Abstract
The thermal decomposition kinetics of entacapone (ENT) have been investigated via thermogravimetric analysis under non-isothermal and isothermal conditions which provide useful stability information for their processing in the pharmaceutical industry and also for predicting shelf life and suitable storage conditions. The determination of the kinetic parameters for the decomposition process under non-isothermal conditions in a nitrogen atmosphere at four heating rates (5, 10, 15, and 20 °C min−1) was performed. Kinetic parameters of the decomposition process for ENT were calculated through Friedman, Flynn–Wall–Ozawa, Kissinger–Akahira–Sunose, and Li–Tang methods. This work demonstrates that the activation energies calculated from the decomposition reactions by different methods are consistent with each other. Moreover, the thermodynamic functions of the decomposition reaction were also calculated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
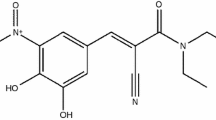
Entacapone (ENT) (see Fig. 1) is an adjunct to standard preparations of levodopa/benserazide or levodopa/carbidopa for use in patients with Parkinson’s disease who have end-of-dose motor fluctuations which cannot be stabilized on those combinations [1]. ENT (Fig. 1) is a specific and mainly peripherally acting catechol-O-methyl transferase (COMT) inhibitor which decreases the metabolic loss of levodopa to 3-O-methyldopa, and it is reported that ENT increases the bio-availability of levodopa to the brain by 5–10% [2]. ENT is practically insoluble in water, but slightly soluble in organic solvents. The aqueous solubility of ENT is very low at acidic pH but increases strongly with increasing pH; it is also slightly soluble in organic solvents [3].
Various analytical methods have been used for ENT determination such as spectrophotometric [4, 5], HPLC [6,7,8,9], micellar capillary chromatography [10, 11], and electrochemical methods [12,13,14,15]. While mainly used for the analytical determination of target analytes, thermal analytical techniques can provide important data regarding the storage and stability of drugs [16,17,18]. The most widely used thermal analysis techniques are thermogravimetry/derivative thermogravimetry (TG/DTG), differential thermal analysis (DTA), and differential scanning calorimetry (DSC) [18,19,20]. These techniques are widely used in the pharmaceutical sciences for the characterization of solid drugs and excipients. The application of thermo-analytical methods may provide new information about the temperature and energy associated with events, such as melting, oxidation and reduction reactions, glass transition, boiling, sublimation, decomposition, crystallization, or gel to liquid crystal transitions [21,22,23]. TG can be considered as a quantitative and comparative analytical technique which can generate fast and reproducible data which leads to the utilization of TG in the quality control of drugs to enhance the final products and for the determination of drug quality via technical parameters [24]. The identification of pharmaceutical and organic compounds can also be performed by differential thermal analysis (DTA) [25].
TG and DTA techniques involve the continuous measurement of physical properties such as mass, volume, heat capacity, as the sample temperature is increased at a predetermined rate; it is possible to calculate the kinetic constants from these techniques by making a number of patterns at different heating rates. TG and DTA are used in the pharmaceutical industry as an analytic tool of high importance, to identify and to test the purity of the active substances with quick and efficient results [26]. Also, DTA can be used to control the quality of the raw materials used to obtain pharmaceutical products [27].
The recommendations of the International Confederation for Thermal Analysis and Calorimetry (ICTAC) offer guidance for the reliable evaluation of kinetic parameters from data obtained by thermogravimetry, differential scanning calorimetry, and differential thermal analysis [28]. The measurement of kinetics and the associated Arrhenius parameters is the essential aspect of the characterization of drug and related compounds [29]. The thermal decomposition of drugs allows the prediction of the degradation rates at marketing temperatures from data collected on accelerated processes that are studied at elevated temperatures. The temperature may increase the chemical reactions, providing sufficient energy (activation energy) required to break chemical bonds and start the decomposition process [30, 31]. In this work, the thermal behavior of ENT is investigated under non-isothermal and isothermal conditions allowing kinetic information to be readily deduced. From isothermal experiments, the activation energy (E) can be obtained from slope of ln t versus 1/T at a constant conversion level. According to the ICTAC recommendations on isothermal analysis, isothermal kinetics data usually are easier to analyze and interpret; gaining worthy isothermal data typically consumes more time and effort than in case of non-isothermal runs [32].
