Abstract
Context
Habitat loss is widely recognized as the main driver of biodiversity loss around the globe, yet the effects of habitat fragmentation on biodiversity have been extensively debated in recent years.
Objectives
We used a robust dataset of medium and large-sized mammals to test (a) the Habitat Amount Hypothesis, which postulates that species richness can be mainly predicted by the total amount of habitat surrounding the sampling site, and (b) the effects of habitat fragmentation per se, which may be expected to be weak or mainly positive on species richness.
Methods
We compiled information on the occurrence of mammal species in 166 forest fragments across the Atlantic Forest. For each forest fragment, we extracted information on patch size, percentage of forest cover (a proxy for habitat amount), and edge density and number of fragments (fragmentation metrics). We related these metrics to mammalian richness considering separately for all species, forest-dependent species, disturbance-tolerant species, and different trophic guilds.
Results
All richness measures strongly declined with decreasing forest cover, yet were unaffected by patch size, number of patches and edge density. The only exception occurred with herbivore richness, which was affected by number of patches. However, we found fragmentation per se effects only for herbivore richness.
Conclusions
Our results show that mammal richness increased with habitat amount at the landscape, whereas habitat fragmentation per se had significant negative impacts on herbivores only. We therefore recommend maintaining highly forested landscapes and restoring severely deforested areas, being essential for ensuring high richness of mammals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rapid loss of natural environments is currently considered the main threat to biodiversity worldwide (Newbold et al. 2015, 2016). In the tropics, large tracts of pristine forests are being replaced by agriculture, pastures and urban expansion areas transforming natural environments into a mosaic of forest patches (Gibbs et al. 2010; Curtis et al. 2018). Habitat loss and fragmentation are different processes, though generally associated, and both can result from anthropogenic land conversion (Fahrig 2003). Recognizing the independent effects of habitat loss and fragmentation on ecological responses is crucial for effective decision making, as species can respond differently to these processes and inappropriate decisions may compromise the efficiency of conservation measures (Lindenmayer and Fischer 2007; Mortelliti et al. 2010).
The Habitat Amount Hypothesis (HAH), proposed by Fahrig (2013), states that species richness should be predicted by the habitat amount in the landscape surrounding the sample site (Fahrig 2013). This hypothesis suggests that the two predictive variables of the Theory of Island Biogeography (MacArthur and Wilson 1967)—patch size and isolation—can be replaced by a single variable, the habitat amount in the landscape. Despite its relatively recent proposition, the HAH has been tested by several studies. For instance, a global synthesis of species density studies of eight taxonomic groups showed that richness was more strongly and positively related to habitat amount, whereas habitat fragmentation metrics unaffected patterns of species richness for the groups evaluated (Watling et al. 2020). Additionally, studies focused on birds (De Camargo et al. 2018), beetles (Seibold et al. 2017) and small mammals (Melo et al. 2017; Vieira et al. 2018) also supported the HAH. Yet some studies failed to support this hypothesis (see Haddad et al. 2016; Bueno and Peres 2019), suggesting that in some cases patch size and isolation might be more decisive than habitat amount.
In contrast to the robust evidence on the pervasive effects of habitat loss on biodiversity, there is no consensus on the effects of fragmentation per se (sensu Fahrig 2003, 2017: the breaking apart of habitat, independent of changes in habitat amount) on biological components. For instance, a recent review showed that when habitat amount is controlled, there are often no additional effects of landscape configuration (i.e. fragmentation) on species’ ecological responses and that when fragmentation effects are detectable, they are mostly positive (Fahrig 2017). This publication ignited an intense debate among researchers (Fahrig 2017; Fletcher Jr et al. 2018; Fahrig et al. 2019) on whether the independent effects of habitat fragmentation per se are positive (Fahrig 2003, 2017) or negative (Reino et al. 2013; Rueda et al. 2013). Disentangling the effects of habitat loss and fragmentation is challenging but necessary for conservation decisions.
An interesting scenario to assess the independent effects of habitat amount and fragmentation per se is the Brazilian Atlantic Forest. This biome extends over 112 Mha distributed along the Brazilian coast; current estimates indicate that 28% of the original native cover remains, with 26% corresponding to forest areas (Rezende et al. 2018). Most of these forest fragments are very small (< 50 ha) and isolated (at an average distance of 1.5 km from one another; Ribeiro et al. 2009). In addition to deforestation, the Atlantic Forest is also highly threatened by poaching (Galetti et al. 2009) and human occupation, severely impacting local fauna (Bogoni et al. 2016). It also harbors one of the highest degrees of biodiversity and endemism for several plant and animal groups, being recognized as a global biodiversity hotspot (Myers et al. 2000). For instance, this biome is home to 321 mammal species, with 89 endemic ones (Graipel et al. 2017).
The importance of mammals for ecosystem structure and functioning is widely recognized (Galetti and Dirzo 2013) due to their key role in community structuring (Jorge et al. 2013), seed predation and dispersal, and their contribution to forest carbon stocks (Bello et al. 2015). Mammals are highly threatened mainly by changes in their habitat (Jorge et al. 2013) and hunting (Cullen Jr et al. 2000; Galetti et al. 2009). Thus, the intense history of deforestation and hunting that occurred in the Atlantic Forest led to pronounced current changes in species composition of mammal assemblages (Canale et al. 2012). Disturbance-sensitive species are the first to disappear in highly disturbed areas, whereas alien and disturbance-tolerant species tend to become dominant in forest fragments (Jorge et al. 2013; Beca et al. 2017). Changes in community composition can also be detected, as functional groups tend to respond non-randomly to changes in their habitat. For example, species that require large home ranges to survive, such as carnivores, tend to be negatively affected by human disturbances, whereas omnivorous and insectivorous species are prone to exhibit great plasticity and be less impacted (Bogoni et al. 2016; Regolin et al. 2017).
