transgenic CTLs contained more IFN-γ secretion T cells than DC-induced AFP-specific CTLs or unspecific activated CTLs.
Functional test of AFP158-166-specific TCR transgenetic CTLs. a Cytotoxic assay using LDH assays to evaluate the cytotoxic effects of DC-induced AFP158-166-specific CTLs and AFP158-166-specific TCR transgenetic CTLs against different target cells, including the following: HepG2, SW480, MCF7, T2, T2 pulsed with AFP158-166 peptides, and T2 pulsed with HER2/neu peptides. The unactivated CTLs and nonspecific activated CTLs were used as control groups. DC-induced AFP-specific CTLs and transgenetic CTLs showed significantly specific cytotoxicity toward AFP+ cells, including HepG2 and T2 pulsed with AFP158-166 peptides. Transgenetic CTLs showed significantly higher cytotoxicity than DC-induced CTLs and control groups toward HepG2 cells at different E/T ratios; (n = 3). b IFN-γ production T cells of DC-induced AFP-specific CTLs and transgenetic CTLs were analyzed by IFN-γ ELISPOT assays. CTLs sensitized with PHA were used as a positive control, and CTLs without sensitized were used as the negative control. The mean (±SEM) number of SFU per million was 1421.5 ± 112.6 in TCR transgenic CTLs versus 785.4 ± 52.5 in DC-induced CTLs and 585.6 ± 36.3 in positive control group (p < 0.01); (n = 3). c Antitumor activity of AFP158-166-specific TCR transgenetic CTLs in vivo. Tumors were measured with caliper every 2 days, starting on day 0. Treatment with nonspecific activated CTLs, DC-induced AFP-specific CTLs, and transgenetic CTLs was administrated at days 0, 7, and 14 by i.v. injection (black arrow indicate the treatment time). PBS was used as control group. The growth of HepG2 cells was significantly inhibited by DC-induced CTLs and transgenetic CTLs. Transgenetic CTLs exhibited significantly higher tumor inhibiting effect than DC-induced CTLs. The growth of HepG2 cells treated with nonspecific activated CTLs or PBS were not inhibited; (n = 6). Error bars indicate SD. *p < 0.01, NS refers to not significant in statistical differences
Transgenic CTLs and DCs induced AFP-specific CTLs displayed HLA-restricted and antigen epitope-specific cytotoxicity. These redirected CTLs selectively lysed the HLA-A*02:01 AFP+ human HCC cell line HepG2 and AFP158-166 peptides pulsed T2, which overexpressed AFP158-166 epitope, but not the AFP158-166 epitope negative cell lines, including the following: SW480, MCF7, T2, and HER2/neu peptides pulsed T2. The specific cytotoxicity of AFP158-166-specific TCR transgenic CTLs toward HepG2 were 11.3 ± 2.9 %, 27.9 ± 7.8 %, and 62.7 ± 10.4 % at the E/T of 10:1, 20:1, and 40:1 respectively, compared to 6.3 ± 1.3 %, 17.9 ± 5.2 %, and 39.2 ± 3.7 % of DCs induced AFP158-166-specific CTLs (p < 0.01) (Fig. 2a).
AFP158-166-specific TCR transgenic CTLs suppress tumor growth in tumor-bearing NOD/SCID mice
As shown in Fig. 2c, after the adoptive transfer of effector T cells, AFP-specific TCR transgenic CTLs and DC-induced AFP-specific CTLs displayed significant tumor regressions in tumor models when compared with unspecific activated CTLs and PBS. AFP-specific TCR-engineered CTLs showed significantly higher tumor inhibiting effect than DCs induced CTLs. The growth of tumors treated with nonspecific activated CTLs or PBS was not inhibited (p < 0.01) (Fig. 2c).
Pathological, morphometric change and lymphocyte infiltration of hepatocarcinoma after treatment
H&E staining showed significantly more necrosis areas and more tumor cells exhibiting morphological changes characteristic of apoptotic processes such as nuclear pyknosis in AFP-specific TCR transgenic CTL group than DCs induced CTL group. But rare morphological changes of apoptosis were found in unspecific activated CTL or PBS group. In the IHC analysis, CD3ζ positive cells were stained brown. The infiltration of CD3-positive T cells in the transgenic CTL group was significantly more than that in DCs induced CTL and unspecific activated CTL group (Fig. 3).
