Abstract
The purpose of this investigation is to calculate the kinetic parameters and the kinetic model for the studied process. The results are further used to predict the system’s behaviour in various circumstances. A kinetic study regarding the ketoprofen—involving active substance’s thermal decomposition—was performed under isothermal conditions and in a nitrogen atmosphere, for the temperature steps: 260; 265; 270; 275; and 280 °C. The thermogravimetry/derivative thermogravimetry data were processed by three differential methods: isothermal–isoconversional, Friedman’s isothermal–isoconversional, and isothermal model-fittings. The obtained results are in good accordance with those obtained under non-isothermal conditions of a previous study, and confirm the necessity for the kinetic parameters to be determined, under different thermal conditions, by the adequate calculation methods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
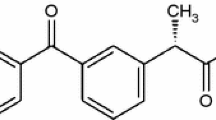
Ketoprofen 2-(3-benzoylphenyl)-propionic acid is one of the most potent non-steroidal anti-inflammatory drugs (NSAIDs), therapeutically used in inflammatory and painful diseases of rheumatic and non-rheumatic origin [1].
The formula of Ketoprofen (KT) is presented in Fig. 1.
The anti-inflammatory activity of NSAIDs and most of its other pharmacological effects are related to the inhibition of the conversion of arachidonic acid to prostaglandins, which are mediators of the inflammatory process. KT is a potent inhibitor of cyclo–oxygenase (COX) enzyme, in vitro and in vivo, thereby decreasing the synthesis of prostaglandins, prostacyclin, and thromboxane products [2, 3].
Two different COX isoforms have been characterized: Cox-1 and Cox-2. Inhibition of the Cox-2 enzyme system results in anti-inflammatory action, whilst inhibition of the Cox-1 enzyme system results in anti-inflammatory action, as well as gastric irritation. Consequently, research efforts have been directed towards evolving compounds which are specific Cox-2 inhibitors [4].
Recent studies have revealed that in addition to arthritis and pain, cancer and neuro-degenerative diseases like Alzheimer’s disease could potentially be treated with Cox-2 inhibitors [5].
Thermal analysis is one of the most frequently used instrumental techniques in the pharmaceutical research, for the thermal characterizations of different materials from solids to semi-solids, which are of pharmaceutical relevance. The term thermal characterization refers to the thermal stability and decomposition of the substances of pharmaceutical interest. The evaluation of the thermal stability of a drug is realized especially by analysing its decomposition in isothermal and non-isothermal conditions. Usually, this takes place by irreversible mass loss. The drugs' decomposition reactions have a theoretical, as well as a practical signification [6–9].
The thermoanalytical techniques, especially thermogravimetry/derivative thermogravimetry (TG/DTG) and differential scanning calorimetry (DSC) or differential thermal analysis (DTA), are used in solving some pharmaceutical problems, e.g. the determination of purity level, the qualitative and quantitative analysis of drug formulations, the polymorphism, the thermal stability with the determination of the corresponding kinetic parameters, etc. [10–14].
Solid-state kinetic studies have assumed increasing importance in thermal analysis, main purposes of which are to calculate the parameters of Arrhenius equation and to determine the mechanism of decomposition reaction. These data can provide valuable information about the time and conditions of storage. The knowledge of such parameters for pure drugs and for drug–excipient mixtures is also meaningful to elucidate their miscibility/incompatibility and their effects on thermal stability [15–20].
In our previous articles [21–28], we have provided the importance and utility of the kinetic analysis in estimation on the thermal behaviour of different pharmaceuticals.
TG is a useful, convenient method for the study of a variety of decomposition processes for pharmaceuticals. Often kinetic data are extracted from a plot of mass loss versus time or temperature. Reliable information may be obtained for well-defined single processes in which the evolution of a well-characterized gaseous product or products reflects the rate of decomposition [9, 10].
In many cases, the decomposition does not correspond to a single, well-defined event. Often many processes occur simultaneously. In these cases, the “kinetic data” obtained are characteristic of no fundamental process and are of limited value [15, 29]. The most appropriate way to obtain reliable kinetic data is to monitor mass loss versus time to obtain rate constants at several temperatures [30–32]. Several repetitions at each temperature provide a good reflection of the uncertainty in the values for the rate constants obtained. This mode of operation corresponds to the theoretical principles of the isothermal methods [15, 31, 32].