The methods proposed for the kinetic study of thermal decomposition are generally classified as model-fitting and model-free methods. In each case, data from isothermal and/or non-isothermal experiments can be used [27, 33]. The kinetic analysis based on an isoconversional method is frequently referred to as “model free” because it is possible to obtain the apparent activation energy (E) as a function of the conversion degree (α) which has a specific interest when the thermal decomposition occurs in more than one step [27].
In this paper, we report the study of the thermal behavior of ENT via TGA under non-isothermal and isothermal conditions. Kinetic parameters are deduced which are of importance to the pharmaceutical industry. The thermo-analytical techniques cannot replace the classical stability studies that usually require weeks or months, but it can provide an early idea to direct the process toward the most successful formulation [34]. Furthermore, to the best of our knowledge, there is no report on the thermal behavior and decomposition kinetic of this target drug, ENT.
Experimental
ENT was kindly supplied from Novartis Pharmaceuticals, Egypt. Thermogravimetry and differential thermal analysis were carried out using simultaneous Shimadzu Thermogravimetric Analyzer TGA-60 H with TA 60 software in a dry nitrogen atmosphere at a flow rate of 30 mL min−1 in a platinum crucible. The experiments were performed from room temperature up to 800 °C at different heating rates (5, 10, 15, and 20 °C min−1). The sample mass was about 5 mg of the drug without any further treatment. In the isothermal condition, the temperatures were 160, 170, 180, 190, and 200 °C with 10 °C temperature increments, under dynamic nitrogen atmosphere with the flow rate of 30 mL min−1. These values were chosen since according to ICTAC recommendation to isothermal analysis, when selecting the temperature range for kinetic experiments, one should be mindful of possible phase transitions that a reactant may undergo within that range. A solid compound can melt. Consequently, we chose temperatures around the melting point as ENT melting point is 162°–163°. The respective rates and Arrhenius parameters for solid and liquid state decomposition can differ significantly. The isothermal holding was monitored based on the time to a mass loss of 5.0% decomposition. The instrument was calibrated at each heating rate considered using a dedicated aluminum oxide standard in a platinum crucible.
Results and discussions
Thermal behavior of ENT
The thermo-analytical graphs of ENT are presented in Fig. 2 which show that ENT decomposes during three steps. The first step shows a mass loss (Δm = 23.4%) in the interval of 185–250 °C, suggesting the release of a diethyl amine molecule (23.6%, calc.). The second decomposition step shows a mass loss (Δm = 14.9%) in the temperature range 251–400 °C, suggesting the release of a nitrite molecule (15.1%, calc.). The third decomposition step shows a mass loss (Δm = 61.6%) in the temperature range 401–800 °C, suggesting complete decomposition of ENT. The DTA curve shows key thermal event when this temperature range is applied. The endothermic peak observed at 163 °C [3] is likely due to the melting of the compound_ENREF_25, while the exothermic peak at 230 °C is attributed to the first decomposition process corresponding to the first mass loss observed in TG/DTG curves as shown in Fig. 2. The sharp exothermic peak at 600 °C is due to the pyrolysis of the compound. The suggested pathway of thermal decomposition of ENT is depicted within Scheme 1.
Kinetic analysis
The kinetic parameters were determined from the TG/DTG curves using the following kinetic methods: Friedman (Fd) [35, 36], Flynn–Wall–Ozawa (FWO) [37,38,39], Kissinger–Akahira–Sunose (KAS) [40, 41], and Li–Tang (LT) [42]. Figure 3 depicts the α–T curves for the non-isothermal decomposition of ENT at different heating rates. The model-free methods (e.g., isoconversional, KAS, and Friedman) allow one to evaluate the activation energy without determining the reaction model [43]. These methods yield the effective activation energy as a function of the extent of conversion allowing the reaction kinetics over a wide temperature region to be predicted [43, 44]. The isoconversional methods give comparable (but not identical) dependences of E on the extent of conversion for isothermal and non-isothermal experiments [45].
