Abstract
Background
Immunotherapy is an effective method for preventing metastasis and recurrence of carcinoma. Hepatocellular carcinoma (HCC) is a common malignancy with a high rate of recurrence, and has not successfully been introduced to immunotherapy.
Methods
Peripheral blood mononuclear cells were isolated from whole blood of HCC patients and stimulated to transform into dendritic cells (DCs). These DCs were then transfected with RNA extracted from HepG-2 hepatoma cells to induce expression of specific antigens.
Results
The transfected DCs stimulated T lymphocytes to produce cytotoxic T lymphocytes, which specifically attacked HepG-2 cells. Injection of T lymphocytes from HCC patients and transfected DCs into severe combined immunodeficiency mice limited the growth of HepG-2 tumors.
Conclusion
A specific immune response against hepatoma can be generated in vivo by administering DCs transfected with RNA from a specific tumor. This method may have therapeutic application in humans to reduce recurrence of HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dendritic cells (DCs) are the most powerful antigen-presenting cells. Peripheral DCs mature during their migration process, expressing a wealth of major histocompatibility complex (MHC) I, II molecules, co-stimulatory molecules, and adhesion molecules. DCs promote generation of T helper (Th) cells and cytotoxic T lymphocytes (CTLs), and induce B cells to produce antibodies [1]. DCs can also secrete high levels of IL-12 to promote a Th1 response, thereby facilitating clearance of viruses and malignant cells [2]. Current research on DCs has focused on areas of transplantation immunology, tumor immunity, and autoimmune disease. The strategy has been to prepare antitumor DCs in vitro, and then use them in vaccines to stimulate the body’s immune response in vivo. Such DC vaccines been used to obtain satisfactory results in clinical treatment of black melanoma, B lymphoma, prostate cancer, and multiple myeloma [3].
The number of DCs found in peripheral blood is very small, and studies have shown that DC function in most cancer patients is inhibited to varying degrees and cannot effectively stimulate T cell immunity [4]. Furthermore, tumor cells usually express only low amounts or no MHC or co-stimulatory molecules. Some tumor cells can secrete cytokines to suppress the maturation and immune function of DCs, leading to escape of tumor cells from immune surveillance and unlimited tumor growth [5, 6]. Therefore, it is necessary to stimulate production of large numbers of DCs in vitro to meet the needs of clinical and scientific research. The classic methods used to obtain large numbers of human DCs in vitro include culturing peripheral blood mononuclear cells (PBMCs) (CD14+, CD1a−) with GM-CSF and IL-4, or inducing CD34+ stem cells with GM-CSF and TNF-α [7–11]. Immature DCs express the antigen CD1a, whereas mature DCs express CD83, P55 (Ig superfamily member), and high levels of co-stimulatory molecules, including CD40, CD80, CD86, and HLA-DR.
Various methods are used to construct DC vaccines, including the use of specific tumor peptides as antigens, use of unspecific tumor peptides as antigens, fusion of DCs and tumor cells, DNA transfection, and RNA transfection. Recent studies suggest that among these methods, vaccine construction by RNA transfection offers the most advantages. When using this method, the amount of RNA can be amplified by cDNA transcription in vitro [12]. Moreover, the half-life of mRNA in mammalian cells is <24 h, and RNA cannot integrate into the cell genome [12]. Additionally, use of RNA-induced DC vaccines does not produce a potential risk of cancer [13, 14]; therefore, RNA is a safe and effective antigen. In this study, DCs transfected with RNA from HepG-2 hepatoma cells were used as a vaccine and tested on HepG-2 cells in vitro and on HepG-2 tumors in vivo.
Methods
Preparation of DCs
Peripheral blood mononuclear cells were isolated from 10 mL of EDTA-treated whole blood from hepatocellular carcinoma (HCC) patients by standardized density gradient centrifugation techniques using Ficoll-Hypaque. The PBMCs were incubated at a density of 1 × 106 cells/mL in standard culture flasks containing RPMI-1640/10 % FCS medium for 2 h at 37 °C to facilitate adherence; non-adherent cells were removed. The adherent cells were cultured in RPMI-1640/10 % FCS medium supplemented with 100 ng/mL of recombinant human granulocyte–macrophage colony-stimulating factor (GM-CSF; Genzyme, Japan) and 500 U/mL of IL-4 (Genzyme, Japan) at 37 °C in a humidified 5 % CO2 atmosphere. Fifty percent of the culture medium was replaced every 2–3 days.
