Abstract
Context
Biodiversity patterns depend on landscape structure, but the spatial scale at which such dependence is strongest (scale of effect, SoE) remains poorly understood, especially for elusive species such as arboreal tropical mammals.
Objectives
To identify the SoE of arboreal mammals and assess whether it depends on the biological response and/or landscape predictor.
Methods
We surveyed arboreal mammals during one year placing camera traps in 100 trees within 20 forest patches in the Lacandona rainforest, Mexico. In each patch, we estimated species richness, total abundance, and species-specific relative abundance. We related each response variable to percent forest cover, matrix openness, patch density, and edge density measured within 13 concentric buffers from the geographic centre of sampling sites (100–1300 m radius). We identified the SoE for each combination of mammal response and landscape predictor.
Results
Edge density tended to have larger SoE than forest cover and matrix openness, and SoE did not differ between species richness and total abundance. SoE tended to be positively related to the body mass of mammals.
Conclusions
The relatively large SoE of edge density suggests that this predictor affects mammals mainly by regulating large-scale processes, such as increasing dispersal rates in landscapes with higher edge density, and not by moderating local-scale processes (e.g. edge effects). Species richness and total abundance seem to be moderated by ecological processes acting across similar spatial scales. SoE tends to increase with body mass, confirming that conservation plans for larger mammals often need to be implemented across larger areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Land-use change is rapidly transforming tropical ecosystems into anthropogenic landscapes. The structure of these emerging landscapes varies not only according to the types and amounts of land covers they contain (e.g. percentage of forest cover, matrix quality, Aide et al. 2013; Hansen et al. 2019), but also according to the spatial arrangement of each land cover (e.g. shape, Moser et al. 2002; patch isolation, Krauss et al. 2003; number of forest patches, Taubert et al. 2018). The effects that landscape structure can have on biodiversity often vary among studies Tscharntke et al. 2012; Galán-Acedo et al. 2019; Arroyo-Rodriguez et al. 2020). For example, while some studies have found strong responses to forest cover (Blanco and Waltert 2013; Piel et al. 2015; Watling et al. 2020), others found no significant responses to this landscape variable (Anzures-Dadda and Manson 2007; Urquiza-Haas et al. 2011). Similarly, while it has long been argued that habitat fragmentation has strong negative effects on biodiversity (reviewed by Fletcher et al. 2018), recent reviews suggest that fragmentation per se (i.e. fragmentation independent of habitat amount) generally has weak effects on biodiversity, and that most significant responses are positive, not negative (Fahrig 2017; Galán-Acedo et al. 2019; 2020).
Detecting the effects of landscape structure is, however, challenging because species’ responses can go undetected if they are not measured at the right spatial scale, the so-called ‘Scale of Effect’ (SoE hereafter; Jackson and Fahrig 2015; Miguet et al. 2016; Martínez-Ruiz et al. 2020). Therefore, to make accurate and more reliable inferences on the effect of landscape structure on biodiversity, we need to use a multiscale approach measuring landscape variables across multiple scales to identify the scale that yields the strongest species-landscape relationship (Jackson and Fahrig 2015). Despite its importance, and some reviews on this topic (e.g. Jackson and Fahrig 2015; Miguet et al. 2016; Martin 2018; Yeiser et al. 2021), our understanding on the SoE is far from complete. Therefore, additional studies are required to better understand species-landscape associations, and thus design more effective management and conservation initiatives.
Identifying the spatial extent at which a given biological response more strongly interacts with a given landscape variable is highly valuable to understand the ecological processes (e.g. dispersal, extinction, births and deaths) that may be regulating such response. As proposed by Miguet et al. (2016) and Martin (2018), responses regulated by local-scale processes are expected to be mainly associated with the spatial context of smaller landscapes, whereas responses driven by large-scale processes should be more strongly related to landscape patterns across larger spatial extents. For example, landscape variables affecting breeding and/or foraging (e.g. habitat fragmentation, edge density) could have smaller SoE than landscape variables related to dispersal success (e.g. habitat amount, matrix contrast; Miguet et al. 2016). With a similar rationale, the abundance of individuals is expected to be more strongly related to local-scale processes (i.e. those affecting the fitness of individuals), while species richness is hypothesized to depend more on processes operating across larger spatial and temporal scales (e.g. dispersal, extinction; Miguet et al. 2016). Therefore, independent of the landscape variable, the SoE should be larger for species richness than for the number of individuals. At the population level, the SoE can be driven by certain species traits, especially by those determining the way species use their home ranges (Miguet et al. 2016). For example, body mass is often related to species’ vagility, with larger species usually moving across larger areas than smaller species (Tucker et al. 2018). Therefore, large-bodied species are expected to have a larger SoE than small-bodied species (Miguet et al. 2016). However, these hypotheses have been tested in few species (Jackson and Fahrig 2015; Miguet et al. 2016; Martin 2018), and we know very little about the SoE for strongly forest-dependent guilds, such as arboreal mammals.