Generally, the kinetics of many reactions (e.g., decomposition, crystallization, polymerization) can be described by the following rate equation [46, 47]:
where t is time, α is the extent of conversion, k(T) is the Arrhenius rate constant, A and E are the Arrhenius parameters (pre-exponential factor and activation energy, respectively), R is the gas constant, and f (α) is the reaction model associated with a certain reaction mechanism. In the case of non-isothermal conditions, dα/dt is replaced with βdα/dT, where β is the heating rate, giving:
Taking the logarithmic form of Eq. (2), the isoconversional Friedman method is based on the following equation:
In order to evaluate the activation energy more precisely, the term ln (βdα/dT) was obtained by numeric derivation of the curve α versus T with respect to T and by subsequently taking logarithms. In the case of α = constant, and using various heating rates, the plot ln (βdα/dT) versus (1/T) is linear, as shown in Fig. 4). The values of the activation energy as obtained from the slopes of the straight lines are listed in Table 1. The general equation of the reaction rate for non-isothermal conditions at constant heating rate is generally written as:
where g(α) is the conversion integral, and p(x) is the temperature integral, x = E/(R·T). The dependence on α is defined by the reaction model, f(α), which can take a variety of mathematical forms [32, 48]. Experimentally, the measured rate is adequate to the actual process kinetics only when the process variables [α, T, and p(x)] are controlled accurately and precisely [32].
Many approximations of the temperature integral p(x) have been suggested in the literature, and as a consequence, it can be approximately represented via different empirical interpolation formulas in terms of Doyle, Agrawal, Gorbatchev, and Frank–Kameneskii approximation [49]. All these methods involve the plot of a logarithmic function (which depends on the approximation for the temperature integral used) versus 1/T α:
where k is a function describing the temperature dependence of the reaction rate, B and C are constants, and the subscript α designates values related to a given extent of conversion. The literature has considered four linear integral isoconversional methods as the most accurate and promised methods which were used in this article. Equation (5) has been derived assuming a constant activation energy [38]. This assumption obviously introduces some systematic error in estimating E α, if the latter varies with α. This error does not appear in the differential isoconversional method of Friedman. For this reason, one can estimate the systematic error of an integral isoconversional method by comparing it against the Friedman method [50].
Flynn–Wall–Ozawa (FWO) method
The FWO method is based on Doyle’s approximation for the temperature integral, \(p\left( x \right) = \exp \left( { - 1.052 \cdot x - 5.331} \right)\). For this method, k = 0 and for constant conversion a, the general linear Eq. (5) becomes:
Utilizing the FWO method, the activation energies were calculated from the slope of the linear fitted function of ln β versus 1/T (as shown in Fig. 5a). The values of the activation energy (E α) are included in Table 1. The order of reaction was determined by Ozawa’s plots in which slope of log heating rate versus 1/T was found to be first order.
Kissinger–Akahira–Sunose (KAS) method
This method sometimes called the generalized Kissinger method is one of the best isoconversional methods [51], and it is based on the equation:
This method utilizes the adequate temperatures (T α) to certain values of the conversion α for experiments effectuated to different rates of heating, β. From the slopes of the straight lines obtained by the graphic representation of the ln β/T 2α versus (1/T α), the activation energy was determined (Fig. 5b). The values of activation energy calculated by means of the integral methods are listed in Table 1.
Li–Tang (LT) method
The approximation proposed by LT for the temperature integral is: −ln p(x) = 0.37774 + 1.89466 ln x − 1.00145x. For this method (k = 1.89466) and at constant conversion α, the general linear Eq. (4) becomes:
For α = constant, the values of E (Table 1) were determined from the slope of the linear fitted function of \(\ln \frac{\beta }{{T_{\upalpha}^{1.894661} }}\) versus 1/T α, (see Fig. 5c).