Here, we gathered and analyzed a robust dataset on the occurrence of medium to large-bodied mammal species for a wide range of forest patches throughout the Atlantic forest to (a) test the habitat amount hypothesis and (b) evaluate the effects of forest fragmentation per se on this group. We evaluated species richness considering the complete assemblage, the forest-dependent and disturbance-tolerant species separately and the species within each trophic guild (i.e., carnivores, insectivores, frugivores, omnivores and herbivores) individually. Specifically, we expected that (a) richness patterns would be better explained by the amount of habitat than by patch size or by fragmentation per se metrics (i.e., edge density and number of patches; Watling et al. 2020) when considering the complete assemblage and investigated groups, and (b) the effects of fragmentation per se on species richness would be mainly positive (Fahrig et al. 2017).
Methods
Data base
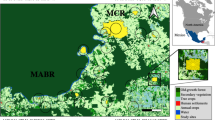
We gathered information on the occurrence of medium- and large-sized mammal species (all terrestrial and arboreal wildlife species larger than 1 kg; see Paglia et al. 2012) within forest fragments (defined as forest patches > 1 ha) from published studies conducted in the Atlantic Forest. In July 2019, we first searched studies using the Scopus and Google Scholar databases, specifying the terms "Atlantic Forest" AND "medium-sized (bodied) mammal" AND/OR "large-sized (bodied) mammal". We further used the same terms in Google Scholar, but only evaluated the first 200 results. We finally included records from the “ATLANTIC-CAMTRAPS” datapaper (Lima et al. 2017). We included studies if they provided (a) the geographic coordinates of the forest fragment(s), (b) the complete list of species recorded per forest fragment(s), and (c) if the data were obtained using camera traps, linear transects, or sign surveys (i.e. feces, playbacks, hair, and footprints), excluding secondary data and interviews. We did not include island patches and reviews. Whenever different studies sampled the same forest fragments, we combined the information and considered them as a single study, resulting in 67 studies carried out in 166 forest fragments (see Appendix S1 in Supporting Information). All forest fragments belonged to either protected or private areas, and were vastly distributed throughout the Brazilian Atlantic Forest, in areas of dense, mixed or semideciduous ombrophilous forest (Fig. 1). The size of forest fragments varied from 1.030 ha to more than 41,700 ha (mean = 2591.19 ha, SD = 6,974.74 ha), and the surrounding land uses consisted mainly of agriculture, pasture, and urban areas.
Geographic distribution of the 166 forest fragments with records on the occurrence of medium and large-sized mammals along the Atlantic Forest, Brazil. The highlighted circular area is an example of landscapes of 0.5, 1, 2, 4 and 6 km of radius around a sampling site where the variables were calculated
We obtained total species richness for each forest fragment by summing all occurrences of the target mammalian group. However, we only considered forest-dwelling species into our database, excluding those species that use forest environments but have preferences for other types of habitats. For example, we excluded the maned wolf (Chrysocyon brachyurus) and the giant anteater (Myrmecophaga tridactyla), as these species most often use open areas like savannas and grasslands. We also excluded species like the Neotropical otter (Lontra longicaudis) and capybara (Hidrochoerus hydrochaeris), which is strongly associated with aquatic environments. Finally, we excluded exotic and opportunistic species such as feral pigs (Sus scrofa feral) and domestic dogs (Canis lupus familiaris) and difficult-to-detect species, such as the maned three-toed sloth (Bradypus torquatus) and the brown-toed sloth (B. variegatus), due to the high probability of false negatives. We thus included 55 species in our study (Table S1).
Although all species included in our database were forest-dwelling species, we further classified them in two categories, according to their tolerance to disturbances based on the literature (see Emmons and Feer 1997; Souza et al. 2019; IUCN 2019): (i) forest-dependent species, which are those found in non-disturbed forests and most frequently in forest interior, and (ii) disturbance-tolerant species, which comprise those more tolerant to anthropogenic disturbances, occurring frequently in disturbed forests or forest edges. In addition, we classified species into trophic guilds: carnivores, insectivores, frugivores, omnivores and herbivores, following Magioli et al. (2015).
Habitat amount and fragmentation metrics
We extracted patch size (in hectares) from the studies or, when not available, calculated it using Google Earth Pro. We then obtained landscape metrics using the maps from MapBiomas Project– Collection [4, 2019] derived from 30-m resolution Landsat imagery, downloaded from the Brazilian Annual Land Use and Land Cover Mapping Project (available at < http://mapbiomas.org >). For each forest fragment, we used the map corresponding to the last year in which data collection was performed in each study.
Forest cover was calculated as the proportion of native forest (excluding agroforestry), considering buffers of 0.5, 1, 2, 4 and 6 km radius around each sampling point provided by the study. These scales were selected based on the literature showing that medium and large-sized mammals best respond to landscape characteristics at large scales and according to the species’ dispersal ability (Lyra-Jorge et al. 2010; Beca et al. 2017; Regolin et al. 2017). To test the fragmentation per se effects, we obtained two fragmentation metrics: the edge density, calculated as the total perimeter of all forest fragments divided by the size of the landscape, and the number of forest patches within each landscape. Analyses were performed in R 3.5.2 (R Core Team 2018) and QGIS® software.