Histologic evaluation of tumor masses in animals treated by AFP158-166-specific TCR transgenetic CTLs. NOD/SCID mice with established tumors of HepG2 cell were treated with nonspecific activated CTLs, DC-induced AFP-specific CTLs, and transgenetic CTLs at days 0, 7, and 14 by i.v. injection. PBS was used as control group. Tumors were excised at day 21. The histopathologic changes of apoptosis were evaluated by H&E staining. Lymphocyte infiltration of tumor tissue was analysis by IHC staining using anti-human CD3ξ antibody. Cells positive for CD3ζ were stained brown. (Scale bar, 100 μm; magnification, 400) H&E staining established significantly more necrosis areas and more tumor cells with morphological changes characteristic of apoptotic processes such as nuclear pyknosis in transgenic CTL group than DCs induced CTL group. But rare morphological changes of apoptosis were found in unspecific activated CTL or PBS group (white arrow indicate pyknosis). In IHC analysis, the infiltration of CD3 positive T cells in the transgenic CTL group were significantly more than that in DCs induced CTL group and unspecific activated CTL group
Discussion
Adoptive transfer of tumor-specific T cells has been investigated as a promising alternative strategy to treat patients suffering from malignancies and emerged in preclinical and clinical evaluation [8, 9]. Although exhibited obvious superiority compared with conventional therapeutic procedures for its antigen-specific cytotoxicity, it also has been limited by the lack of ability to isolate and expand high-affinity T cells specific for tumor-associated antigens [10]. Most tumor-associated antigens recognized by tumor-specific T cell are also expressed in self tissue at lower density. Therefore, autologous T cells targeting these tumor antigen epitopes are normally of low to intermediate affinity for the peptide/MHC complex because the high-affinity clones have been deleted from the T cell repertoire by the thymic negative selection or rendered non-responsive to self molecules due to peripheral tolerance induction for preventing autoimmunity. Moreover, they often show a severe functional impairment caused by several immunosuppressive mechanisms, such as regulatory T cells (Tregs) and inhibitory receptors. Therefore, rare functional tumor-specific T cells could be isolated from patients with malignancy. Genetically redirected T cells with high-affinity TCR or CAR by transgenic technologies could confer T cell novel tumor-specific cytotoxicity targeting tumor-associated antigens [11–13]. In 1999, the first successful study had demonstrated that transfer of an HLA-A2-restricted MART-1 antigen-specific TCR into human T cells could confer them the specificity toward malignant melanomas [14]. Several preclinical and clinical studies have also exhibited that genetic modification with a tumor antigen-specific TCR could yield an antigen-specific T cell population [15–21].
Butterfield and colleagues first reported AFP could serve as a potential tumor-associated antigen and cause the responses of both murine and human T cells in tumor-bearing murine model, and identified four HLA-A*02:01-restricted antigen epitope peptides derived from AFP that could stimulate specific T cell responses [22]. In our study, activation and proliferation of AFP-specific T cells were stimulated by AFP158-166 peptides pulsed DCs. As described above, these populations were so little that the positive rate was only 1.7 %. In the step of isolation and collection of positive clones, we preferred FACS to the limited dilution assays which was more widely applied in the cell cloning culture in the past decades because the previous procedure could obtain a more comprehensive AFP-specific CTL repertoire in an efficient way.
The TCR was a complex of trans-membrane dimeric proteins and most of them expressed by circulating T cells comprising of α and β chains. The combinatorial rearrangement of the V, (D) and J genes and the mechanisms of trimming lead to the huge diversity of TCR repertoires and hampered the cloning of TCR repertoires by considerable workloads. The set of primers for amplification and cloning TCR genes that reported by Ilenia Boria et al. could minimize the workload dramatically and facilitate the creation of more diverse TCR library in our study [23].
As a result, the TCR α, β, and the full length of TCR chain genes from this library are polyclonal. The shortcoming of this research was the pair of TCR α and β genes was only selected randomly for the follow-up experiment but failed to be evaluated the affinity by phage display technology. Future approaches should therefore be focused on the screening of high avidity TCR able to recognize minute amounts of MHC-AFP158-166 on the surface of tumor cells by phage display technology.