The values of the activation energy (E) obtained by the four methods are in good agreement and the weak variation of E versus α. The values of E show a considerable thermal stability of the ENT-active substance. According to Galwey [52], the numbers of reactions for which the reported E values occurred within steps of equal increment (30.0 kJ mol−1) through distribution of magnitudes of activation energies graph, a broad flat maximum, for which 65% were between 100 < E < 230 kJ mol−1. No particularly preferred magnitude of E can be identified. The overall mean magnitude of E was 175 kJ mol−1. There was a small proportion (8%) of relatively large E values, >300 kJ mol−1.
The change in entropy (ΔS), enthalpy (ΔH), and free energy (ΔG) were calculated using the following relations [53, 54]:
where h is the Planck constant, K is the Boltzmann constant, T is the temperature, and A is the Arrhenius constant. The calculated kinetic parameters for ENT are also included in Table 2. A comparison between the results obtained by applying different kinetic methods reveals that the values of activation energies calculated for ENT are very close to each other. The obtained kinetic parameters were used to evaluate the thermodynamic parameters of activation. The entropy values (ΔS) for the ENT decomposition are negative. In terms of the activated complex theory (transition theory) [55, 56], a negative value of ΔS indicates a highly ordered activated complex. The result may be interpreted as a “slow” stage. The positive values of ΔH and ΔG for the decomposition show that it is connected with the introduction of heat and it is a non-spontaneous process.
It is known that the thermal decomposition of drugs is a complex process which tends to take place in many steps with different heating rates [26]. Through this complex process, simultaneously competitive and consecutive reactions (parallel) could occur. For ENT-active substance, the competitive reactions can be excluded because the total mass lost for the four heating rates is the same. It is difficult to specify the nature of the decomposition products because of a possible process of condensation between reacted and non-reacted molecules of the ENT, followed by their decomposition process [26].
The isothermal TG curves superimposed of ENT are illustrated in Fig. 6 and were recorded at 160, 170, 180, 190, and 200 °C. These curves show mass loss rate dependence in temperature function of isothermal; the higher the temperature lower will be the necessary time so that occur the same mass loss. The curves were used to obtain the graphic of lnt versus 1/T (K−1) at a constant conversion level 5.0% [19, 57]. From this linear regression method, the equation for the line is y = –13,800·3x + 25.47 and R = 0.9993 are obtained. The value of the activation energy can be calculated from the product of the slope with the molar gas constant (R = 8.314). The calculated activation energy was found to be 114.73 kJ mol−1. This result is in agreement with the values obtained from the dynamic methods, and this is an important experimental finding, Fig. 6.
Calculation of rate constant
The values of rate constants (k) for thermal decomposition of ENT were calculated at the room temperature of 25 °C using the following equation and the previously mentioned values for activation energies (E) and Arrhenius factors (A) [58]:
The calculated k values are listed in Table 2. By using the calculated values of k, the half-life of ENT at 25 °C was estimated and found to be 4.73 years and deviation from this temperature; say as a consequence of storing ENT at higher temperatures will result in reducing its half-life progressively. ENT is a more heat-sensitive drug compared with other drugs [59], which require more care during storage.
Conclusions
We have explored the thermal decomposition of ENT via TGA allowing thermal decomposition kinetics to be readily deduced. The thermal decomposition mechanism of ENT is deduced to proceed through three key steps (see Scheme 1). The half-life of ENT (at 25 °C) was deduced to be 4.73 years indicating that it is largely unaffected by heat in comparison with other drugs—this information is valuable to the pharmaceutical industry.
References
Männistö P, Ulmanen I, Lundström K, Taskinen J, Tenhunen J, Tilgmann C, et al. Characteristics of catechol O-methyltransferase (COMT) and properties of selective COMT inhibitors. Progress in drug research/Fortschritte der Arzneimittelforschung/Progrès des recherches pharmaceutiques. Berlin: Springer; 1992. p. 291–350.