Data analysis
We firstly evaluated the scale of effect—the spatial extent at which a certain landscape characteristic has the strongest impact on the dependent variable (Martin and Fahrig 2012; Fahrigh 2013) for each landscape variable. For this, we adjusted Generalized Linear Models (GLM) using the 'Multifit' function (Huais 2018), which automates the selection process at various scales and relates the response variable to each explanatory variable. We then used Akaike's Information Criterion (AIC) to classify the models, selecting the ‘best’ scale as the one showing the lowest AIC. The scale of effects varied between 0. 5, 1, 2 and 4 km, for different metrics (Table S2).
Forest fragments were considered spatially nested within several regions. Using QGIS software, we distinguished regions by combining groups of forest fragments according to the presence of large geographical barriers such as (1) large rivers, belonging to the main hydrographic basins that occur in the Atlantic Forest, (2) mountainous areas, using relief information, and (3) fragments belonging to extensive areas of forest, using Google Earth images. We extracted this information from the Brazilian Institute of Geography and Statistics (IBGE 2017) and Google Earth Pro tools. We transformed patch size values into logarithm (Log10) to homogenize the spread of the data. Due to differences in measurement units between the explanatory variables, we standardized their values by subtracting the mean and dividing by the standard deviation. Although sampling effort varied among surveyed forest fragments, we were unable to obtain this information for most of fragments included in our database. Additionally, different sampling techniques were used among studies, which also implies in different units of sampling effort. Therefore, we were unable to account for sampling effort in the GLMs. We used Variance Inflation Factors (VIF) (Zuur et al. 2009) to verify the collinearity between the explanatory variables. The VIF was always below 3, and, considering the ecological importance of all variables, we kept all of them in further analyses (Dormann et al. 2013) (Table S3).
To test the HAH on mammal richness, we first adjusted GLMs, with negative binomial distribution, containing all explanatory variables (i.e., patch size, forest cover, edge density and number of patches), for each response variable (richness of all species, forest-dependent and disturbance-tolerant species, and different trophic guilds), with no interactions. Subsequently, we verified the residual spatial autocorrelation using Moran's I and semivariograms. As the residuals were spatially autocorrelated, we adjusted Generalized Linear Mixed Models (GLMMs), which are appropriate to control for spatial non-independence (Zuur et al. 2009). Thus, we incorporated the terms 'region' and 'data type' into the models as random factors. The variable 'data type' corresponds to the different data collection methods (camera trap, linear transect or sign surveys). For ‘data type’, we included all methods cited in each study analyzed. We re-evaluated spatial autocorrelation using the same procedure as above, and no further corrections were necessary.
We performed Multimodel Inference (Burnham and Anderson 2002) using the function 'dredge', which compares all subsets of models with all possible combinations of explanatory variables plus a null model, resulting in a total of 16 models being compared for each response variable. Afterwards, we used the function 'importance' to calculate the relative importance of each explanatory variable (Burnham and Anderson 2002). This function sums the Akaike weights of models including each explanatory variable; variables with larger summed weights are more important than variables with smaller weights (Burnham and Anderson 2002). Finally, we evaluated the significance (p < 0.05) and direction (positive or negative) of the effect of each explanatory variable, using the model averaging approach, which calculates the average of the coefficients of all models weighted by the Akaike weights (Burnham and Anderson 2002).
To test the fragmentation per se effects on mammal richness, we adjusted GLMMs (with negative binomial distribution and the same random factors as above) containing habitat amount as the only explanatory variable. We then extracted the model Pearson residuals (calculated as the observed minus the expected value, divided by the square root of the variance – Zuur et al. 2009) in order to control the effects of habitat amount on the response variables. Finally, we used linear regressions to relate the residuals to fragmentation metrics (edge density and number of patches). With this, we aimed to assess whether the fragmentation metrics can explain variation that was not explained by habitat amount. We assessed whether there was an effect by analyzing the p-value; when it was significant (p ≤ 0.05) we also checked whether the relation was positive or negative. All analyses were carried out in R 3.5.2 (R Core Team 2018), using the lme4 (Bates et al. 2015), MASS (Venables and Ripley 2002) and MuMIn (Barton 2020) packages.
Results
From the total of 55 mammal species recorded at least once in all forest fragments included in our final databse, 33 were classified as forest-dependent species while 22 comprise disturbance-tolerant species. They belong to nine orders: Primates, Rodentia, Cingulata, Lagomorpha, Cetartiodactyla, Perissodactyla, Pilosa, Carnivora and Didelphimorphia. The most common species were the nine-banded armadillo (Dasypus novemcinctus) (62%), followed by the South-American coati (Nasua nasua) (56%), the crab-eating fox (Cerdocyon thous) (49%) and the crab-eating raccoon (Procyon cancrivorus) (44%), whereas the agouti (Dasyprocta iacki) and the southern long-nosed armadillo (Dasypus hybridus) had a single record each.
Forest cover had positive effects and was the most important variable explaining richness patterns of medium and large-bodied mammals, independent of the disturbance tolerance or the trophic guild evaluated (Fig. 2). The exception was the insectivores, in which edge density was the most important variable. For omnivores and herbivores, respectively, edge density and number of patches were also important. The second most important variable was the number of patches, partially explaining richness patterns of the overall community, forest-dependent species, carnivores, frugivores and herbivores. However, only herbivores richness was significantly affected by the number of patches. The patch size and edge density did not significantly explain species richness of any group (Fig. 2; Tables S4; S5).