Given their strong dependence on forest canopy, arboreal mammals can be particularly sensitive to disturbances caused by land-use change (Whitworth et al. 2016; Bolt et al. 2018; Schüßler et al. 2018; Galán-Acedo et al. 2019). Arboreal mammals constitute a large proportion of vertebrate species in tropical forests (Kays and Allison 2001), and are involved in crucial ecological roles in the upper rainforest strata, such as pollination (e.g. Janson et al. 1981; Ganesh and Devy 2000), seed dispersal (e.g. Andresen et al. 2018), and herbivory (e.g. Chapman et al. 2013). Yet, several groups of arboreal mammals are highly threatened with extinction (e.g. primates, Estrada et al. 2017; marsupials, Wayne et al. 2016; sloths, Superina et al. 2010), while for others we have insufficient data to assess their population trends (e.g. anteaters and porcupines, IUCN 2019).
Here, we determined the SoE of four landscape variables (percent forest cover, matrix openness, forest patch density, and forest edge density) for arboreal mammals in the Lacandona rainforest—a biodiversity hotspot in southeastern Mexico. To our knowledge, only three studies have studied the SoE for arboreal mammals, but all focused on primates (Ordóñez-Gómez et al. 2015; Galán-Acedo et al. 2018; Gestich et al. 2019). We measured each landscape variable at 13 spatial scales (circular landscapes with radii of 100 to 1300 m). We considered two responses at the community level (number of species and total abundance of all species per site) and one at the population level (species-specific relative abundance index). Following Miguet et al. (2016), we predicted that forest patch density and forest edge density would have smaller SoE values than forest cover and matrix openness. We also expected that SoE would be higher for species richness than for total abundance. Finally, regarding the relative abundance index of individual species, we predicted that SoE would increase with the average body mass of each species.
Methods
Study region
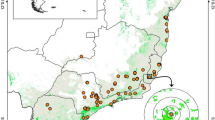
The Lacandona rainforest, Chiapas, Mexico (91˚6’42.8”–90˚41’8.7’’ W; 16˚19’17.1”–16˚2’49.3” N) has a warm (mean annual temperature 24–26 °C) and humid climate (mean annual precipitation: 2500–3500 mm). The original vegetation is tall evergreen rainforest (Carabias et al. 2015). The Lacantún River separates a large protected forest tract on the western side of the study area, the Montes Azules Biosphere Reserve, from the Marqués de Comillas region on the eastern side. The latter, is a heavily deforested area with approximately 50% of remaining forest cover (203,999 ha; Arce-Peña et al. 2019), dominated by cattle ranches, annual crops and oil palm plantations. We conducted this study in 20 forest patches in the Marqués de Comillas region. Patches ranged in size from 5 to 2300 ha and were separated from each other by a distance of at least 2.5 km, measured from their geographical centres (Fig. 1).
Location of the 20 study patches (yellow polygons) in the Lacandona rainforest, Mexico. The circles around the patches indicate the maximum spatial extent (landscape size), and the inset shows an example of the 13 concentric buffers (range = 100–1300 m radii) where landscape variables were measured. MABR = Montes Azules Biosphere Reserve; MCR = Marqués de Comillas Region. Map created with Sentinel-2 satellite images
Arboreal mammal surveys
Mammal surveys are detailed elsewhere (Cudney-Valenzuela et al. 2021), but a brief overview is given here. As suggested by Fahrig (2013), sampling was not proportional to patch size, but instead, we used a standardized sample size across landscapes to avoid potential confounding effects related to the so-called ‘sample-area effect’. At the geographical centre of each patch, and avoiding vegetation gaps, we selected five trees with suitable climbing conditions (branches ≥ 20 cm wide, preferably hard wood species) and whose architecture allowed to install a camera trap facing other main branches. At each tree, we established a single-rope climbing system. Focal trees in the same patch were separated by 30 to 150 m. Of the five focal trees per patch, four reached the canopy (mean tree height ± SD = 21.8 ± 6.2 m, range = 10.2 to 36.6 m) and one the midstory (9.1 ± 4.7 m, 3.4 to 19.6 m). This allowed us to capture a greater vertical range of strata potentially used by arboreal mammals.