Mannisto P. Catechol O-methyltransferase: characterization of the protein, its gene, and the preclinical pharmacology of COMT inhibitors. Adv Pharmacol. 1998;42:324–8.
Savolainen J, Leppanen J, Forsberg M, Taipale H, Nevalainen T, Huuskonen J, et al. Synthesis and in vitro/in vivo evaluation of novel oral N-alkyl-and N,N-dialkyl-carbamate esters of entacapone. Life Sci. 2000;67(2):205–16.
Rajeswari K, Sankar G, Rao AL, Rao J. A new spectrophotometric method for the determination of entacapone in pure and tablet dosage form. Int J Chem Sci. 2006;4(3):694–6.
Paim C, Gonçalves H, Lange A, Miron D, Steppe M. Validation of UV spectrophotometric method for quantitative determination of entacapone in tablets using experimental design of Plackett–Burman for robustness evaluation and comparison with HPLC. Anal Lett. 2008;41(4):571–81.
Paim C, Gonçalves H, Miron D, Sippel J, Steppe M. Stability-indication LC determination of entacapone in tablets. Chromatographia. 2007;65(9–10):595–9.
Karlsson M, Wikberg T. Liquid chromatographic determination of a new catechol-O-methyltransferase inhibitor, entacapone, and its Z-isomer in human plasma and urine. J Pharm Biomed Anal. 1992;10(8):593–600.
Keski-Hynnilä H, Raanaa K, Forsberg M, Männistö P, Taskinen J, Kostiainen R. Quantitation of entacapone glucuronide in rat plasma by on-line coupled restricted access media column and liquid chromatography–tandem mass spectrometry. J Chromatogr B Biomed Sci Appl. 2001;759(2):227–36.
Ramakrishna N, Vishwottam K, Wishu S, Koteshwara M, Chidambara J. High-performance liquid chromatography method for the quantification of entacapone in human plasma. J Chromatogr B. 2005;823(2):189–94.
Lehtonen P, Mälkki-Laine L, Wikberg T. Separation of the glucuronides of entacapone and its (Z)-isomer in urine by micellar electrokinetic capillary chromatography. J Chromatogr B Biomed Sci Appl. 1999;721(1):127–34.
Keski-Hynnilä H, Raanaa K, Taskinen J, Kostiainen R. Direct analysis of nitrocatechol-type glucuronides in urine by capillary electrophoresis–electrospray ionisation mass spectrometry and tandem mass spectrometry. J Chromatogr B Biomed Sci Appl. 2000;749(2):253–63.
Abasq ML, Courtel P, Burgot G. Determination of entacapone by differential pulse polarography in pharmaceutical formulation. Anal Lett. 2008;41(1):56–65.
Jain R, Yadav RK, Dwivedi A. Square-wave adsorptive stripping voltammetric behaviour of entacapone at HMDE and its determination in the presence of surfactants. Colloids Surf A. 2010;359(1):25–30.
Rizk M, Attia AK, Elshahed MS, Farag AS. Validated voltammetric method for the determination of antiparkinsonism drug entacapone in bulk, pharmaceutical formulation and human plasma. J Electroanal Chem. 2015;743:112–9.
Salama NN, Azab SM, Mohamed MA, Fekry AM. A novel methionine/palladium nanoparticle modified carbon paste electrode for simultaneous determination of three antiparkinson drugs. RSC Adv. 2015;5(19):14187–95.
Gaisford S, Buanz AB. Pharmaceutical physical form characterisation with fast (>200 C min−1) DSC heating rates. J Therm Anal Calorim. 2011;106(1):221–6.
Neto HS, Novák C, Matos J. Thermal analysis and compatibility studies of prednicarbate with excipients used in semi solid pharmaceutical form. J Therm Anal Calorim. 2009;97(1):367–74.
Salama NN, Mohammad MA, Fattah TA. Thermal behavior study and decomposition kinetics of amisulpride under non-isothermal and isothermal conditions. J Therm Anal Calorim. 2015;120(1):953–8.