The methods proposed for the kinetic study of thermal decomposition are generally classified as model-fitting and model-free methods. In each case, data from isothermal and/or non-isothermal experiments can be used [33–35]. The model-fitting defines a single-reaction step, whereas the model-free approach represented by isothermal–isoconversional methods gave the dependence of the activation energy with the extent of conversion [15–20]. The model-free isoconversional methods are considered as also being the most trusted, especially the Friedman (Fd) method [36], because of their theoretical and experimental advantages.
The kinetic parameters, the rate constant (k), the activation energy (E), the pre-exponential factor (A) and the reaction order (n) may be calculated using different methods according to the mentioned classification, under isothermal and/or non-isothermal conditions [15, 21–31].
In a previous study, a kinetic study of decomposition of KT was realized under non-isothermal conditions [26].
The purpose of the present article is to determine kinetic parameters for thermal decomposition of KT under isothermal conditions and to establish the nature of the decomposition process (single or multi-step) on the base of the obtained values (especially n and E). Also, the values of the kinetic parameters are compared to the adequate values determined under non-isothermal conditions.
Experimental
Materials
The KT is available as pure compound, able to be used for medical purpose. It was obtained from S.I.M.S., Italy, lot: 138315.
Methods
Thermal decomposition was carried out using the Netzsch-STA 449 TG/DTA instrument. The isothermal TG curves were measured at 260; 265; 270; 275; and 280 °C with a heating rate of 10 °C min−1 until isothermal temperature, under a dynamic atmosphere of nitrogen with 99.999% purity (Linde) at a flow of 20 mL min−1 and utilizing platinum crucibles with ≈20 mg of samples. The curves were recorded by stepwise isothermal heating. The stepwise isothermal approach consists of heating at a constant rate, until a mass change begins, and then holding isothermally until the mass change is complete. This approach has significant advantages over the other techniques as the onset and complete reactions can be very carefully controlled.
Results and discussion
Experimental data processing strategy
It is well known that solid compounds submitted to heating treatment undergo single- or multi-step thermal decomposition processes in relation to the complexity of their structures.
Kinetic analysis of decomposition process is traditionally expected to produce an adequate kinetic description of the process in terms of the reaction model and the Arrhenius parameters using a single-step kinetic equation:
where t is the time, T is the temperature, α is the conversion degree and f(α) is the reaction model. The temperature dependence of the rate constant is introduced by replacing k(T) with Arrhenius equation, which gives as follows:
where E (the activation energy) and A (the pre-exponential factor) are the Arrhenius parameters, and R is the gas constant. For non-isothermal conditions, d/dt in Eq. 2 is replaced with β dα/dT where β is the heating rate giving
where the three components (f(α), E and A) called “kinetic triplet” define both in 2 and 3 a single-step reaction that disagrees with the multi-step nature of decomposition that usually occurs in solid state.
For compounds having complex structures, it can be hypothesized that several steps with different energies will be involved.
If a process involves several steps with different activation energies, the relative contributions of these steps to the overall reaction rate will vary with both the temperature and the extent of conversion. This means that the effective activation energy, determined from the analysis of the results, will also be a function of these two variables. The use of Eqs. 2 and 3 determines reactions model that does not represent multi-step kinetics.
For the above reason, one cannot justify the establishment of the reaction mechanism from f(α) alone.
An alternative approach to kinetic analysis is to use model-free methods that allow for evaluating Arrhenius parameters without choosing the reaction model. The isoconversional methods make up the best representation of the model-free approach.
These methods yield the variation of the effective activation energy as a function of the extent of conversion.
The knowledge of the dependence E on α allows detecting multi-step processes and predicting some mechanistic conclusions on the reaction kinetics over a wide temperature range.
The isoconversional methods could also yield similar dependencies of the activation energy on the extent of conversion for isothermal and non-isothermal experiments.
Kinetic analysis
The thermal curves of this substance obtained under non-isothermal conditions in a dynamic nitrogen atmosphere and the samples of ≈20 mg are presented in Fig. 2.
The DTA curve of active substance (Fig. 2) shows an endothermic sharp event between 89.4 and 128.6 °C indicating the melting (T peak = 96.8 °C), which corresponds to the values indicated in the literature (94–97 °C). In this temperature range, the TG/DTG curves did not show mass loss.
The second broad event, observed in DTA curve, was also an endothermic one which corresponds to the thermal decomposition process. This is confirmed by TG/DTG curves that indicate thermal decomposition in the following temperature range and mass loss: 235–400 °C (Δm = 86%). For the decomposition process, the thermal curves indicate the following temperatures of the peaks: T peak DTG = 361.4 °C; and T peak DTA = 365.0 °C. The DTA data combined with TG allow evidencing a thermal stability of KT active substance up to 235 °C.