Arboreal camera trapping allows to collect data reducing human interference and effort; which is critical for detecting rare, elusive and nocturnal species such as arboreal mammals (Moore et al. 2021). We used one camera trap (Bushnell Trophy Cam HD Aggressor Low Glow ©) per patch. Within each patch, we rotated the location of the camera once a month among the five focal trees, except from October to December when they remained on the same focal tree. We placed camera traps at varying heights depending on the characteristics of the focal tree (camera height of canopy and midstory trees was 15 ± 4.3 m and 2 ± 0.6 m, respectively). We set the cameras to be continuously active from May 2018 to May 2019, and we serviced them once a month (change of batteries, downloading of pictures, replacement of malfunctioning cameras). Total sampling effort was 7387 camera trap nights (average per patch = 369 ± 12 nights), with 6233 active camera trap nights (average per patch = 312 ± 20 nights).
To increase the probability of photo-capture we used baits in the midstory trees (tuna fish, peanut butter with oatmeal and a banana). As revealed by photographs, bait was consumed by mammals during the first two nights in all cases. Since we did not provide more bait while the camera was active on that tree and no camera malfunctioned during the baited period, all sites had the same bait sampling effort. We processed all photographs with the program Digikam© and extracted photograph metadata with the package ‘camtrapR’ (Niedballa et al. 2016). We considered photo captures as independent events when there was at least a 24 h interval between captures of the same species, since individuals photographed on the same day are likely the same ones (Royle et al. 2009). We identified each mammal species using Reid’s (2009) field guide, and obtained their average body mass from Ceballos and Oliva (2006; Supplementary Material, Table S1). Except for the Mexican hairy porcupine (Coendou mexicanus) and squirrels, we excluded all other rodents from the analyses due to imprecision in identification. Ten species of small rodents are reported for the area, and three of these are considered arboreal (Medellín 1994). For further analyses, we excluded rare species that appeared in less than 5 out of 20 sites (i.e. Eira barbara, n = 4 sites, 7 records; Leopardus wiedii, n = 2 sites, 2 records; Procyon lotor, n = 1 site, 5 records) to avoid spurious relationships. Ultimately, we included 12 species in the analyses.
Landscape variables
We adopted a site-landscape approach (sensu Brennan et al. 2002), with response variables measured in same-sized sample sites (i.e. five focal trees at the centre of each forest patch), and landscape variables measured within the 13 concentric circular landscapes measured from the geographical centre of each site (Fig. 1). We used 2016 high-resolution Sentinel S2 satellite images to produce land cover maps of each landscape surrounding the focal patches using ENVI 5.0 software. We used a supervised classification corroborated in field to classify land covers into six types: (i) old-growth forest cover; (ii) secondary vegetation; (iii) tree crops (e.g. oil palm plantations); (iv) annual crops and cattle pastures; (v) human settlements; and (vi) water bodies (Fig. 1). We calculated the area covered by each land cover type using ArcGIS software with the ‘Patch Analyst’ extension. We then estimated the following landscape variables: (i) percent forest cover, calculated as the area covered by old-growth forest divided by landscape size × 100 (forest cover hereafter), (ii) matrix openness, calculated as the area covered by treeless land (i.e. cattle pastures, annual crops, and water) divided by matrix area × 100, (iii) patch density, calculated as the number of old-growth forest patches completely or partially within the landscape, divided by landscape size, and (iv) edge density, calculated as the sum of the length of all old‐growth forest edges completely or partially within the landscape, divided by landscape size. We did not assess patch area effects since they are contained within the effects of landscape forest cover (Fahrig 2013), and we are interested in assessing the scale of landscape effects.
Data analyses
We calculated the number of arboreal species per forest patch based on photographic records, excluding rarely recorded species to avoid spurious relationships (see above). We also calculated each species’ relative abundance index (O’Brien 2011) by dividing the number of events for a given species by the number of days the camera was active in the patch, multiplied by 100. This index is widely used as a proxy of mammal abundance in studies using camera traps (e.g. Srbek-Araujo and Chiarello 2005; Cassano et al. 2012; Mandujano and Pérez-Solano 2019; Benchimol and Peres 2021). We rounded up each species’ relative abundance index to the nearest whole number to calculate species-specific abundance per patch, and summed the relative abundance index of all species in each patch to calculate total abundance per patch.
To identify the SoE, i.e. the scale at which each landscape variable best predicted each response (total abundance, species richness, and relative abundance index of each mammal species), we used generalized linear models with a Poisson distribution error. We excluded the smallest scale from the analysis (100 m radius) because at the 100 m scale all landscapes, except one, had the same value for patch density (Fig. S1). We first quantified the relationship between each landscape variable and each response at each scale: 4 landscape variables × 12 landscape buffers × 14 response variables (i.e. the relative abundance index of each of the 12 species, plus species richness and total abundance) = 672 models. To identify the SoE, we calculated the percentage of explained deviance by each landscape variable measured at each scale, and considered the SoE as the scale at which each landscape variable best predicted each response variable (i.e. with highest explained deviance; Fig. 2). For the analyses at the population level, we only considered this scale as the SoE if it showed a relatively higher empirical support (i.e. it showed a difference in Akaike Information Criterion (ΔAIC) > 2) when compared with the null model (i.e. including only the intercept) (see Table S2). This allows discerning spurious landscape-species associations when there is no actual landscape effect. Then, following the protocol proposed by Galán-Acedo et al. (2018), San-José et al. (2019) and Martínez-Ruiz et al. (2020), we used ANOVA to test whether the SoE differed among landscape variables (each landscape SoE as a data point), and a t-test to verify whether species richness had greater SoE than total abundance (each species-specific SoE as a data point). Finally, we used a linear regression to assess whether the species-specific SoE increased with body mass.