Mohamed MA, Attia AK. Thermal behavior and decomposition kinetics of cinnarizine under isothermal and non-isothermal conditions. J Therm Anal Calorim. 2017;127(2):1751–6.
Beyer H, Meini S, Tsiouvaras N, Piana M, Gasteiger H. Thermal and electrochemical decomposition of lithium peroxide in non-catalyzed carbon cathodes for Li-air batteries. Phys Chem Chem Phys. 2013;15(26):11025–37.
Brown ME, Glass BD. Decomposition of solids accompanied by melting—Bawn kinetics. Int J Pharm. 2003;254(2):255–61.
Rodomonte A, Antoniella E, Bertocchi P, Gaudiano MC, Manna L, Bartolomei M. Different crystal morphologies arising from different preparation methods of a same polymorphic form may result in different properties of the final materials: the case of diclofenac sodium trihydrate. J Pharm Biomed Anal. 2008;48(2):477–81.
Salvio Neto H, Matos JDR. Compatibility and decomposition kinetics studies of prednicarbate alone and associated with glyceryl stearate. J Therm Anal Calorim. 2011;103(1):393–9.
Macêdo R, Gomes do Nascimento T, Soares Aragăo C, Barreto Gomes A. Application of thermal analysis in the characterization of anti-hypertensive drugs. J Therm Anal Calorim. 2000;59(3):657–61.
O’Neil MJ. The Merck index: an encyclopedia of chemicals, drugs, and biologicals. Whitehouse Station, NJ: Merck; 2006.
Marian E, Tiţa B, Jurca T, Fuliaş A, Vicaş L, Tiţa D. Thermal behaviour of erythromycin-active substance and tablets. J Therm Anal Calorim. 2013;111(2):1025–31.
Tiţa D, Fuliaş A, Tiţa B. Thermal stability of ketoprofen. J Therm Anal Calorim. 2013;111(3):1979–85.
Dickinson C, Heal G. A review of the ICTAC Kinetics Project, 2000: Part 1. Isothermal results. Thermochim Acta. 2009;494(1):1–14.
Venkatesh M, Ravi P, Tewari SP. Isoconversional kinetic analysis of decomposition of nitroimidazoles: Friedman method versus Flynn–Wall–Ozawa method. J Phys Chem A. 2013;117(40):10162–9.
Burnham L, Dollimore D, Alexander KS. Kinetic study of the drug acetazolamide using thermogravimetry. Thermochim Acta. 2002;392:127–33.
Felix FS, da Silva LC, Angnes L, Matos J. Thermal behavior study and decomposition kinetics of salbutamol under isothermal and non-isothermal conditions. J Therm Anal Calorim. 2009;95(3):877–80.
Vyazovkin S, Chrissafis K, Di Lorenzo ML, Koga N, Pijolat M, Roduit B, et al. ICTAC Kinetics Committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim Acta. 2014;590:1–23.
Cai J, Liu R. On evaluate of the integral methods for the determination of the activation energy. J Therm Anal Calorim. 2009;96(1):331–3.
Chaves LL, Rolim LA, Gonçalves ML, Vieira AC, Alves LD, Soares MF, et al. Study of stability and drug-excipient compatibility of diethylcarbamazine citrate. J Therm Anal Calorim. 2013;111(3):2179–86.
Friedman HL. New methods for evaluating kinetic parameters from thermal analysis data. J Polym Sci, Part C: Polym Lett. 1969;7(1):41–6.
Friedman HL, editor. Kinetics of thermal degradation of char‐forming plastics from thermogravimetry. Application to a phenolic plastic. In: Journal of Polymer Science Part C: Polymer Symposia, Wiley Online Library; 1964.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38(11):1881–6.
Flynn JH, Wall LA. General treatment of the thermogravimetry of polymers. J Res Natl Bur Stand. 1966;70(6):487–523.