Although 85.4 % T cells were transfected with lentiviral vector and redirected, the AFP158-166-specific TCR-expressed CTLs was only 4.2 % according to the MHC-Pentamer staining. After sorting CD8 and eGFP double positive population by FACS, the Pentamer positive CTLs were only raised to 9.86 %. The low level of transgenic TCR expression could due to undesired formation of mixed TCR dimers by mispairing of the endogenous chains with genetic transfered chains. Cross-pairing could not only minimize the tumor-specific cytotoxicity but also generate unpredictable TCRs against self-antigens and cause autoimmunity. But several strategies have already been investigated to avoid TCR mispairing in previous studies [24–31]. Another potential issue that may affect the positive rate of engineered TCR expression was the low affinity between AFP158-166-specific TCR and MHC-Pentamer. The result of flow cytometry analysis could not reflect the real level of exogenous TCR expression. This assumption also explained that although the proportion of MHC-Pentamer positive AFP158-166-specific CTLs was very low, the transgenic T cell showed significant specific cytotoxicity in follow-up experiments in vivo and in vitro.
In this study, we have cloned AFP158-166-specific TCR gene and demonstrated the feasibility of adoptive therapy using AFP158-166-specific TCR gene transfer CTLs. These redirects CTLs displayed specific cytotoxicity against AFP+ HCC cells. It might be a potential approach for the treatment of HCC with significant value of clinical application. Further studies are therefore warranted to investigate the utility of this therapy in the clinic.
References
Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47(Suppl):S2–6.
Cucchetti A, Qiao GL, Cescon M, Li J, Xia Y, Ercolani G, et al. Anatomic versus nonanatomic resection in cirrhotic patients with early hepatocellular carcinoma. Surgery. 2014;155:512–21.
Weiner LM, Dhodapkar MV, Ferrone S. Monoclonal antibodies for cancer immunotherapy. Lancet. 2009;373:1033–40.
Gehring AJ, Xue SA, Ho ZZ, Teoh D, Ruedl C, Chia A, et al. Engineering virus-specific T cells that target HBV infected hepatocytes and hepatocellular carcinoma cell lines. J Hepatol. 2011;55:103–10.
Kalos M, June CH. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity. 2013;39:49–60.
El-Serag HB, Kanwal F. α-Fetoprotein in hepatocellular carcinoma surveillance: mend it but do not end it. Clin Gastroenterol Hepatol. 2013;11:441–3.
Butterfield LH, Ribas A, Meng WS, Dissette VB, Amarnani S, Vu HT, et al. T-cell responses to HLA-A*0201 immunodominant peptides derived from alpha-fetoprotein in patients with hepatocellular cancer. Clin Cancer Res. 2003;9:5902–8.
Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–5.
Visioni A, Zhang M, Graor H, Kim J. Expansion of melanoma-specific T cells from lymph nodes of patients in stage III: implications for adoptive immunotherapy in treating cancer. Surgery. 2012;152:557–65. discussion 565-6.
Bonaccorsi I, Pezzino G, Morandi B, Ferlazzo G. Novel perspectives on dendritic cell-based immunotherapy of cancer. Immunol Lett. 2013;155:6–10.
Yang S, Dudley ME, Rosenberg SA, Morgan RA. A simplified method for the clinical-scale generation of central memory-like CD8+ T cells after transduction with lentiviral vectors encoding antitumor antigen T-cell receptors. J Immunother. 2010;33:648–58.
Wu F, Zhang W, Shao H, Bo H, Shen H, Li J, et al. Human effector T cells derived from central memory cells rather than CD8(+)T cells modified by tumor-specific TCR gene transfer possess superior traits for adoptive immunotherapy. Cancer Lett. 2013;339:195–207.
Perro M, Tsang J, Xue SA, Escors D, Cesco-Gaspere M, Pospori C, et al. Generation of multi-functional antigen-specific human T-cells by lentiviral TCR gene transfer. Gene Ther. 2010;17:721–32.
Clay TM, Custer MC, Sachs J, Hwu P, Rosenberg SA, Nishimura MI. Efficient transfer of a tumor antigen-reactive TCR to human peripheral blood lymphocytes confers antitumor reactivity. J Immunol. 1999;163:507–13.
Frankel TL, Burns WR, Peng PD, Yu Z, Chinnasamy D, Wargo JA, et al. Both CD4 and CD8 T cells mediate equally effective in vivo tumor treatment when engineered with a highly avid TCR targeting tyrosinase. J Immunol. 2010;184:5988–98.
Chinnasamy N, Wargo JA, Yu Z, Rao M, Frankel TL, Riley JP, et al. A TCR targeting the HLA-A*0201-restricted epitope of MAGE-A3 recognizes multiple epitopes of the MAGE-A antigen superfamily in several types of cancer. J Immunol. 2011;186:685–96.
