Abstract
In this study, phenylalanine dithiocarbamate (PHEDTC) ligand was successfully synthesized and then radiolabeled with [99mTcO]3+ core and [99mTc≡N]2+ core to produce 99mTcO–PHEDTC and 99mTcN–PHEDTC, respectively. Both complexes were prepared with high radiochemical purity and had good stability. The partition coefficient results showed they were hydrophilic, while 99mTcN–PHEDTC was more hydrophilic than 99mTcO–PHEDTC. The biodistribution study in mice bearing S 180 tumor showed that 99mTcO–PHEDTC and 99mTcN–PHEDTC had high tumor uptake at 2 h post-injection, 1.91 and 1.21, respectively. The good uptake and retention in tumor together with favorable tumor-to-muscle ratios make them promising candidates for further evaluation as potential tumor imaging agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The sugar-based PET radiotracer [18F]fluorodeoxyglucose (18F-FDG), the golden standard tracer for tumor detection and staging clinically, still has some limitations, like producing false-positive or -negative results, low image contrast in brain tumor diagnosis and poor differentiation of tumor from inflammatory [1–4]. Currently, great researches are being performed, with the aim to develop the alternative tracer of 18F-FDG. So far, there has been an increasing interest on a wide range of radiolabeled amino acids, because they are the substrates of various amino acids transport systems, which can be upregulated in certain tumors. Recently, since the success of 6-[F-18]fluoro-3,4-dihydroxy-l-phenylalanine (18F-DOPA) for tumor imaging, many studies have been focused on 18F labeled l-phenylalanine derivates [5–13]. However, the limited availability (requiring cyclotron) and high costs of 18F restrict its wide application in clinical practice, especially in many developing countries. Compared to radionuclide 18F, 99mTc is more widely accessible because it can be produced from a commercial 99Mo–99mTc generator and 99mTc labeled imaging agents can be easily prepared by using available kits, thus making 99mTc labeled tracers the mainstay of nuclear medicine imaging worldwide. Recently, we have reported the synthesis and evaluation of 99mTcN–PRODTC (PRODTC: proline dithiocarbamate) as a potential tumor imaging agent [14]. As reported, 99mTcN–PRODTC can be synthesized through easy procedures and exhibited relatively high tumor-to-muscle and tumor-to-blood ratio at 2 h post-injection. However, the uptake and retention in tumor of 99mTcN–PRODTC was not satisfactory. Thus, it may be of great interest to probe other better 99mTc labeled amino acids derivatives for tumor imaging. Among all the radiolabeled amino acids, radiolabeled aromatic amino acids have the tendency to be more suitable for tumor imaging [15]. Bearing in mind l-phenylalanine is a natural aromatic amino acid and its molecular structure has an active amine group, thus making it possible to react with carbon disulfide in NaOH solutions to produce the phenylalanine dithiocarbamate (PHEDTC). Based on our previous reported work [16, 17], it can be assumed that PHEDTC can also be used to form stable 99mTcO and 99mTcN complexes on the basis of efficient binding of the group to four sulfur atoms. This background encouraged us to synthesize 99mTcO–PHEDTC and 99mTcN–PHEDTC by ligand-exchange reaction to find good tumor imaging agents. In this study, the synthesis and biological evaluation of novel 99mTc-oxo and 99mTc-nitrido complexes with PHEDTC for tumor imaging are reported for the first time.
Experimental
Materials and methods
l–phenylalanine was purchased from J&K CHEMICA, China. Succinic dihydrazide (SDH) kit and glucoheptonate (GH) kit were obtained from Beijing Shihong Pharmaceutical Center, Beijing Normal University, China. All other chemicals were of reagent grade and were used without further purification. 99Mo/99mTc generator was obtained from the China Institute of Atomic Energy (CIAE). IR spectrum was obtained with an AVATAR 360 FT-IR spectrometer using KBr pellets. NMR spectrum was recorded on a 500 MHz Bruker Avance spectrophotometer. Elemental analyses were performed on a Vario EL elemental analyzer model.