According to the mentioned temperature range, the following temperatures were chosen: 260; 265; 270; 275; and 280 °C for the experiments which were effectuated in isothermal conditions.
The kinetic parameters were determined from TG curves, using the methods: isothermal–isoconversional, Fds isothermal–isoconversional and isothermal model-fitting.
Isothermal–isoconversional method
The isothermal–isoconversional method was used to verify that activation energy value E related to decomposition process remains constant, and a single mechanism occurs in the experimental temperature range.
It is well known that isothermal kinetics of solid-state reactions can be represented by the equation:
where k is the specific constant rate and g(α) is an integral mathematical expression related to a mechanism of solid phase reactions.
From the isothermal TG curves, a set of temperature T and t values were obtained for fixed values of α. Substituting k = A·exp(−E/RT) in Eq. 4 one obtains
where the obtained t and T are the time and temperature values, respectively, which yield constant of the function g(α). By means of the logarithmic form of Eq. 5, it can be written as
and rearranging the above, one obtains
By plotting lnt versus 1/T according to Eq. 7, the activation energies were found at any given α value from the slope of a regression straight line (Fig. 3). The activation energy values are presented in Table 1. The variation range of E with α does not reveal a multistage process.
Friedman isothermal–isoconversional method
This method is based on the relation:
and for f(α) = (1 − α)n, at a constant conversion and with temperature dependence according to Arrhenius equation, the reaction rate is
By plotting the left member versus 1/T, the activation energy could be obtained at different conversion degrees (Table 1), and from the weak variation of E with α, it was shown that the process takes place in a single decomposition stage.
The activation’s energy values determined by the two isoconversional methods are concordant.
Isothermal model-fitting method
This method relies on the choice of the kinetic equation which describes the reaction’s mechanism, which is done after the verification of several possible kinetic equations, taking into account that, in isothermal conditions, where the rate constant is independent of the reaction time, the graphical representation of g(α) versus time gives a straight line for the correct chosen form of g(α).
According to the squared correlation coefficient values r 2 (Table 2), which were calculated from plottings for various possible kinetic functions, it was revealed that the decomposition process is most likely at n = 0.5 order kinetic (r 2 = 0.983). The squared correlation coefficient was calculated according to the experimental values from Fig. 4.
For decomposition processes, following first-order reaction, g(α) = −ln(1 − α); for n ≠ 1 and the g(α) = [1−(1 − α)1 − n]/(1 − n). the reaction rate is described by
where k(T) = the rate constant at temperature T and (1 – α)n = f(α) the differential conversion function. By linearization, it becomes
and by plotting ln(dα/dt) versus ln(1 − α) (Fig. 5), the values of lnk and n for each temperature can be obtained (Table 3).
Considering the temperature dependence of k to be of Arrhenius type, by plotting lnk(T) versus 1/T (Fig. 6), the activation energy E and the pre-exponential factor A will be obtained (Table 3).
According to the values presented in Table 3 (in which the mean for n is 0.497 ± 0.017), significant variation of the reaction order versus temperature of reaction was not observed and, according to Sbirrazzuoli [37], this denotes the presence of a process which takes place in a single step.
The values of n and lnA (10–20) correspond to the reaction order model, which agrees with an homogenous decomposition of purely condensed phase (the case of KTs decomposition in molten phase) with additional transport.
The values of activation energy, pre-exponential factor and reaction order are in agreement with those obtained under non-isothermal conditions: Chang method (E = 78.1 ± 1.2; lnA = 11.3 ± 0.9; n = 0.513 ± 0.018); Coats–Redfern method (E = 76.0 ± 1.7; lnA = 10.9 ± 0.9); Madhusudanan method (E = 76.7 ± 1.6; lnA = 11.1 ± 0.8).
The activation energy’s values determined by the three isothermal methods are in agreement between them, as well as with the values determined in non-isothermal conditions [26] (Table 4).
Conclusions
We performed a kinetic study for the thermal decomposition of KT active substance, in a nitrogen atmosphere and under isothermal conditions, in comparison to the study performed in non-isothermal conditions. In the literature, there are plenty of articles that show the superiority of the isothermal methods to those which are non-isothermal because of the way of experimental data processing.
The study suggests that the decomposition of KT occurs after melting in a single step. The TG/DTG data obtained in the above mentioned conditions have been processed by three methods (characteristic to the isothermal study): the one of isothermal model fitting, isothermal–isoconversional and Fd isothermal–isoconversional.