Relative importance of each spatial variables: (PS) patch size, (FC) forest cover, (ED) edge density and (NP) number of patches for mammal species richness considering: a all species, b forest-dependent, c disturbance-tolerant, d carnivores, e insectivores, f frugivores, g omnivores and h herbivores. Values correspond to the sum of Akaike weights of the models containing each explanatory variable. The (+) and (−) corresponds to the variables that had a significant positive and negative effect, respectively
Regarding the fragmentation per se effects, our results showed no relationship between the residuals of species richness and the fragmentation metrics except for herbivores that were negatively influenced by the number of patches. This shows that, in general, habitat fragmentation per se had no effect on mammal richness after accounting for the effects of habitat amount (Fig. 3).
Relationship between forest cover model residuals and fragmentation variables (ED—edge density and NP—number of patches), for medium and large-sized mammals of the Atlantic Forest in relation to the richness of: a all species, b forest-dependent, c disturbance-tolerant, d carnivores, e insectivores, f frugivores, g omnivores and h herbivores
Discussion
Our results show that habitat amount is the most important predictor explaining the richness of medium and large-sized mammals inhabiting forest patches in the Brazilian Atlantic Forest. We also found that fragmentation per se had no effects on mammal richness, except for herbivores. The combination of these results reinforces the importance of habitat amount over fragmentation to explain species richness, independent of the functional differences between the groups analyzed. Our study thus adds more evidence to the importance of forest cover to safeguard biodiversity, with direct and simple conservation strategies focused on mammals ‒ maximizing the habitat amount in the landscape.
Habitat amount hypothesis
Our results support the HAH, as forest cover, used as a proxy for habitat amount, was the most important predictor of mammal species richness. The same pattern has been observed for medium and large-sized mammals in the southern region of Brazil (Regolin et al. 2017) and small specialist mammals in the Brazilian Cerrado (Melo et al. 2017). Yet forest-dependent small mammals responded equally to habitat amount and isolation in another study (Vieira et al. 2018). In addition to mammals, other groups have also responded positively to increases in habitat amount, including saprophytic beetles (Seibold et al. 2017), moths (Merckx et al. 2019) and birds (Lindenmayer et al. 2020). Although sampling effort was not included in our data analyses given the absence of this information on most studies coupled with the inclusion of different sampling methodologies in our database, we still found a strong relationship between species richness and habitat amount. In fact, this reinforces the hypothetical consistency of the habitat amount effects, since other studies took into account the sampling intensity (De Camargo et al. 2018; Palmeirim et al. 2019).
Our results also showed that species exhibiting contrasting responses to environmental disturbances, such as forest-dependent and disturbance-tolerant species, responded similarly to the habitat amount in the landscape. In general, forest cover was also the most important variable explaining richness of all functional groups analyzed, especially the richness of carnivores, frugivores, omnivores and herbivores. These results reinforce the importance of highly forested landscapes to safeguard large-bodied mammal species, a unique ecological group but which had their ranges intensely reduced due to the loss and degradation of their habitats (Canale et al. 2012; Jorge et al. 2013). In fact, the disappearance of frugivore and herbivore species from forest fragments can have pervasive implications for forest functionality. For example, the extinction of the white-lipped peccary, a large-bodied herbivore that is also an important seed disperser and predator, had strong negative impacts on the composition of small mammal communities in southeastern Brazil (Galetti et al. 2015), while the disappearance of species such as tapir can compromise populations of species with large seeds and long-distance dispersal (Galetti et al. 2001).
Patch size did not affect medium and large-sized mammals in the Atlantic forest. This contrasts to other studies that have found that patch size is a key predictor of mammal richness (Benchimol and Peres 2015; Michalski and Peres 2007). Conversely, edge density was an important variable to explain richness patterns of insectivores, also being the second variable with the highest weight for omnivores, but without significant effects. Conversely, the number of patches was the second most important variable to explain species richness of most of the groups analyzed, having a significant negative effect only for herbivores. Although edge density did not have a significant effect, such negative relationships with edge density have been observed for the richness of carnivorous mammals in areas of Atlantic Forest and Savanna, in Brazil (Lyra-Jorge et al. 2010). This negative effect may be related to microclimate and forest structure changes due to edge effects (Murcia 1995; Harper et al. 2005). In addition, species responses to edge density can generally be related to how species exploit resources in the landscape and the availability of resources at the edges and in the matrix (Ries and Sisk 2004). For example, more habitat-generalist species with high dispersion capacity tend to benefit at the edges (Lyra-Jorge et al. 2010; Pfeifer et al. 2017). However, this has not been confirmed here, even for groups that have lower specific diets, such as insectivores and omnivores. Similarly, the negative effect of the number of patches on species richness, especially for herbivores, might be explained by the matrix surrounding the forest fragments analyzed here, which mainly consisted of agriculture and cattle pasture areas. As the matrix quality can influence the connectivity between habitat fragments in the landscape (Arroyo-Rodríguez et al. 2020), it is possible that croplands and pastures represent a less permeable matrix for these species, as these environments reduces connectivity between forest fragments (Watling et al. 2011). This negative relationship with edge density and number of fragments in the landscape may accentuate the need for forest areas for these mammal species, since species that use the matrix tend to require smaller amounts of forest to persist (Arroyo-Rodríguez et al. 2020).
Overall, our study contrasts with some studies that did not corroborate the HAH, such as the experimental study with plants and microarthropods performed by Haddad et al. (2016). A possible explanation for the results found by Haddad et al. (2016) is that they detected a smaller richness due to the high turnover of species in the studied landscapes, which would lead to a decline in species richness (see De Camargo et al. 2018). Another factor that can be determinant is the matrix type surrounding the patch. For instance, a study with insectivorous birds in the Amazon found that bird richness was predicted mainly by the fragment size and not by habitat amount, which can be justified by the aquatic matrix that substantially contrasts with the birds' habitat and the low dispersion capacity of the species analyzed (Bueno and Peres 2019). Here, we did not include islands in the database, but we suggest that future studies should investigate this relationship.