Straetemans T, van Brakel M, van Steenbergen S, Broertjes M, Drexhage J, Hegmans J, et al. TCR gene transfer: MAGE-C2/HLA-A2 and MAGE-A3/HLA-DP4 epitopes as melanoma-specific immune targets. Clin Dev Immunol. 2012;2012:586314.
Batchu RB, Gruzdyn OV, Moreno-Bost AM, Szmania S, Jayandharan G, Srivastava A, et al. Efficient lysis of epithelial ovarian cancer cells by MAGE-A3-induced cytotoxic T lymphocytes using rAAV-6 capsid mutant vector. Vaccine. 2014;32:938–43.
Jakka G, Schuberth PC, Thiel M, Held G, Stenner F, Van Den Broek M, et al. Antigen-specific in vitro expansion of functional redirected NY-ESO-1-specific human CD8+ T-cells in a cell-free system. Anticancer Res. 2013;33:4189–201.
Schuberth PC, Jakka G, Jensen SM, Wadle A, Gautschi F, Haley D, et al. Effector memory and central memory NY-ESO-1-specific re-directed T cells for treatment of multiple myeloma. Gene Ther. 2013;20:386–95.
Hillerdal V, Nilsson B, Carlsson B, Eriksson F, Essand M. T cells engineered with a T cell receptor against the prostate antigen TARP specifically kill HLA-A2+ prostate and breast cancer cells. Proc Natl Acad Sci U S A. 2012;109:15877–81.
Butterfield LH, Ribas A, Dissette VB, Lee Y, Yang JQ, De la Rocha P, et al. A phase I/II trial testing immunization of hepatocellular carcinoma patients with dendritic cells pulsed with four alpha-fetoprotein peptides. Clin Cancer Res. 2006;12:2817–25.
Boria I, Cotella D, Dianzani I, Santoro C, Sblattero D. Primer sets for cloning the human repertoire of T cell receptor variable regions. BMC Immunol. 2008;9:50.
Fang J, Qian JJ, Yi S, Harding TC, Tu GH, VanRoey M, et al. Stable antibody expression at therapeutic levels using the 2A peptide. Nat Biotechnol. 2005;23:584–90.
Cohen CJ, Zhao Y, Zheng Z, Rosenberg SA, Morgan RA. Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability. Cancer Res. 2006;66:8878–86.
Cohen CJ, Li YF, El-Gamil M, Robbins PF, Rosenberg SA, Morgan RA. Enhanced antitumor activity of T cells engineered to express T-cell receptors with a second disulfide bond. Cancer Res. 2007;67:3898–903.
Kuball J, Dossett ML, Wolfl M, Ho WY, Voss RH, Fowler C, et al. Facilitating matched pairing and expression of TCR chains introduced into human T cells. Blood. 2007;109:2331–8.
Voss RH, Willemsen RA, Kuball J, Grabowski M, Engel R, Intan RS, et al. Molecular design of the Calphabeta interface favors specific pairing of introduced TCRalphabeta in human T cells. J Immunol. 2008;180:391–401.
Okamoto S, Mineno J, Ikeda H, Fujiwara H, Yasukawa M, Shiku H, et al. Improved expression and reactivity of transduced tumor-specific TCRs in human lymphocytes by specific silencing of endogenous TCR. Cancer Res. 2009;69:9003–11.
Ochi T, Fujiwara H, Okamoto S, An J, Nagai K, Shirakata T, et al. Novel adoptive T-cell immunotherapy using a WT1-specific TCR vector encoding silencers for endogenous TCRs shows marked antileukemia reactivity and safety. Blood. 2011;118:1495–503.
Provasi E, Genovese P, Lombardo A, Magnani Z, Liu PQ, Reik A, et al. Editing T cell specificity towards leukemia by zinc finger nucleases and lentiviral gene transfer. Nat Med. 2012;18:807–15.
Acknowledgments
This work was supported by National Natural Science Foundation of China Grants No.81172167.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, L., Guo, H., Jiang, R. et al. Engineered cytotoxic T lymphocytes with AFP-specific TCR gene for adoptive immunotherapy in hepatocellular carcinoma. Tumor Biol. 37, 799–806 (2016). https://doi.org/10.1007/s13277-015-3845-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3845-9