Effect of four landscape variables (indicated with different colors) measured across different spatial scales on the abundance of 12 arboreal mammals in the Lacandona rainforest, Mexico. The strength of landscapes effects is measured with the explained deviance (%) of each generalized linear model. Species = Alouatta pigra (black howler monkey; a), Ateles geoffroyi (spider monkey; b), Tamandua mexicana (northern tamandua; c), Nasua narica (white-nosed coati; d), Potos flavus (kinkajou; e), Coendou mexicanus (Mexican hairy porcupine; f), Didelphis marsupialis (common opossum; g), Philander opossum (four-eyed gray opossum; h), Caluromys derbianus (wooly opossum; i), Sciurus aureogaster (gray squirrel; j), Sciurus deppei (Deppe’s squirrel; k), Marmosa mexicana (Mexican mouse opossum; l). The scale of effect (SoE) of each landscape attribute on each species is indicated with big colored points. Dashed lines indicate the cases in which the model with highest explained deviance showed a similar plausibility than the null model (i.e. ΔAIC < 2)
Results
We obtained 1672 independent photo-captures of 15 species. The most frequently recorded species were the Deppe’s squirrel (Sciurus deppei; 18.5% of records), the kinkajou (Potos flavus; 16.6% of records), and the black howler monkey (Alouatta pigra; 10.2% of records), together representing ~ 45% of all records. Rarely recorded species were the margay (Leopardus wiedii; 0.1% of records), the Northern raccoon (Procyon lotor; 0.3% of records) and the tayra (Eira barbara; 0.4% of records), together representing ~ 0.8% of the records. The complete dataset can be found in Cudney-Valenzuela et al. (2021)
We found a large variation in SoE among species and landscape variables. No single landscape variable had empirically supported SoE models for all species (Fig. 2, Table S2). Twenty out of 48 species-specific SoE models showed a greater empirical support than the null model (i.e. ΔAIC < 2). Only 3 out of 12 species – the common opossum (Didelphis marsupialis), the white-nosed coati (Nasua narica), and the Mexican mouse opossum (Marmosa mexicana) – did not show empirically supported SoE models with any landscape variable (Fig. 2).
The SoE did not differ among landscape metrics (F = 1.39, p = 0.28; Fig. 3). However, the median value of SoE of edge density was > 2 times higher than the SoE of forest cover and matrix openness, and > 3 times higher than the SoE of patch density (Fig. 3). Moreover, the SoE of forest cover and matrix openness showed a relatively smaller variance than that of the other landscape metrics, ranging between 200 and 700 m radii and 400 and 700 m radii, respectively. We also found no differences in SoE between response variables at the community level; species richness and total abundance had similar SoE (t = − 0.58, p = 0.59; Fig. 4). After excluding the cases in which the model with highest explanatory power did not show stronger empirical support than the null model, the SoE for the relative abundance index of individual species tended to be positively related to body mass (R2 = 0.16; F(1,18) = 3.56, p = 0.07; Fig. 5).
Relationship between body mass and the scale of effect of landscape structure on the relative abundance index of arboreal tropical mammals in the Lacandona rainforest, Mexico. The gray area indicates the standard error of the linear regression model. We only include SoE values for which the model showed a higher plausibility than the null model (i.e. ΔAIC < 2). Also note that we assessed four landscape variables, so a single mammal species can have up to four data points depending on the number of landscape variables for which we detected the scale of effect. For example, Caluromys derbianus (average weight = 307.5 g) has two scales of effect of 200 m, which overlap into a single point. Potos flavus (average weight = 3000 g) has three scales of effect of 400 m, which also overlap into a single point. This creates a graph with 17 visible points but constructed with 20 items
Discussion
In this study, we assessed the potential determinants of the scale of landscape effect (SoE) on arboreal mammals – a threatened and understudied group. Although we did not find significant differences in SoE among landscape variables, the SoE of edge density tended to be higher than the SoE of forest cover, matrix openness and patch density. Unexpectedly, the SoE was also independent of the response variable at the community level. At the population level, we found that, as predicted, the SoE for the relative abundance index of individual species tended to increase with increasing body mass. We discuss below the ecological and conservation implications of these findings.