Effects of fragmentation per se
Our results showed that fragmentation per se had no effect on medium and large-sized mammal richness. Even for groups for which edge density and number of patches had a relatively high importance, these effects were insignificant when removing the effects of forest cover, except for herbivore richness, which was negatively affected by the number of patches in the landscape. This result corroborates previous evidence that the effects of fragmentation per se are in general non-significant (Fahrig 2003, 2017), implying that medium to large-bodied Atlantic Forest mammals respond more strongly to habitat loss than to fragmentation. On the other hand, other studies have found strong effects of fragmentation per se on mammal richness. For example, Regolin et al. (2020) found positive effects of landscape configuration, measured as edge density, on terrestrial forest mammals in the southwestern region of Brazil, whereas Palmeirim et al. (2019) unveiled that the number of patches positively affected patterns of small mammal species richness.
Considering the ongoing debate on the effects of habitat fragmentation, it is important to understand under which circumstances, for which group and type of landscapes the fragmentation effects are more important. Our results contribute by adding more evidence that fragmentation has weak effects for large-bodied mammals in the Atlantic Forest and emphasizes the greater importance of landscape forest cover over landscape configuration in explaining richness patterns of forest-dweller mammal assemblages in forest fragments of the threatened Atlantic forest. We therefore reinforce the importance of focusing conservation efforts on all habitat patches, even if they are small and regardless of how they are distributed in the landscape (Fahrig et al. 2019). This is truly important for the Atlantic Forest, which is highly fragmented and has more than 80% of its remaining area in patches smaller than < 50 ha (Ribeiro et al. 2009). We also highlight the need to recover degraded areas, to increase the habitat amount in the landscape. These approaches would maximize the habitat amount available, maintaining the minimum conditions necessary to ensure high richness of mammals and also favor the persistence of other groups that occur in the Atlantic Forest (see Morante-Filho et al. 2020).
Additionally, we suggest that future studies include other features of the landscape that tend to interact with fragmentation, as this knowledge can maximize species retention in fragmented landscapes (Lees and Peres 2009; Miller-Rushing et al. 2019). For example, with the intense landscape modification, habitat remnants end up immersed within different land use types, and several studies have demonstrated the effect of matrix quality on species persistence (Brady et al. 2011; Beca et al. 2017). Finally, we agree that this is a relevant debate for conservation. Particularly, it is essential to enhance our understanding regarding the circumstances under which fragmentation per se affects biodiversity, and, when these effects are detected, whether they are mostly positive or negative. This knowledge can thus be translated into effective and vital conservation actions (Fletcher Jr et al. 2018; Fahrig et al. 2019).
Conclusions
Our results showed that mammal species richness responds more strongly to habitat amount in the surrounding landscapes and that fragmentation per se affected only herbivores, whose richness decreased with increasing number of patches. Thus, these results reinforce the HAH and support the idea that fragmentation per se has consistently weak effects on the ecological responses of species when the habitat amount is controlled (see Fahrig 2013, 2017). Understanding the responses of mammal assemblages to changes in their habitat is essential to define better conservation and management strategies for the Brazilian Atlantic Forest. These results contribute to simplification in decision making policy conservation actions, since efforts can focus on preventing habitat loss, as well as increasing or maintaining the total habitat amount in the landscape and restoring degraded habitats. These measures have direct implications for safeguard richer mammal assemblages, and this is especially important for the Atlantic Forest that has already been extensively devastated (Ribeiro et al. 2009) and has a large number of species either locally extinct or severely threatened with extinction at the national level (MMA 2014).
Data availability
Data will be available from the Figshare Repository.
References
Arroyo-Rodríguez V, Fahrig L, Tabarelli M, Watling JI, Tischendorf L, Benchimol M, Cazetta E, Faria D, Leal IR, Melo FPL, Morante-Filho JC, Santos BA, Arasa-Gisbert R, Arce-Peña N, Cervantes-López MJ, Cudney-Valenzuela S, Galán-Acedo C, San-José M, Vieira ICG, Slik JWF, Nowakowski AJ, Tscharntke T (2020) Designing optimal human-modified landscape for forest biodiversity conservation. Ecol Lett 23:1404–1420