Preparation and culture of lymphocytes
Peripheral blood mononuclear cells were isolated from 20 mL of heparin-treated whole blood obtained from HCC patients. An equal volume of PBS was added to the blood. The diluted blood was added to Lymphoprep centrifugation tubes and centrifuged at 2,500 rpm for 20 min. PBMCs were collected, diluted, and washed 2× with PBS. The density of cells was adjusted to 1 × 106/mL, and the cells were cultured for 2 h. Non-adherent cells were then collected as peripheral blood lymphocytes (PBLs) and cultured in RPMI-1640/10 % FCS medium supplemented with human recombinant interleukin-2 (rhIL-2, 20 U/L; Genzyme, Japan).
Culture of HepG-2 and SGC-7801 cells and isolation of total RNA
HepG-2 cells and SGC-7801 cells were cultured in RPMI-1640/10 % FCS medium at 37 °C in a humidified 5 % CO2 atmosphere. Suspended cells (1 × 106 cells in 1 mL medium), were added to 1 mL Trizol and mixed for 5 min. Total RNA was then isolated using a standard protocol and dissolved in diethyl pyrocarbonate-treated water.
Transfection of DCs in vitro
Dendritic cells were transfected with total RNA using a method described by Boczkowski et al. [15]. On day 7 of culture, the DCs were suspended in 2 mL of Opti-MEM at a density of 1 × 106/mL. RNA (10 μg) and DOTAP (30 μL) were separately diluted in Opti-MEM (100 μL), and maintained at room temperature for 20 min. Then, the RNA and DOTAP diluents were mixed together and incubated for another 15 min. These mixtures were then added to DCs, followed by incubation at 37 °C for 4 h. After 4 h of incubation, the DCs were washed with PBS and cultured in RPMI-1640/10 % FCS medium supplemented with 100 ng/mL of GM-CSF and 500 U/mL of IL-4. On day 2 following transfection, the DCs were sorted by flow cytometry.
Flow cytometry
Dendritic cells were collected, diluted to a density of 1 × 105 cells, and washed with Hanks solution. The DCs were then incubated with monoclonal antibodies against HLA-DR, CD14, CD83, and CD86 for 30 min and washed with PBS. The cells were then sequentially incubated with FITC-labeled mouse anti-human secondary antibodies for 30 min. After washing with PBS, the DCs were suspended in 300 μL PBS and sorted using a fluorescence-activated cell sorter (Beckman-Coulter, Brea, CA, USA).
CTL preparation and cytotoxicity assay
Dendritic cells or transfected DCs were cultured with lymphocytes at a ratio of 1:10, respectively, in RPMI-1640 with rhIL-2 (20 ku/L) for 1 week. The lymphocytes were harvested as CTLs, functioning as effector cells. The MTT method was used to determine the cytotoxicity of CTLs. Each well of a 96-well plate was plated with 2 × 105 HepG-2 or SGC-7901 cells as target cells in 100 μL medium. The same volume of CTLs with different cell densities (effector cell-target cell ratios of 2.5:1, 5:1, 10:1, or 20:1) was then added. The plates were then incubated at 37 °C for 16 h. After 16 h, 10 μL MTT (5 g/L) was added to the wells, followed by incubation for 8 h. The plates were centrifuged (2,000 rpm/min, 10 min), and the supernatants were discarded. Then 100 μL of DMSO was added to each well to dissolve formazan crystals, and absorption values at 570 nm (A) were recorded for each well sample. Each experiment was performed in triplicate and the mean OD values were recorded. Cytotoxicity was then calculated using the equation: Cytotoxicity (%) = [1 − (experimental A − effector A)/target A] × 100 %.
Construction of a HCC PBL-SCID mosaic model and tumor examination
PBLs (2 × 107) were injected into the abdominal cavity of SCID mice. One week later, the mice were divided into three groups of ten mice each and injected with either DCs, DCs transfected with HepG-2 RNA, or PBS via the tail vein, and a second injection was conducted 1 week later. One week after the second injection, HepG-2 cells (1 × 106 cells in 0.1 mL PBS) were subcutaneously injected into the left inguinal region of each mouse. One mouse was euthanized 1 week after injection with HepG-2 cells. During the following 3 weeks, three mice in each injection group were euthanized each week and examined for tumor formation. Tumor growth was measured every week for 4 weeks using a slide gauge. Tumor volume (V) was calculated according to the equation: V (mm3) = π/6 × a × b 2 [where “a” is the larger diameter and “b” is the diameter perpendicular to “a” (mm)]. The resulting data were used to construct a tumor growth curve.