Contrary to our predictions, the SoE did not differ significantly among landscape variables. However, forest edge density tended to have larger SoE than forest cover, matrix openness and patch density. This is consistent with previous studies of arboreal primates (Galán-Acedo et al. 2018) and suggests that, contrary to what is usually argued (e.g. Fletcher et al. 2018), edge density affects biodiversity by mainly moderating large-scale processes, not local-scale processes (e.g. edge effects). Large-scale processes could include more frequent dispersal of individuals due to higher connectivity in landscapes with more edge density (reviewed by Ewers and Didham 2006). This issue can be particularly relevant in the context of fragmentation studies, which usually extrapolate empirical evidence obtained at the local scale to the landscape scale (reviewed by Fahrig et al. 2019). For example, a common extrapolation in these studies is that the species that have a lower abundance along forest edges than in the forest interior cannot persist in fragmented landscapes with higher edge density (Fletcher et al. 2018; Phalan 2018). Nevertheless, we suggest that, in agreement with Fahrig et al. (2019), this extrapolation is unreliable because it overlooks other mechanisms at large scales (e.g. increased landscape connectivity and habitat heterogeneity, enhanced landscape complementation and supplementation dynamics) that can counteract local edge effects. In fact, edge density is largely determined by shape complexity of remaining patches in the landscape, and shape complexity is known to facilitate the movements of individuals among habitat patches and between patches and the surrounding anthropogenic matrix (Collinge and Palmer 2002; Ewers and Didham 2006). Therefore, as argued by Galán-Acedo et al. (2018), it seems reasonable to consider edge density as a connectivity-related landscape variable, whose effects on arboreal mammals can be more evident at relatively large spatial areas.
This does not mean, however, that edge density does not shape local-scale patterns and processes. For example, the remaining forest patches in temperate and tropical forests can show a negative edge-interior temperature gradient (reviewed by Arroyo-Rodríguez et al. 2017). This gradient can promote significant community and ecosystem shifts along forest edges (Tuff et al. 2016), including an increased mortality of canopy trees (Laurance et al. 2002), which could negatively affect arboreal mammals (Cudney-Valenzuela et al. 2021). Therefore, although as argued above it seems to be reasonable that edge density is more strongly related to large-scale processes, this and other fragmentation-related metrics (e.g. patch density) could also be at least partially related to local-scale processes. This can explain the relatively large SoE variance of edge density and patch density, as these two metrics could be moderating processes across different spatial scales (Fahrig et al. 2019).
The lack of differences in SoE between species richness and total abundance is not totally surprising. Although these findings contradict our predictions based on previous theoretical models (Miguet et al. 2016), they align with a recent review showing that the SoE of richness-related response variables is not always larger than the SoE of abundance-related responses (Martin 2018). Such lack of differences in SoE can be explained by three factors. First, the landscape-scale processes regulating these two responses may act at similar scales. For example, both species richness and abundance may depend on migrations across short and large scales, and the landscape processes affecting the fitness of individuals at the population level can ultimately affect species richness. Second, given the relatively short history of land-use change in the region (< 40 years), it is reasonable to expect that there is a relatively high extinction debt, which means that the effects of extinctions (i.e. a process usually associated to large temporal and spatial scales; Miguet et al. 2016) on species richness has not been fully expressed. Finally, another non-exclusive explanation of the lack of differences in the SoE between community responses is that in their calculation we pooled the responses of species that differ greatly in their dispersal abilities. In fact, as discussed below, we found that large species tended to have larger SoE than small species. Therefore, when assessing the SoE of total abundance and species richness we are combining the responses of individual species that differ in key traits related to space-use and could prevent us from detecting community-landscape associations.
The hypothesis that body mass can determine the SoE for arboreal mammals was supported by our results. In particular, larger species tended to have larger SoE. This finding aligns with numerous studies of different animal groups that demonstrate the positive association between body mass and the landscape size that is used by the species (e.g. mammals, Tucker et al. 2014; birds, Thornton and Fletcher 2014; reptiles, Mitrovich et al. 2011; fishes, Nash et al. 2015). This can be related to the fact that larger species have larger home ranges and can travel further than smaller ones (e.g. Jetz et al. 2004; Laforge et al. 2021), as this implies that they interact with the spatial structure of larger areas.
In summary, we suggest that the SoE can depend on both landscape variables and the biological responses at the population level. These findings can be applied to guide conservation actions. Based on the fact that the SoE of edge density tended to be higher than the SoE of other landscape variables, we suggest that to prevent negative responses of biodiversity to edge density, the management and conservation actions should be designed and implemented across relatively large spatial extents. The fact that SoE is positively related to body mass is congruent with the fact that larger species have larger spatial requirements and move over larger distances. Therefore, conservation actions for larger species might be more efficient if planned and implemented across larger spatial scales.