Barton K (2020) Multi-Model Inference: Package 'MuMIn'. R package version 1.43.17. https://cran.r-project.org/web/packages/MuMIn/MuMIn.pdf
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Soft 67:1–48
Beca G, Vancine MH, Carvalho CS, Pedrosa F, Alves RSC, Buscariol D, Peres CA, Ribeiro MC, Galetti M (2017) High mammal species turnover in forest patches immersed in biofuel plantations. Biol Conserv 210:352–359
Bello C, Galetti M, Pizo MA, Magnago LFS, Rocha MF, Lima RAF, Peres CA, Ovaskainen O, Jordano P (2015) Defaunation affects carbon storage in tropical forests. Sci Adv 1:e1501105
Benchimol M, Peres CA (2015) Widespread forest vertebrate extinctions induced by a mega hydroelectric dam in lowland Amazonia. PLoS ONE 10:e0129818
Bogoni JA, Cherem JJ, Giehl ELH, Oliveira-Santos LG, De Castilho PV, Filho VP, Fantacini FM, Tortato MA, Luiz MR, Rizzaro R, Graipel ME (2016) Landscape features lead to shifts in communities of medium-to large-bodied mammals in subtropical Atlantic Forest. J Mamm 97:713–725
Brady MJ, McAlpline CA, Possingham HP, Miller CJ, Baxter GS (2011) Matrix is important for mammals in landscapes with small amounts of native forest habitat. Landsc Ecol 26:617–628
Bueno AS, Peres CA (2019) Patch-scale biodiversity retention in fragmented landscapes: Reconciling the habitat amount hypothesis with the island biogeography theory. J Biogeogr 46:621–632
Burnham KP, Anderson DR (2002) Model Selection and Multimodel Inference: a pratical information—theoric approach, 2nd edn. Springer, New York
Canale GR, Peres CA, Guidorizzi CE, Gatto CAF, Kierulff MCM (2012) Pervasive defaunation of forest remnants in a tropical biodiversity hotspot. PLoS ONE 7:e41671
Core Team R (2018) R: A language and Environment for Statistical Computing. Viena, Austria: R Foundation for Statistical Computing. Retrieved from https://www.r-project.org/
Cullen L Jr, Bodmer RE, Pádua CV (2000) Effects of hunting in habitat fragments of the Atlantic forests, Brazil. Biol Cons 95:49–56
Curtis PG, Slay CM, Harris NL, Tyukavina A, Hansen MC (2018) Classifying drivers of global forest loss. For Ecol 361:1108–1111
De Camargo RX, Boucher-Lalonde V, Currie DJ (2018) At the landscape level, birds respond strongly to habitat amount but weakly to fragmentation. Divers Distrib 24:629–639
Dormann CF, Elith J, Bacher S, Buchmann C, Carl G, Carré G, Marquéz JRG, Gruber B, Lafourcade B, Leitão PJ, Münkemüller T, McClean C, Osborne PE, Reineking B, Schröder B, Skidmore AK, Zurell D, Lautenbach S (2013) Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36:27–46
Emmons L, Feer F (1997) Neotropical rainforest mammals: a field guide, 2nd edn. University of Chicago Press, Chicago
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34:487–515
Fahrig L (2013) Rethinking patch size and isolation effects: the habitat amount hypothesis. J Biogeogr 40:1649–1663
Fahrig L (2017) Ecological responses to habitat fragmentation per se. Annu Rev Ecol Evol Syst 48:1–23
Fahrig L, Arroyo-Rodríguez V, Bannett JR, Boucher-Lalonde V, Cazetta E, Currie DJ, Eigenbrod F, Ford AT, Harrison SP, Jaeger JAG, Koper N, Martin AE, Martin JL, Metzger JP, Morrison P, Rhodes JR, Saunders DA, Simberloff D, Smith AC, Tischendorf L, Vellend M, Watling JI (2019) Is habitat fragmentation bad for biodiversity? Biol Conserv 230:179–186
Fletcher RJ, Didham RK, Ewers RM, Ries L, Laurance WF, Banks-Leite C, Barlow J, Ewers RM, Rosindell J, Holt RD, Gonzalez A, Pardini R, Damschen EI, Melo FPL, Ries L, Prevedello JA, Tscharntke T, Laurance WF, Lovejoy T, Haddad NM (2018) Is habitat fragmentation good for biodiversity? Biol Conserv 226:9–15
Galetti M, Keuroghlian A, Hanada L, Morato MI (2001) Frugivory and seed dispersal by the lowland tapir (Tapirus terrestris) in Southeast Brazil. Biotropica 33:723–726
Galetti M, Giacomini HC, Bueno RS, Bernardo CSS, Marques RM, Bovendorp RS, Steffer CE, Rubim P, Gobbo SK, Donatti CI, Begotti RA, Meirelles F, Nobre RA, Chiarello AG, Peres CA (2009) Priority areas for the conservation of Atlantic forest large mammals. Biol Conserv 142:1229–1241
Galetti M, Dirzo R (2013) Ecological and evolutionary consequences of living in a defaunated world. Biol Conserv 163:1–6
Galetti M, Guevara R, Neves CL, Rodarte RR, Bovendorp R, Moreira M, Hopkins JB III, Yeakel JD (2015) Defaunation affects the populations and diets of rodents in Neotropical rainforests. Biol Conserv 190:2–7
Gibbs HK, Ruesch AS, Clayton MK, Holmgren P, Foley JA, Ramankutty N, Achard F (2010) Tropical forests were the primary sources of new agricultural land in the 1980s and 1990s. Proc Natl Acad Sci USA 107:16732–16737
Graipel ME, Cherem JJ, Monteiro-Filho ELA, Carmignotto AP (2017) Mamíferos da Mata Atlântica. In: Monteiro-Filho ELA, Conte CE (eds) Revisões de Zoologia: Mata Atlântica (pp 391–482). Ed. UFPR
Haddad NM, Gonzalez A, Brudvig LA, Burt MA, Levey DJ, Damschen EI (2016) Experimental evidence does not support the Habitat Amount Hypothesis. Ecography 40:48–55