Synthesis of phenylalanine dithiocarbamate (PHEDTC)
The synthesis of PHEDTC was carried out according to the literature [18]. Carbon disulfide (0.35 mL, 5.70 mmol) was dissolved in 5 mL anhydrous diethyl ether and cooled to 0 °C in an ice bath. l-phenylalanine (936 mg, 5.70 mmol) and NaOH (456 mg, 11.4 mmol) were dissolved in 10 mL anhydrous methanol and added dropwise to the carbon disulfide solution. The mixture was stirred for 3 h in an ice bath. The solvent was removed and the resulting residue was triturated with diethyl ether. The white solid was filtered and washed with Et2O and dried in vacuo to yield PHEDTC as a pale yellow solid (0.631 g, 39 %).
Radiolabeling of 99mTcO–PHEDTC and 99mTcN–PHEDTC and quality control techniques
99mTcO–PHEDTC and 99mTcN–PHEDTC were prepared by using GH kit and SDH kit, separately.
For preparing 99mTcO–PHEDTC, 1 mL of saline containing 99mTcO4 − (370 MBq) was added to a GH kit containing 0.1 mg of stannous chloride dihydrate, 20.0 mg of GH. The reaction vial was incubated at room temperature for 15 min. Successively, 1.0 mg of PHEDTC dissolved in 1.0 mL water was added and the resulting solution was heated at 100 °C for 30 min.
As for preparing 99mTcN–PHEDTC, 1 mL of saline containing [99mTcO4]− (370 MBq) was added to a SDH kit, which contained 0.05 mg of stannous chloride dihydrate, 5.0 mg of SDH and 5.0 mg of propylenediamine tetraacetic acid (PDTA). The mixture was kept at room temperature for 15 min. Then, 1.0 mg of PHEDTC dissolved in 1.0 mL water was added to the mixture and the reaction vial was incubated for 20 min on a boiling water bath.
The radiochemical purities of the complexes were routinely checked by thin layer chromatography (TLC). TLC was performed on a polyamide strip and eluted with saline and acetonitrile, respectively.
In vitro stability study
99mTcO–PHEDTC and 99mTcN–PHEDTC were kept in the labeling milieu at room temperature (25 °C) and the radiochemical purities were assayed by TLC analysis for up to 6 h after labeling. To test the stabilities of the complexes in serum, 0.5 mL of each labeled complex was separately incubated in 1.0 mL human serum (1 mg/mL) at 37 °C for 4 h and then the radiochemical purity of each complex was analyzed by TLC.
Human serum albumin binding assay
10 µL of labeled complex (370 KBq) was added in 100 µL human serum albumin (100 mg/mL) in the centrifuge tube. After the mixture was incubated at 37 °C for 2 h, the serum protein was precipitated by adding 1 mL trichloroacetic acid (250 mg/mL) to the mixture. The supernatant and precipitate were separated by centrifugation at 2,000 g for 5 min. Then the radioactivity of each phase was measured separately. This experimental procedure was repeated three times and the percentage of human serum protein binding was determined as the following equation:
Determination of the partition coefficient
The partition coefficient was determined by mixing the complex with an equal volume of 1-octanol and phosphate buffer (0.025 mol/L, pH 7.4). In a centrifuge tube, containing 2 mL of each phase, 0.1 mL of the labeled complex solution was added, and the mixture was shacked on a Vortex mixer for 1 min and then centrifuged at 5,000 g for 5 min. Three samples (0.1 mL each) from each layer were counted in a well gamma γ-counter. The partition coefficient, P, was calculated as the mean value of counts per minute in octanol divided by that of the buffer. Usually the final partition coefficient value was expressed as log P. The log P value was reported as an average of three measurements plus the standard deviation.