The model-free isoconversional methods, especially Fd isothermal–isoconversional method, offer the best results. The great advantage of the model-free analysis is found in its simplicity and the avoidance of the errors connected with the selection of a kinetic model.
For the choice of the kinetic equation which describes the reaction mechanism, several equations were verified by plotting, and the adequate correlation coefficient was calculated. Based on these values, the reaction model that was chosen corresponds to n = 1/2.
The kinetic parameter values determined under isothermal conditions by the above mentioned methods are in good accordance with those determined in non-isothermal conditions by applying the above mentioned methods.
The concordance of the values obtained for the kinetic parameters and the dependence mode of the activation energy on the conversion degree show the correctness of the applied methods and the fact that the thermal decomposition process of the KT is indeed a unitary process.
The activation energy’s values and the pre-exponential factor, as well as the range of decomposition indicate a lower thermal stability of KT. The correlation between the kinetic parameter values determined under different thermal conditions confirms the need to use different methods for experimental data processing.
The activation energy's values can be used in the quality control of drug, with a view to the improvement of the final product and for the determination of drug quality via the technological parameters. Finally, kinetic study can be a fast alternative or a complementary method to estimate the shelf-life of medicines.
References
Kovala-Demertzi D. Recent advances on non-steroidal anti-inflammatory drugs, NSAIDs: organotin complexes of NSAIDs. J Organomet Chem. 2006;691:1767–74.
Ying YC, Yi L, Cheng ZJ, Dan Z. Inhibitory effect of copper complex of indomethacin on bacteria studied by microcalorimetry. Biol Trace Elem Res. 2008;122:82–8.
Teslyuk OI, Beltyukova SV, Yegorova AV, Yagodkin BN. Complex compounds of terbium(III) with some nonsteroidal anti-inflammatory drugs and their analytical applications. J Anal Chem. 2007;62:330–5.
Kafarska K, Czakis-Sulikowska D, Wolf WM. Novel Co(II) and Cd(II) complexes with non-steroidal anti-inflammatory drugs. Synthesis, properties and thermal investigation. J Therm Anal Cal. 2009;96:617–21.
Dutta S, Padhye S, McKee V. Structural characterization and SOD activity of copper–oxaprozinate. Inorg Chem Commun. 2004;7:1071–4.
Felix FS, Cides da Silva LC, Angnes L, Matos JR. Thermal behavior study and decomposition kinetics of salbutamol under isothermal and non-isothermal conditions. J Therm Anal Cal. 2009;95:877–80.
Neto HS, Barros FAP, de Sousa Carvalho FM, Matos JR. Thermal analysis of prednicarbate and characterization of thermal decomposition product. J Therm Anal Cal. 2010;102:277–83.
Macêdo RO, Aragão CFS, do Nascimento TG, Macêdo AMC. Application of thermogravimetry in the quality control of chloramphenicol tablets. J Therm Anal Cal. 1999;56:1323–7.
Moura EA, Correia LP, Pinto MF, Procopio JVV, de Sousa FS, Macedo RO. Thermal characterization of the solid state and raw material fluconazole by thermal analysis and pyrolysis coupled to GC/MS. J Therm Anal Cal. 2010;100:289–93.
Picciochi R, Diogo HP, da Piedade MEM. Thermochemistry of paracetamol. J Therm Anal Cal. 2010;99:391–401.
Barboza F, Vecchia DD, Tagliari MP, Silva MAS, Stulzer HK. Differential scanning calorimetry as a screening technique in compatibility studies of acyclovir extended release formulations. Pharm Chem J. 2009;43:363–8.
Mura P, Gratteri P, Faucci TM. Compatibility studies of multicomponent tablet formulations. DSC and experimental mixture design. J Therm Anal Cal. 2002;68:541–51.
Giordano F, Rossi A, Pasquali I, Bettini R, Frigo E, Gazzaniga A, Sangalli ME, Miles V, Catinella S. Thermal degradation and melting point determination of diclofenac. J Therm Anal Cal. 2003;73:509–18.
Fini A, Fazio G, Benetti L, Chedini V. Thermal analysis of some diclofenac salts with alkyl and alkylhydroxy amines. Thermochim Acta. 2007;464:65–74.
Ortega A. A simple and precise linear integral method for isoconversional data. Termochim Acta. 2008;474:81–6.