Harper KA, Macdonald SE, Burton PK, Chen JQ, Brosofske KD, Saunders SC, Euskirchen ES, Roberts D, Jaiteh MS, Esseen PA (2005) Edge influence on forest structure and composition in fragmented landscapes. Conserv Biol 19:768–782
Huais PY (2018) Multifit: an R function for multi-scale analysis in landscape ecology. Landsc Ecol 33:1023–1028
IBGE (2017) Base cartográfica Nacional IBGE. http://forest-gis.com/download-de-shapefiles/
IUCN (2019) The IUCN red list of threatened species. https://www.iucnredlist.org/
Jorge MLSP, Galetti M, Ribeiro MC, Ferraz KMPMB (2013) Mammal defaunation as surrogate of trophic cascades in a biodiversity hotspot. Biol Conserv 163:49–57
Lees AC, Peres CA (2009) Gap-crossing movements predict species occupancy in Amazonian forest fragments. Oikos 118:280–290
Lima F, Beca G, Muylaert RL, Jenkins CN, Perili MLL, Paschoal AMO, Massara RL, Paglia AP, Chiarello AG, Graipel ME, Cherem JJ, Regolin AL, Santos LGRO, Brocardo CR, Paviolo A, Di Bitetti MS, Scoss LM, Rocha FL, Fusco-Costa R, Rosa CA, Da Silva MX, Hufnagell L, Santos PM, Duarte GT, Guimarães LN, Bailey LL, Rodrigues FHG, Cunha HM, Fantacini FM, Batista GO, Bogoni JA, Tortato MA, Luiz MR, Peroni N, De Castilho PV, Maccarini TB, Filho VP, De Angelo C, Cruz P, Quiroga V, Iezzi ME, Varela D, Cavalcanti SMC, Martensen AC, Maggiorini EV, Keesen FF, Nunes AV, Lessa GM, Cordeiro-Estrela P, Beltrão MG, De Albuquerque ACF, Ingberman B, Cassano CR, Cullen Jr LC, Ribeiro MC, Galetti M (2017) ATLANTIC-CAMTRAPS: a dataset of medium and large terrestrial mammal communities in the Atlantic Forest South America. Ecology 98:1–32
Lindenmayer DB, Fischer J (2007) Tackling the habitat fragmentation panchreston. Trends Ecol Evol 22:127–132
Lindenmayer DB, Blanchard W, Foster CN, Scheele BC, Westgate MJ, Stein J, Crane M, Florance D (2020) Habitat amount versus connectivity: an empirical study of bird responses. Biol Conserv 241:108377
Lyra-Jorge MC, Ribeiro MC, Ciochete G, Tambosi LR, Pivello VR (2010) Influence of multi-scale landscape structure on the occurrence of carnivorous mammals in a human-modified savanna, Brazil. Eur J Wild Res 56:359–368
MacArthur RH, Wilson EO (1963) An equilibrium theory of insular zoogeography. Evolution (NY) 17: 373–387.
Magioli M, Ribeiro MC, Ferraz KMPM, Rodrigues MG (2015) Thresholds in the relationship between functional diversity and patch size for mammals in the Brazilian Atlantic Forest. Anim Conser 18:499–511
Martin AE, Fahrig L (2012) Measuring and selecting scales of effect for landscape predictors in species–habitat models. Ecol Appl 22:2277–2292
Melo GL, Sponchiado J, Cáceres NC, Fahrig L (2017) Testing the habitat amount hypothesis for South American small mammals. Biol Conserv 209:304–314
Merckx T, Miranda MD, Pereira HM (2019) Habitat amount, not patch size and isolation, drives species richness of macro-moth communities in countryside landscapes. J Biogeogr 46:956–967
Michalski F, Peres CA (2007) Disturbance-mediated mammal persistence and abundance-area relationships in Amazonian Forest fragments. Conserv Biol 21:1626–1640
Miller-Rushing AJ, Primack RB, Devictor V, Corlett RT, Cumming GS, Loyola R, Maas B, Pejchar L (2019) How does habitat fragmentation affect biodiversity? A controversial question at the core of conservation biology. Biol Conser 232:271–273
MMA (2014) Lista Nacional Oficial de Espécies Ameaçadas de Extinção. http://pesquisa.in.gov.br/imprensa/jsp/visualiza/index.jsp?jornal=1&pagina=121&data=18/12/2014
Morante-Filho JC, Benchimol M, Faria D (2020) Landscape composition is the strongest determinant of bird occupancy patterns in tropical forest patches. Landsc Ecol. https://doi.org/10.1007/s10980-020-01121-6
Mortelliti A, Fagiani S, Battisti C, Capizzi D, Boitani L (2010) Independents effects of habitat loss, habitat fragmentation and structural connectivity on forest-dependent birds. Divers Distrib 16:941–951
Myers N, Mittermeier RA, Mittermeier CG, Da Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Murcia C (1995) Edge effects in fragmented forests: implications for conservation. TREE 10:58–62
Newbold T, Hudson LN, Arnell AP, Contu S, Palma A, Ferrier S, Hill SLL, Hoskins AJ, Lysenko I, Phillips HRP, Burton VJ, Chng CWT, Emerson S, Gao D, Pask-Hale G, Hutton J, Jung M, Sanchez-Ortiz K, Simmons BI, Whitmee S, Zhang H, Scharlemann JPW, Purvis A (2016) Has land use pushed terrestrial biodiversity beyond the planetary boundary? A global assessment. Science 353:288–291
Newbold T, Ingram DJ, Collen B, Newbold T, Mace GM, De Palma A , Díaz S, Echeverria-Londoño S, Edgar MJ, Feldman A, Garon M, Harrison MLK, Alhusseini T, Ingram DJ, Itescu Y, Kattge J, Kemp V, Kirkpatrick L, Kleyer M, Correia DLP, Martin CD, Meiri S, Novosolov M, Pan Y, Phillips HRP, Purves DW, Robinson A, Simpson J, Tuck SL, Weiher E, White HJ, Ewers RM, Mace GM, Scharlemann JPW, Purvis A (2015) Global effects of land use on local terrestrial biodiversity. Nature 520:45–50