References
Aide TM, Clark ML, Grau HR, López-Carr D, Levy MA et al (2013) Deforestation and Reforestation of Latin America and the Caribbean (2001–2010). Biotropica 45:262–271
Andresen E, Arroyo-Rodríguez V, Ramos-Robles M (2018) Primate seed dispersal: old and new challenges. Int J Primatol 39:443–465
Anzures-Dadda A, Manson RH (2007) Patch- and landscape-scale effects on howler monkey distribution and abundance in rainforest fragments. Anim Conserv 10:69–76
Arce-Peña NP, Arroyo-Rodríguez V, San-José M, Jiménez-González D, Franch-Pardo I et al (2019) Landscape predictors of rodent dynamics in fragmented rainforests: testing the rodentization hypothesis. Biodivers Conserv 28:655–669
Arroyo-Rodríguez V, Saldaña-Vázquez RA, Fahrig L, Santos BA (2017) Does forest fragmentation cause an increase in forest temperature? Ecol Res 32:81–88
Arroyo-Rodríguez V, Fahrig L, Tabarelli M, Watling JI, Tischendorf L et al (2020) Designing optimal human-modified landscapes for forest biodiversity conservation. Ecol Lett 23:1404–1420
Benchimol M, Peres CA (2021) Determinants of population persistence of terrestrial and arboreal vertebrates stranded in tropical forest land-bridge islands. Conserv Biol 35:870–883
Blanco V, Waltert M (2013) Does the tropical agricultural matrix bear potential for primate conservation? A baseline study from Western Uganda. J Nat Conserv 21:383–393
Bolt LM, Schreier AL, Voss KA, Sheehan EA, Barrickman NL et al (2018) The influence of anthropogenic edge effects on primate populations and their habitat in a fragmented rainforest in Costa Rica. Primates 59:301–311
Brennan JM, Bender DJ, Contreras TA, Fahrig L (2002) Focal patch landscape studies for wildlife management: optimizing sampling effort across scales. In: Liu J, Taylor WW (eds) Integrating: landscape ecology into natural resource management. Cambridge University Press, Cambridge, UK, pp 68–91
Carabias J, de la Maza J, Cadena R (2015) Conservación y desarrollo sustentable en la Selva Lacandona. 25 años de actividades y experiencias. Natura y Ecosistemas Mexicanos A.C., Mexico City, Mexico
Cassano CR, Barlow J, Pardini R (2012) Large Mammals in an agroforestry mosaic in the Brazilian Atlantic Forest. Biotropica 44:818–825
Ceballos G, Oliva G (2006) Los mamíferos silvestres de México. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad. Fondo de Cultura Económica, Mexico City, Mexico
Chapman CA, Bonnell TR, Gogarten JF, Lambert JE, Omeja PA et al (2013) Are primates ecosystem engineers? Int J Primatol 34:1–14
Collinge SK, Palmer TM (2002) The influences of patch shape and boundary contrast on insect response to fragmentation in California grasslands. Landsc Ecol 17:647–656
Cudney-Valenzuela SJ, Arroyo-Rodríguez V, Andresen E, Toledo-Aceves MT, Mora-Ardila F et al (2021) Does patch quality drive arboreal mammal assemblages in fragmented rainforests? Perspect Ecol Conserv 19:61–68
Estrada A, Garber PA, Rylands AB, Roos C, Fernandez-Duque E et al (2017) Impending extinction crisis of the world’s primates: why primates matter. Sci Adv 3:e1600946
Ewers RM, Didham RK (2006) Confounding factors in the detection of species responses to habitat fragmentation. Biol Rev 81:117–142
Fahrig L (2013) Rethinking patch size and isolation effects: the habitat amount hypothesis. J Biogeogr 40:1649–1663
Fahrig L (2017) Ecological responses to habitat fragmentation per se. Annu Rev Ecol Evol Syst 48:1–23
Fahrig L (2020) Why do several small patches hold more species than few large patches? Glob Ecol Biogeogr 29:615–628
Fahrig L, Arroyo-Rodríguez V, Bennett JR, Boucher-Lalonde V, Cazetta E et al (2019) Is habitat fragmentation bad for biodiversity? Biol Conserv 230:179–186
Fletcher RJ, Didham RK, Banks-Leite C, Barlow J, Ewers RM et al (2018) Is habitat fragmentation good for biodiversity? Biol Conserv 226:9–15
Galán-Acedo C, Arroyo-Rodríguez V, Estrada A, Ramos-Fernández G (2018) Drivers of the spatial scale that best predict primate responses to landscape structure. Ecography 41:2027–2037
Galán-Acedo C, Arroyo-Rodríguez V, Cudney-Valenzuela SJ, Fahrig L (2019) A global assessment of primate responses to landscape structure. Biol Rev 94:1605–1618