Flynn JH, Wall LA. A quick, direct method for the determination of activation energy from thermogravimetric data. J Polym Sci, Part C: Polym Lett. 1966;4(5):323–8.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29(11):1702–6.
Akahira T, Sunose T. Method of determining activation deterioration constant of electrical insulating materials. Res Rep Chiba Inst Technol (Sci Technol). 1971;16:22–31.
Li C-R, Tang TB. A new method for analysing non-isothermal thermoanalytical data from solid-state reactions. Thermochim Acta. 1999;325(1):43–6.
Vyazovkin S. Computational aspects of kinetic analysis: Part C. The ICTAC Kinetics Project—the light at the end of the tunnel? Thermochim Acta. 2000;355(1–2):155–63.
Vyazovkin S. Kinetic concepts of thermally stimulated reactions in solids: a view from a historical perspective. Int Rev Phys Chem. 2000;19(1):45–60.
Vyazovkin S, Wight CA. Isothermal and non-isothermal kinetics of thermally stimulated reactions of solids. Int Rev Phys Chem. 1998;17(3):407–33.
Brown ME, Dollimore D, Galwey AK. Reactions in the solid state (comprehensive chemical kinetics), Chap. 2, vol. 22. 1st ed. Amsterdam: Elsevier; 1980.
Brown M. Thermal decomposition of ionic solids, vol. 86. 1st ed. Amsterdam: Elsevier Science; 1999.
Ferreira B, Araujo B, Sebastião R, Yoshida M, Mussel W, Fialho S, et al. Kinetic study of anti-HIV drugs by thermal decomposition analysis. J Therm Anal Calorim. 2017;127(1):577–85.
Gai C, Dong Y, Zhang T. The kinetic analysis of the pyrolysis of agricultural residue under non-isothermal conditions. Biores Technol. 2013;127:298–305.
Vyazovkin S. Modification of the integral isoconversional method to account for variation in the activation energy. J Comput Chem. 2001;22(2):178–83.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520(1):1–19.
Galwey AK. Magnitudes of Arrhenius parameters for decomposition reactions of solids. Thermochim Acta. 1994;242:259–64.
Mahfouz RM, Al-Farhan KA, Hassen GY, Al-Wassil AI, Alshehri SM, Al-Wallan AA. Preparation and characterization of new In(III), Re(III), and Re(V) complexes with thenoyltrifluoroacetone and some bidentate heterocyclic ligands. Synth React Inorg Met-Org Chem. 2002;32(3):489–508.
Sekerci M, Yakuphanoglu F. Thermal analysis study of some transition metal complexes by TG and DSC methods. J Therm Anal Calorim. 2004;75(1):189–95.
Vlase T, Vlase G, Doca M, Doca N. Specificity of decomposition of solids in non-isothermal conditions. J Therm Anal Calorim. 2003;72(2):597–604.
Vlase T, Jurca G, Doca N. Non-isothermal kinetics by decomposition of some catalyst precursors. Thermochim Acta. 2001;379(1):65–9.
Fandaruff C, Araya-Sibaja A, Pereira R, Hoffmeister C, Rocha H, Silva M. Thermal behavior and decomposition kinetics of efavirenz under isothermal and non-isothermal conditions. J Therm Anal Calorim. 2014;115(3):2351–6.
Krabbendam-LaHaye E, De Klerk W, Krämer R. The kinetic behaviour and thermal stability of commercially available explosives. J Therm Anal Calorim. 2005;80(2):495–501.
Shamsipur M, Pourmortazavi SM, Beigi AAM, Heydari R, Khatibi M. Thermal stability and decomposition kinetic studies of acyclovir and zidovudine drug compounds. AAPS PharmSciTech. 2013;14(1):287–93.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohamed, M.A., Atty, S.A. & Banks, C.E. Thermal decomposition kinetics of the antiparkinson drug “entacapone” under isothermal and non-isothermal conditions. J Therm Anal Calorim 130, 2359–2367 (2017). https://doi.org/10.1007/s10973-017-6664-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6664-y