Enzyme-linked immunosorbent assay (ELISA) of Ig, IL-2, and IFN-γ
Aliquots of mouse serum were placed in 96-well plates and let stand for 90 min, followed by incubation with primary antibodies against human Ig, IL-2, or IFN-γ for 180 min. The wells were then washed four times with PBS. Secondary antibodies were then added to the wells and incubated for 30 min at 37 °C. The wells were washed, substrate reagent was added, and the samples were incubated at 37 °C for 15 min. Stop solution was then immediately added to wells, and the absorbance of each well at 450 nm was recorded.
Statistical analyses
Normally distributed continuous variables were compared by one-way analysis of variance. When a significant difference was seen between groups, a multiple comparison of the means was performed using the Bonferroni procedure with a type-I error adjustment. Data are presented as the mean ± SD. All statistical assessments were two-sided; a P value ≤0.05 was considered statistically significant. Statistical analyses were performed using SPSS 15.0 statistics software (SPSS Inc., Chicago, IL).
Results
Culture and identification of DCs from HCC patients
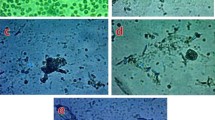
Peripheral blood mononuclear cells were isolated from HCC patients and cultured with IL-4 and GM-CSF. As shown in Fig. 1a, on day 2 of culture, PBMCs appeared as aggregates of 10–20 cells each during examination with an inverted light microscope. Some suspended cells were also observed in the culture medium. On day 5 of culture, both the size and number of cells in the PBMC aggregates had increased, and the size of the aggregates had reached a plateau (Fig. 1b). On day 7 of culture, many large cells had been released from the cell aggregates, and showed morphology typical of DCs, including branching and pseudopod formation (Fig. 1c).
Culture and identification of dendritic cells from HCC patients. PBMCs were isolated from HCC patients and cultured with IL-4 and GM-CSF. a–c PBMCs examined with inverted light microscope on days 2, 5, and 7 of culture. d The DC-like cells were then sorted using flow cytometry with CD14, CD83, CD86, and HLA-DR antibodies
The DC-like cells were sorted using flow cytometry with CD14, CD83, CD86, and HLA-DR antibodies. These cells demonstrated high expression of HLA-DR (99.7 ± 9.7 %), CD83 (51.1 ± 3.9 %), CD86 (73.6 ± 8.7 %), and low expression of CD14 (1.53 ± 1.48 %) (Fig. 1d), and thus were confirmed as DCs. These results indicate that DCs could be successfully developed from PBMCs of HCC patients.
DCs transfected with total RNA from tumors induce specific immune responses against HCC in vitro
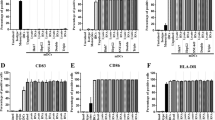
To test whether specific immune responses could be generated by DCs against HCC, DCs from HCC patients were transfected with total RNA extracted from the HepG-2 hepatoma cell line or the human hepatic Chang cell line. At 48 h following transfection, the DCs were examined for the presence of HLA-DR, CD14, CD83, and CD86 on the cell surface by flow cytometry. As shown in Fig. 2, 54.1 % of non-transfected DCs and 30 % of empty transfected DCs expressed CD83; 52.2 % non-transfected cells and 60.2 % of empty transfected cells expressed CD86, and ~99 % of both non-transfected and empty transfected cells expressed HLA-DR (DC + OPTI-MEM and DC + DOTAP, the first and second row). Following transfection with total RNA extracted from Chang cells, 41.2 % of DCs expressed CD83, 53.9 % expressed CD86, and 99.4 % expressed HLA-DR (DC + Chang RNA, the third row). Following transfection with RNA extracted from HepG-2 cells, 80.0 % of DCs expressed CD83, 97.4 % expressed CD86, and 99.9 % expressed HLA-DR (DC + HepG-2 RNA, bottom row). In conclusion, the percentages of DCs expressing CD83 and CD86 dramatically increased when DCs were transfected with total RNA extracted from HepG-2 cells. Although the percentage of DCs expressing HLA-DR was not affected by RNA transfection, the total numbers of DCs increased when DCs were transfected with HepG-2 RNA. These results indicate that DCs transfected with HepG-2 RNA were induced and ready to activate T lymphocytes.