Paglia AP, da Fonseca GA, Rylands AB, Herrmann G, Aguiar LM, Chiarello AG, Leite YLR, Costa LP, Siciliano S, Kierulff MCM, Mendes SL, Tavares VC, Mittermeier RA, Patton JL (2012) Lista anotada dos mamíferos do Brasil/Annotated checklist of Brazilian mammals (2nd ed.). Arlington, Occasional Papers in Conservation Biology, 6
Palmeirim AF, Figueiredo MSL, Grelle CEV, Carbone C, Vieira MV (2019) When does habitat fragmentation matter? A biome-wide analysis of small mammals in the Atlantic Forest. J Biogeogr 46:2811–2825
Pfeifer M, Lefebvre V, Peres CA, Banks-Leite C, Wearn OR, Marsh CJ, Butchart SHM, Arroyo-Rodríguez V, Barlow J, Cerezo A, Cisneros L, D'Cruze N, Faria D, Hadley A, Harris SM, Klingbeil BT, Kormann U, Lens L, Medina-Rangel GF, Morante-Filho JC, Olivier P, Peters SL, Pidgeon A, Ribeiro DB, Scherber C, Schneider-Maunoury L, Struebig M, Urbina-Cardona N, Watling JI, Willing MR, Wood EM, Ewers RM (2017) Creation of forest edges has a global impact on forest vertebrates. Nature 551:187–191
Projeto MapBiomas (2019) MapBiomas Project, Collection [4] of the Annual Land Use Land Cover Maps of Brazil. http://mapbiomas.org
QGIS Development Team (2019) Quantum GIS Geographic Information System. Open Source Geospatial Foundation Project. https://qgis.org/en/site/
Regolin AL, Cherem JJ, Graipel ME, Bogoni JA, Ribeiro JW, Vancine MH, Tortato MA, Oliveira-Santos LG, Fantacini FM, Luiz MR, De Castilho PV, Ribeiro MC, Cáceres NC (2017) Forest cover influences occurrence of mammalian carnivores within Brazilian Atlantic Forest. J Mammal 98:1721–1731
Regolin AL, Ribeiro MC, Martello F, Melo GL, Sponchiado J, Campanha LFC, Sugai LSM, Silva TSF, Cáceres NC (2020) Spatial heterogeneity and habitat configuration overcome habitat composition influences on alpha and beta mammal diversity. Biotropica. https://doi.org/10.1111/btp.12800
Reino L, Beja P, Araújo MB, Dray S, Segurado P (2013) Does local habitat fragmentation affect large-scale distributions? The case of a specialist grassland bird. Divers Distrib 19:423–432
Rezende CL, Scarano FR, Assad ED, Joly CA, Metzger JP, Strassburg BBN, Tabarelli M, Fonseca GA, Mittermeier RA (2018) From hotspot to hopespot : An opportunity for the Brazilian Atlantic. PECON 16:208–214
Ribeiro MC, Metzger JP, Martensen AC, Ponzoni FJ, Hirota MM (2009) The Brazilian Atlantic Forest: how much is left, and how is the remaining forest distributed? Implications for conservation. Biol Conserv 142:1141–1153
Ries L, Sisk TD (2004) A predictive model of edge effects. Ecology 85:2917–2926
Rueda M, Hawkins BA, Morales-Castilla I, Vidanes RM, Ferrero M, Rodríguez MÁ (2013) Does fragmentation increase extinction thresholds ? A European-wide test with seven forest birds. Glob Ecol Biogeogr 22:1282–1292
Seibold S, Bässler C, Brandl R, Fahrig L, Heurich M, Hothorn T, Scheipl F, Thorn S, Müller J (2017) An experimental test of the habitat-amount hypothesis for saproxylic beetles in a forested region. Ecology 98:1613–1622
Souza Y, Gonçalves F, Lautenschlager L, Akkawi P, Mendes C, Carvalho MM, Bovendorp RS, Fernandes-Ferreira H, Rosa C, Graipel ME, Peroni N, Cherem JJ, Bogoni JA, Brocardo CR, Miranda J, Silva LZ, Melo G, Cáceres N, Sponchiado J, Ribeiro MC, Galetti M (2019) ATLANTIC MAMMALS: a data set of assemblages of medium- and large-sized mammals of the Atlantic Forest of South America. Ecology 100:e02785
Venables WN, Ripley BD (2002) Modern Applied Statistics with S. (4nd ed.) ISBN 0-387-95457-0. Springer, New York.
Vieira MV, Almeida-Gomes M, Delciellos AC, Cerqueira R, Crouzeilles R (2018) Fair tests of the habitat amount hypothesis require appropriate metrics of patch isolation: An example with small mammals in the Brazilian Atlantic Forest. Biol Conser 226:264–270
Watling JI, Arroyo-Rogríguez V, Pfeifer M, Baeten L, Banks-Leite C, Cisneros LM, Fang R, Hamel-Leigue AC, Lachat T, Leal IR, Lens L, Possingham HP, Raheem DC, Ribeiro DB, Slade EM, Urbina-Cardona JN, Wood EM, Fahrig L (2020) Support for the habitat amount hypothesis from a global synthesis of species density studies. Ecol Lett 23:674–681
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GH (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
We thank the Centre for Research and Conservation, Royal Zoological Society of Antwerp for the doctoral scholarship, the Universidade Estadual de Santa Cruz—(PROPP, 00220.1100.1840), The Rufford Foundation (24655-1) and Idea Wild for funding. E.C. thanks the Conselho Nacional de Desenvolvimento Científico for the productivity fellowship (306373/2018-1). We thank the anonymous reviewer and Victor Arroyo-Rodríguez for their valuable comments on an earlier version of the ms.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rios, E., Benchimol, M., Dodonov, P. et al. Testing the habitat amount hypothesis and fragmentation effects for medium- and large-sized mammals in a biodiversity hotspot. Landscape Ecol 36, 1311–1323 (2021). https://doi.org/10.1007/s10980-021-01231-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-021-01231-9