Ganesh T, Devy MS (2000) Flower use by arboreal mammals and pollination of a rain forest tree in South Western Ghats, India. Selbyana 21:60–65
Gestich CC, Arroyo-Rodríguez V, Ribeiro M, da Cunha RGT, Setz EZF (2019) Unraveling the scales of effect of landscape structure on primate species richness and density of titi monkeys (Callicebus nigrifrons). Ecol Res 34:150–159
Hansen A, Barnett K, Jantz P, Phillips L, Goetz SJ et al (2019) Global humid tropics forest structural condition and forest structural integrity maps. Sci Data 6:232
IUCN Red List version (2019) The IUCN Red List of Threatened Species. International Union for Conservation of Nature and Natural Resources (IUCN). https://iucnredlist.org (Accessed 09/29/2019)
Jackson HB, Fahrig L (2015) Are ecologists conducting research at the optimal scale? Glob Ecol Biogeogr 24:52–63
Janson CH, Terborgh J, Emmons LH (1981) Non-flying mammals as pollinating agents in the Amazonian forest. Biotropica 13:1–6
Jetz W, Carbone C, Fulford J, Brown JH (2004) The scaling of animal space use. Science 306:266–268
Kays R, Allison A (2001) Arboreal tropical forest vertebrates: current knowledge and research trends. Plant Ecol 153:109–120
Krauss J, Steffan-Dewenter I, Tscharntke T (2003) How does landscape context contribute to effects of habitat fragmentation on diversity and population density of butterflies? J Biogeogr 30:889–900
Laforge A, Archaux F, Coulon A, Sirami C, Froidevaux J et al (2021) Landscape composition and life-history traits influence bat movement and space use: Analysis of 30 years of published telemetry data. Glob Ecol Biogeogr 30:2442–2454
Laurance WF, Lovejoy TE, Vasconcelos HL, Bruna EM, Didham RK et al (2002) Ecosystem decay of Amazonian forest fragments: a 22-year investigation. Conserv Biol 16:605–618
Mandujano S, Pérez-Solano LA (2019) Fototrampeo en R. Organización y análisis de datos. Vol. I. Instituto de Ecología A.C., Xalapa, México
Martin AE (2018) The spatial scale of a species’ response to the landscape context depends on which biological response you measure. Curr Landsc Ecol Rep 3:23–33
Martínez-Ruiz M, Arroyo-Rodríguez V, Franch I, Renton K (2020) Patterns and drivers of the scale of landscape effect on diurnal raptors in a fragmented tropical dry forest. Landsc Ecol 35:1309–1322
Medellín RA (1994) Mammal diversity and conservation in the selva Lacandona. Chiapas Mexico Conserv Biol 8:780–799
Miguet P, Jackson HB, Jackson ND, Martin AE, Fahrig L (2016) What determines the spatial extent of landscape effects on species? Landsc Ecol 31:1177–1194
Mitrovich MJ, Gallegos EA, Lyren LM, Lovich RE, Fisher RN (2011) Habitat use and movement of the endangered arroyo toad (Anaxyrus californicus) in coastal Southern California. J Herpetol 45:319–328
Moore JF, Soanes K, Balbuena D, Beirne C, Bowler M et al (2021) The potential and practice of arboreal camera trapping. Methods Ecol Evol 12:1768–1779
Moser D, Zechmeister HG, Plutzar C, Sauberer N, Wrbka T et al (2002) Landscape patch shape complexity as an effective measure for plant species richness in rural landscapes. Landsc Ecol 17:657–669
Nash LK, Welsh JQ, Graham NAJ, Bellwood DR (2015) Home-range allometry in coral reef fishes: comparison to other vertebrates, methodological issues and management implications. Oecologia 177:73–83
Niedballa J, Sollmann R, Courtiol A, Wilting A (2016) camtrapR: an R package for efficient camera trap data management. Methods Ecol Evol 7:1457–1462
O’Brien TGO (2011) Abundance, density, and relative abundance: a conceptual framework. In: O’Connell AF, Nichols JD, Karanth UK (eds) Camera Traps in Animal Ecology. Methods and Analyses. Springer, New York, USA, pp 71–96
Ordóñez-Gómez JD, Arroyo-Rodríguez V, Nicasio-Arzeta S, Cristóbal-Azkarate J (2015) Which is the appropriate scale to assess the impact of landscape spatial configuration on the diet and behavior of spider monkeys? Am J Primatol 77:56–65
Phalan BT (2018) What have we learned from the land sparing-sharing model? Sustainability 10:1760
Piel AK, Cohen N, Kamenya S, Ndimuligo SA, Pintea L, Stewart FA (2015) Population status of chimpanzees in the Masito-Ugalla Ecosystem, Tanzania. Am J Primatol 77:1027–1035
Reid FA (2009) A field guide to the mammals of Central America and Southeast Mexico. Oxford University Press, New York, USA