Presence of HLA-DR, CD14, CD83, and CD86 on cell surface of DCs following RNA transfection. DCs from HCC patients were transfected with total RNA extracted from HepG-2 cells (DC + HepG-2) or Chang cells (DC + Chang RNA) or transfected without RNA (DC + DOTAP). At 48 h post-transfection, the DCs were analyzed for the presence of CD14, CD83, CD86, and HLA-DR by flow cytometry
The DCs induced by RNA transfection were incubated with lymphocytes at a ratio 1:10 for 7 days. T lymphocytes were activated by stimulation of induced DCs and transformed into CTLs. The CTLs were then mixed with hepatoma cell line HepG-2 or gastric carcinoma cell line SGC-7901 at ratios of 2.5:1, 5:1, 10:1, and 20:1. After 16 h of incubation, cell viability was tested using the MTT assay. As shown in Fig. 3a, when HepG-2 cells were mixed with CTLs which were activated by HepG-2 RNA-transfected DCs, the cytotoxicity to HepG-2 cells increased in parallel with increasing numbers of CTLs (HepG-2 RNA). When the ratio of activated CTLs to HepG-2 cells was increased from 2.5:1 to 20:1, the cytotoxicity of HepG-2 cells was increased from 5 to 35 %. In contrast to that increase, when HepG-2 cells were mixed with CTLs which were activated by induced DCs with Chang RNA transfection, or by induced DCs with empty transfection, the cytotoxicity to HepG-2 cells remained at 5 % regardless of the numbers of activated CTLs (Chang RNA and DOTAP). When SGC-7901 cells were mixed with CTLs activated by induced DCs with HepG-2 RNA, Chang RNA, or empty transfection, cytotoxicity to SGC-7901 cells remained at 5 % (Fig. 3b, HepG-2 RNA, Chang RNA, or DATOP). These results demonstrated that only CTLs stimulated by HepG-2 RNA-transfected DCs specifically attacked HepG-2 cells, and also suggest that DCs transfected with RNA from HepG-2 cells can express HepG-2-specific antigens on their surface.
CTL cytotoxicity to HepG-2 and SGC-971cells. CTLs induced by DCs with HepG-2 RNA, Chang RNA, or empty transfection (HepG-2 RNA/DC, Chang RNA/DC, or DOTAP/DC) were incubated with HepG-2 cells (a) or SGC-7901 cells (b) at ratios of 2.5:1, 5:1, 10:1, and 20:1. CTLs were effectors (E), and HepG-2 cells or SGC-7901 cells were targets (T). *Significant difference between the indicated E:T and E:T of 2.5:1, P < 0.017. Multiple comparisons between groups were determined using Bonferroni’s test with α = 0.017 adjustment
DCs transfected with total tumor RNA induce specific immune responses against HCC in vivo
We next conducted experiments using SCID mice to test whether DCs transfected with HepG-2 RNA could stimulate T lymphocytes to transform into CTLs, and test whether those CTLs could specifically attack tumors formed by HepG-2 cells in vivo. PMBCs and PBLs were isolated from HCC patients and injected into the intraperitoneal abdomen of mice to create a human immune system in the mice. To further immunize the mice, 7 days after the injection, DCs transfected with HepG-2 RNA, naïve DCs developed from PMBCs (HepG-2 RNA DC and DC), or PBS buffer (PBS) were also injected two times into the tail veins of the mice, with a 1 week interval between injections. Seven days after the second DC injection, HepG-2 cells (1 × 107) were introduced into the immunized mice via left inguinal subcutaneous injection.