Chrissafis K. Kinetics of thermal degradation of polymers. Complementary use of isoconversional and model-fitting methods. J Therm Anal Cal. 2009;95:273–83.
Saha B, Maiti AK, Ghoshal AK. Model-free method for isothermal and non-isothermal decomposition kinetics analysis of PET sample. Thermochim Acta. 2006;444:46–52.
Dickinson CF, Heal GR. A review of the ICTAC Kinetics Project, 2000: Part 1. Isothermal results. Thermochim Acta. 2009;494:1–14.
Dickinson CF, Heal GR. A review of the ICTAC Kinetics Project, 2000: Part 2. Non-isothermal results. Thermochim Acta. 2009;494:15–25.
Budrugeac P. Differential non-linear isoconversional procedure for evaluating the activation energy of non-isothermal reactions. J Therm Anal Cal. 2002;68:131–9.
Tiţa B, Fuliaş A, Marian E, Tiţa D. Thermal behaviour of acetylsalicylic acid—active substance and tablets. Kinetic study under non-isothermal conditions. Rev Chim (Bucureşti). 2009;60:419–23.
Tiţa B, Fuliaş A, Marian E, Tiţa D. Thermal stability and decomposition kinetics under non-isothermal conditions of sodium diclofenac. Rev Chim (Bucureşti). 2009;60:524–8.
Tiţa B, Fuliaş A, Rusu G, Tiţa D. Thermal behaviour of indomethacin—active substance and tablets kinetic study under non-isothermal conditions. Rev Chim (Bucureşti). 2009;60:1210–5.
Tiţa B, Fuliaş A, Bandur G, Rusu G, Tiţa D. Thermal stability of ibuprofen. Kinetic study under non-isothermal conditions. Rev Roum Chim. 2010;55:553–8.
Tiţa B, Fuliaş A, Tiţa D. Kinetic study of indomethacin under isothermal conditions. Rev Chim (Bucureşti). 2010;61:1037–41.
Tiţa D, Fuliaş A, Tiţa B. Thermal stability of ketoprofen—active substance and tablets. Part 1. Kinetic study of the active substance under non-isothermal conditions. J Therm Anal Cal. 2011;105:501–8.
Tiţa B, Fuliaş A, Ştefănescu M, Marian E, Tiţa D. Kinetic study of decomposition of ibuprofen under isothermal conditions. Rev Chim (Bucureşti). 2011;62:216–21.
Tiţa B, Fuliaş A, Ştefănescu M, Marian E, Tiţa D. Kinetic study of sodium diclofenac under isothermal conditions. Rev Chim (Bucureşti). 2011;62:31–6.
Soliman MH, Mohamed GG, Mohamed EA. Metal complexes of fenoterol drug: preparation, spectroscopic, thermal, and biological activity characterization. J Therm Anal Cal. 2010;99:639–47.
Foppoli A, Zema L, Maroni A, Sangalli M, Caira M, Gazzaniga A. Dehydration kinetics of theophylline-7-acetic acid monohydrate. J Therm Anal Cal. 2010;99:649–54.
Avula GS, Alexander K, Riga A. Predicting eutectic behavior of drugs and excipients by unique calculations. J Therm Anal Cal. 2010;99:655–8.
Oliveira PR, Stulzer HK, Bernardi LS, Borgmann SHM, Cardoso SG, Silva MAS. Sibutramine hydrochloride monohydrate: thermal behavior, decomposition kinetics and compatibility studies. J Therm Anal Cal. 2010;100:277–82.
Chen HX, Liu NA. New approximate formulae for the generalized temperature integral. J Therm Anal Cal. 2009;96:175–8.
Cai JM, Liu RH. On evaluate of the integral methods for the determination of the activation energy. J Therm Anal Cal. 2009;96:331–3.
Li X, Wu Y, Gu D, Gan F. Thermal decomposition kinetics of nickel (II) and cobalt (II) azo barbituric acid complex. Thermochim Acta. 2009;493:85–9.
Friedman HL. New methods for evaluating kinetic parameters from thermal analysis data. J Polym Sci. 1965;6C:183–7.
Sbirrazzuoli N, Vincent L, Vyazovkin S. Comparison of several computational procedures for evaluating the kinetics of thermally stimulated condensed phase reactions. Chenom Intell Lab Syst. 2000;54:53–60.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tiţa, D., Fuliaş, A. & Tiţa, B. Thermal stability of ketoprofen. J Therm Anal Calorim 111, 1979–1985 (2013). https://doi.org/10.1007/s10973-011-2147-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-2147-8