Royle JA, Nichols JD, Karanth KU, Gopalaswamy AM (2009) A hierarchical model for estimating density in camera-trap studies. J Appl Ecol 46:118–127
San-José M, Arroyo-Rodríguez V, Jordano P, Meave JA, Martínez-Ramos M (2019) The scale of landscape effect on seed dispersal depends on both response variables and landscape predictor. Landsc Ecol 34:1069–1080
Schüßler D, Radespiel U, Ratsimbazafy JH, Mantilla-Contreras J (2018) Lemurs in a dying forest: factors influencing lemur diversity and distribution in forest remnants of north-eastern Madagascar. Biol Conserv 228:17–26
Srbek-Araujo AC, Chiarello AG (2005) Is camera-trapping an efficient method for surveying mammals in Neotropical forests? A case study in south-eastern Brazil. J Trop Ecol 21:121–125
Superina M, Plese T, Moraes-Barros N, Abba AM (2010) The 2010 sloth red list assessment. Edentata 11:115–134
Taubert F, Fischer R, Groeneveld J, Lehmann S, Müller MS et al (2018) Global patterns of tropical forest fragmentation. Nature 554:519–522
Thornton DH, Fletcher RJ (2014) Body size and spatial scales in avian response to landscapes: a meta-analysis. Ecography 37:454–463
Tscharntke T, Tylianakis JM, Rand TA, Didham RK, Fahrig L et al (2012) Landscape moderation of biodiversity patterns and processes - eight hypotheses. Biol Rev 87:661–685
Tucker MA, Ord TJ, Rogers TL (2014) Evolutionary predictors of mammalian home range size: body mass, diet and the environment. Glob Ecol Biogeogr 23:1105–1114
Tucker MA, Böhning-Gaese K, Fagan WF, Fryxell JM, Van Moorter B, et al (2018) Moving in the Anthropocene: Global reductions in terrestrial mammalian movements. Science 359:466–469
Tuff KT, Tuff T, Davies KF (2016) A framework for integrating thermal biology into fragmentation research. Ecol Lett 19:361–374
Urquiza-Haas T, Peres CA, Dolman PM (2011) Large vertebrate responses to forest cover and hunting pressure in communal landholdings and protected areas of the Yucatan Peninsula, Mexico. Anim Conserv 14:271–282
Watling JI, Arroyo-Rodríguez V, Pfeifer M, Baeten L, Banks-Leite C et al (2020) Support for the habitat amount hypothesis from a global synthesis of species density studies. Ecol Lett 23:674–681
Wayne AF, Cowling A, Lindenmayer DB, Ward CG, Vellios CV et al (2016) The abundance of a threatened arboreal marsupial in relation to anthropogenic disturbances at local and landscape scales in Mediterranean-type forests in south-western Australia. Biol Conserv 127:463–476
Whitworth A, Villacampa J, Brown A, Huarcaya RP, Downie R et al (2016) Past human disturbance effects upon biodiversity are greatest in the canopy; a case study on rainforest butterflies. PLoS Biol 11:e0150520
Yeiser JM, Chandler RB, Martin JA (2021) Distance-dependent landscape effects in terrestrial systems: a review and a proposed spatio-temporal framework. Curr Landsc Ecol Rep 6:1–8
Acknowledgements
We especially thank Audón Jamangapé, Adolfo Jamangapé and Marta Aguilar for their invaluable field assistance and accommodation in the Marqués de Comillas Region. We also thank the landowners for allowing us to collect data on their properties. We thank Carmen Galán-Acedo for her help digitizing the map and obtaining landscape variables.
Funding
This research was supported by the Rufford Small Grant no. 23706-1, SEP-CONACyT (Project 2015-253946), and IdeaWild. SCV obtained a graduate scholarship from CONACyT. This paper constitutes a partial fulfilment of the PhD program of the Posgrado en Ciencias Biológicas, Universidad Nacional Autónoma de México.
Author information
Authors and Affiliations
Contributions
SCV and VAR developed the idea of the study, with support from EA and TTA. SCV collected and analysed the data with guidance from VAR. All authors made substantial contributions to the intellectual content, interpretation and editing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cudney-Valenzuela, S.J., Arroyo-Rodríguez, V., Andresen, E. et al. What determines the scale of landscape effect on tropical arboreal mammals?. Landsc Ecol 37, 1497–1507 (2022). https://doi.org/10.1007/s10980-022-01440-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-022-01440-w