The mice were then examined every week for the presence of HepG-2 tumors, starting 1 week after the injection. Tumor volumes (V) were calculated using the equation “V (mm3) = π/6 × a × b 3”, where a is the longest tumor axis, and b is the shortest tumor axis. As shown in Fig. 4a, tumors began to form 6–7 days following injection, and mice injected with PBS buffer (PBS) or naïve DCs (DCs) developed tumors of similar size and with growth curves over time. Tumor sizes in mice injected with HepG2 cells reached ~500 mm3 at 4 weeks following injection. Significantly, mice injected with HepG-2 RNA-transfected DCs (HepG-2 RNA DCs) showed much slower tumor growth, requiring 9–10 days to reach ~200 mm3 in size. The mice were euthanized 4 weeks after injection of HepG-2 cells, and the tumors were collected and photographed (Fig. 4b, PBS, DCs, HepG-2 RNA DCs, from left to right). All tumor masses had intact capsules. Some tumor cells were observed peripheral to the tumor, and caseous necrosis was found in the center of tumors (data not shown). The tumors were localized, and no lymphatic metastases were observed in the liver, spleen, pancreas or lungs.
Tumor growth during 4 weeks after injection of HepG-2. PBLs and DCs with or without HepG-2 RNA transfection (HepG-2 RNA DC and DC) or PBS buffer (PBS) were sequentially injected into SCID mice. Seven days after DC or PBS injection, 1 × 107 HepG-2 cells were subcutaneously injected into the left inguinal region of the mice. Starting 1 week after injection of HepG-2 cells, the sizes of tumors formed by HepG-2 cells were examined every week, and tumor growth curves were plotted (a). Four weeks after injection of HepG-2 cells, the mice were euthanized, and tumors were collected and photographed (b; PBS, DCs, HepG-2 RNA DCs, from left to right). Compared with PBS and DCs groups, tumor growth in the HepG-2 RNA/DCs group was significantly inhibited. *Significant difference between the indicated group and the PBS group. †Significant difference between the indicated group and the DC group. Multiple comparisons between groups were conducted using Bonferroni’s test with α = 0.017 adjustment
The levels of human IL-2 and IFN-γ in the SCID mouse sera were then assayed to determine whether the immune system had been activated. As shown in Fig. 5, the levels of IL-2 (Fig. 5a) and IFN-γ (Fig. 5b) in mice injected with HepG-2 RNA-transfected DCs (HepG-2 RNA DCs) were enhanced approx. three- to fivefold compared with levels of IL-2 and IFN-γ in mice injected with PBS buffer (PBS) or naïve DCs (DCs).
Samples of serum from the three groups of mice used in previous experiments (PBS, DCs, HepG-2 RNA DCs) were collected, and levels of IL-2 and IFN-γ were tested by ELISA. Serum levels of IL-2 and IFN-γ in the HepG-2 RNA/DCs group were significant higher than those in the other groups. *Significant difference between the indicated group and the PBS group. †Significant difference between the indicated group and the DC group. Multiple comparisons between groups were conducted using Bonferroni’s test with α = 0.017 adjustment
Discussion
In this study, naïve DCs from HCC patients were transfected with HepG-2 hepatoma RNA. These transfected DCs stimulated T lymphocytes to become CTLs in vitro. These trained CTLs could then specifically attack HepG-2 cells in vitro or HepG-2 tumors in vivo. This is the first report showing that DCs transfected with hepatoma RNA can activate immunity against specific hepatic tumors in vivo. In this experiment, SCID mice were used to build a HCC human PBL immune model. The PBL-SCID mice were then implanted with HepG-2 cells, which subsequently formed tumor masses. When the PBL-SCID mice were injected with HepG-2 RNA-transfected DCs, tumor growth was inhibited ~50 % relative to tumor growth in mice injected with naive DCs or PBS. Thus, the RNA DC vaccine delivered specific antigens to the immune system and significantly inhibited tumor growth in vivo, indicating its possible efficacy for treating hepatoma.
Boczkowski et al. [15] reported that DCs transfected with tumor RNA could prevent tumor progression in a mouse metastasis model. Injection of tumor-pulsed DCs into mice not only caused a tumor-specific CTL reaction, but also reduced the incidence of lung metastasis, and extended the survival of tumor-bearing mice [16]. Additionally, another study showed that RNA-transfected DCs could elicit anti-tumor immunity [17]. The DC vaccine used in that study was made using Mucin 1 (MUC1) RNA transfected into mouse bone marrow-derived DCs, and was injected at the root of the tail. The DCs then migrated to the groin lymph node area. The injection of MUC1 RNA-transfected DCs into C57BL/6 mice-generated immunity to MUC1-positive MC38 tumor cells, but produced no immunity against MUC1-negative tumor cells [17]. Nair et al. [18] reported that carcinoembryonic antigen (CEA) RNA-transfected peripheral blood-derived DCs from healthy subjects and cancer patients could induce a specific CD8 + CTL immune response in vitro, and they proposed that CEA RNA-transfected DCs were a suitable target for use in cytotoxicity assays; this method is now widely used. Nair and his colleagues also attempted to transfect CEA mRNA, human telomerase reverse transcriptase enzyme mRNA, and autologous tumor total RNA into DCs from cancer patients, and those transfected DCs induced specific CTLs, which dissolved tumor cells in vitro [19–21]. In those studies by Nair et al., total RNA extracted from frozen sections of tissue was amplified by in vitro transcription. The amplified RNA products were then transfected in DCs, leading to a tumor-specific CTL immune response [22]. Results from previous studies have shown that DCs transfected with total RNA from kidney carcinoma and prostate cancer tumors can stimulate anti-tumor polyclonal T cell responses, and those T cells can dissolve both primary and the metastatic tumor cells in vitro [23, 24].
RNA-transfected DC vaccines have also been used for clinical treatment of metastatic prostate cancer and metastatic renal cell carcinoma [25, 26]. Human DCs transfected with RNA encoding prostate-specific antigen can stimulate prostate-specific CTL responses in vitro, and such DCs have been given to patients with metastatic prostate cancer. Results of those studies showed that patients given the DC vaccine gained immunity against prostate-specific antigens, and displayed no obvious side effects [25]. DC vaccines made with total RNA of metastatic renal cell carcinomas have also demonstrated good therapeutic effects [26]. Besides priming effector T cells, it is also desirable if a vaccine induces formation of memory T cells for sustaining immunity. Recent studies have shown that DC vaccines do not directly prime CD8+ T cells, but rather facilitate transfer of antigens to endogenous antigen-presenting cells, which are then responsible for the subsequent activation of T cells [27]. Such findings suggest that agents other than DC vaccines should be considered for use in anti-cancer immunotherapy; however, DC vaccines can be used as platform for developing new targeted therapies.
References
Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811.
Heufler C, Koch F, Stanzl U, Topar G, Wysocka M, Trinchieri G, et al. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. Eur J Immunol. 1996;26:659–68.
Nouri-Shirazi M, Banchereau J, Fay J, Palucka K. Dendritic cell based tumor vaccines. Immunol Lett. 2000;74:5–10.
Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, et al. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6:1755–66.
Halliday GM, Le S. Transforming growth factor-beta produced by progressor tumors inhibits, while IL-10 produced by regressor tumors enhances, Langerhans cell migration from skin. Int Immunol. 2001;13:1147–54.
Ohm JE, Carbone DP. VEGF as a mediator of tumor-associated immunodeficiency. Immunol Res. 2001;23:263–72.
Cao H, Verge V, Baron C, Martinache C, Leon A, Scholl S, et al. In vitro generation of dendritic cells from human blood monocytes in experimental conditions compatible for in vivo cell therapy. J Hematother Stem Cell Res. 2000;9:183–94.
Garderet L, Cao H, Salamero J, Vergé V, Tisserand E, Scholl S, et al. In vitro production of dendritic cells from human blood monocytes for therapeutic use. J Hematother Stem Cell Res. 2001;10:553–67.
Zheng Z, Takahashi M, Narita M, Toba K, Liu A, Furukawa T, et al. Generation of dendritic cells from adherent cells of cord blood by culture with granulocyte-macrophage colony-stimulating factor, interleukin-4, and tumor necrosis factor-alpha. J Hematother Stem Cell Res. 2000;9:453–64.
Ramadan G, Schmidt RE, Schubert J. In vitro generation of human CD86+ dendritic cells from CD34 + haematopoietic progenitors by PMA and in serum-free medium. Clin Exp Immunol. 2001;125:237–44.
Hagihara M, Li C, Gansuvd B, Munkhbat B, Inoue H, Shimakura Y, et al. Extensive and long-term ex vivo production of dendritic cells from CD34 positive umbilical cord blood or bone marrow cells by novel culture system using mouse stroma. J Immunol Methods. 2001;253:45–55.
Mitchell DA, Nair SK. RNA-transfected dendritic cells in cancer immunotherapy. J Clin Invest. 2000;106:1065–9.
Saenz-Badillos J, Amin SP, Granstein RD. RNA as a tumor vaccine: a review of the literature. Exp Dermatol. 2001;10:143–54.
Kariko K, Kuo A, Barnathan ES, Langer DJ. Phosphate-enhanced transfection of cationic lipid-complexed mRNA and plasmid DNA. Biochim Biophys Acta. 1998;1369:320–34.
Boczkowski D, Nair SK, Snyder D, Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med. 1996;184:465–72.
Zhang W, He L, Yuan Z, Xie Z, Wang J, Hamada H, et al. Enhanced therapeutic efficacy of tumor RNA-pulsed dendritic cells after genetic modification with lymphotactin. Hum Gene Ther. 1999;10:1151–61.
Koido S, Kashiwaba M, Chen D, Gendler S, Kufe D, Gong J. Induction of antitumor immunity by vaccination of dendritic cells transfected with MUC1 RNA. J Immunol. 2000;165:5713–9.
Nair SK, Boczkowski D, Morse M, Cumming RI, Lyerly HK, Gilboa E. Induction of primary carcinoembryonic antigen (CEA)-specific cytotoxic T lymphocytes in vitro using human dendritic cells transfected with RNA. Nat Biotechnol. 1998;16:364–9.
Nair SK, Hull S, Coleman D, Gilboa E, Lyerly HK, Morse MA. Induction of carcinoembryonic antigen (CEA)-specific cytotoxic T-lymphocyte responses in vitro using autologous dendritic cells loaded with CEA peptide or CEA RNA in patients with metastatic malignancies expressing CEA. Int J Cancer. 1999;82:121–4.
Nair SK, Morse M, Boczkowski D, Cumming RI, Vasovic L, Gilboa E, et al. Induction of tumor-specific cytotoxic T lymphocytes in cancer patients by autologous tumor RNA-transfected dendritic cells. Ann Surg. 2002;235:540–9.
Nair SK, Heiser A, Boczkowski D, Majumdar A, Naoe M, Lebkowski JS, et al. Induction of cytotoxic T cell responses and tumor immunity against unrelated tumors using telomerase reverse transcriptase RNA transfected dendritic cells. Nat Med. 2000;6:1011–7.
Boczkowski D, Nair SK, Nam JH, Lyerly HK, Gilboa E. Induction of tumor immunity and cytotoxic T lymphocyte responses using dendritic cells transfected with messenger RNA amplified from tumor cells. Cancer Res. 2000;60:1028–34.
Heiser A, Maurice MA, Yancey DR, Coleman DM, Dahm P, Vieweg J. Human dendritic cells transfected with renal tumor RNA stimulate polyclonal T-cell responses against antigens expressed by primary and metastatic tumors. Cancer Res. 2001;61:3388–93.
Heiser A, Maurice MA, Yancey DR, Wu NZ, Dahm P, Pruitt SK, et al. Induction of polyclonal prostate cancer-specific CTL using dendritic cells transfected with amplified tumor RNA. J Immunol. 2001;166:2953–60.
Heiser A, Dahm P, Yancey DR, Maurice MA, Boczkowski D, Nair SK, et al. Human dendritic cells transfected with RNA encoding prostate-specific antigen stimulate prostate-specific CTL responses in vitro. J Immunol. 2000;164:5508–14.
Su Z, Dannull J, Heiser A, Yancey D, Pruitt S, Madden J, et al. Immunological and clinical responses in metastatic renal cancer patients vaccinated with tumor RNA-transfected dendritic cells. Cancer Res. 2003;63:2127–33.
Yewdall AW, Drutman SB, Jinwala F, Bahjat KS, Bhardwaj N. CD8+ T cell priming by dendritic cell vaccines requires antigen transfer to endogenous antigen presenting cells. PLoS One. 2010. doi:10.1371/journal.pone.0011144.
Acknowledgments
The study was supported by grants from the National Natural Science Foundation of China (No. 30600156); the Science and Technology Projects Foundation of Guangdong Province (No. 2011B031800022); and the Youth Foundation of Jiangxi Province Education Department (No. Gjj10232).
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
B. H. Xie and J. Y. Yang contributed equally to this study.
Rights and permissions
About this article
Cite this article
Xie, B.H., Yang, J.Y., Li, H.P. et al. Dendritic cells transfected with hepatocellular carcinoma (HCC) total RNA induce specific immune responses against HCC in vitro and in vivo. Clin Transl Oncol 16, 753–760 (2014). https://doi.org/10.1007/s12094-013-1145-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-013-1145-7