Biodistribution study
All biodistribution studies were carried out in compliance with the national laws related to the conduct of animal experimentation. 0.1 mL of 99mTcO–PHEDTC (7.4 × 105 Bq) was injected into the Kunming male mice (18–20 g) bearing S180 tumor via a tail vein. The mice were sacrificed in groups of five at 2 and 4 h post-injection. The tumor, other organs of interest and blood were collected, weighed and measured for radioactivity. The counting tubes, including a standard equivalent to 1 % of the injected dose, were assayed in a well-type NaI (Tl) detector and the results were expressed as the percent uptake of injected dose per gram of tissue (% ID/g). The final results are expressed as mean ± standard deviation. The biodistribution study of 99mTcN–PHEDTC was conducted in the same way.
Results and discussion
Synthesis and radiolabeling
PHEDTC was prepared by reacting l-phenylalanine with an equivalent amount of carbon disulfide in NaOH solutions. The reaction equation is shown in Scheme 1. It was characterized by IR, 1H NMR, 13C NMR and Elemental analysis. Specific rotation [α] 25D (H2O) = +53.9°. IR(KBr)/cm−1: 3418 (N–H), 1651 (C=O), 1614 (C=C), 1111 (C=S). 1H-NMR (D2O) δ: 7.11–7.26 (m, 5H), 4.77–4.80 (dd, 1H), 2.88–3.17 (m, 2H). 13C-NMR (D2O) δ: 210.54, 177.79, 137.55, 129.342, 129.37, 129.02, 128.57, 126.74, 63.61, 37.24. Elemental analysis calculated (%) for C10H9NNa2O2S2·0.5H2O: C, 40.82; N, 4.76; H, 3.40. Found: C, 41.21; N, 4.82; H, 3.98.
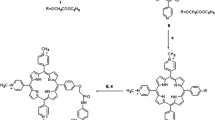
Synthesis of PHEDTC The preparations of 99mTcO–PHEDTC and 99mTcN–PHEDTC were carried out using the following procedure in Scheme 2
For preparing 99mTcO–PHEDTC, the method is based on the reaction of [99mTcO4]− with GH in the presence of stannous chloride as reducing agent to form 99mTc(V)–GH which contains the [99mTcO]3+ core and 99mTc(V)–GH is a suitable substrate for the substitution reaction with PHEDTC to produce the final complex 99mTcO–PHEDTC.
As for 99mTcN–PHEDTC, it was prepared by adding PHEDTC to the [99mTcN]2+ intermediate, which was produced by the reaction of [99mTcO4]− with SDH in the presence of stannous chloride as reducing agent. The [99mTcN]2+ core is an appropriate substrate for the substitution reaction with PHEDTC to obtain 99mTcN–PHEDTC with high yield.
Based on a previous characterization of the molecular structure of 99mTc(V)O-DMSA (DMSA: dimercaptosuccinic acid) and bis(N-ethoxy, N-ethyl dithiocarbamato) nitrido technetium-99m complex [99mTcN(NOEt)2] [19–21], it can be presumed that 99mTcO–PHEDTC or 99mTcN–PHEDTC would have a square pyramidal geometry with an apical Tc=O bond or Tc≡N bond and two PHEDTC ligands spanning the four positions in the basal plane through the four sulfur atoms. Clearly, its detailed structure needs to be determined by further research work.
The radiochemical purities of the complexes were routinely checked by TLC. For 99mTcO–PHEDTC, in saline, 99mTcO4 − and 99mTcO–PHEDTC migrated at R f = 0.1 while 99mTc–GH migrated at R f = 0.8–1.0. In acetonitrile, 99mTcO4 − migrated at R f = 0.3–0.5, while 99mTcO–PHEDTC and 99mTc–GH migrated at R f = 0.1. For 99mTcN–PHEDTC, in saline, 99mTcO4 − and 99mTcN–PHEDTC remained at the origin while [99mTcN]2+ migrated with the front. In acetonitrile, 99mTcO4 − migrated at R f = 0.3–0.5, while 99mTcN–PHEDTC and [99mTcN]2+ remained at the origin.
Stability study
The radiochemical purities of 99mTcO–PHEDTC and 99mTcN–PHEDTC were both over 90 % at 6 h after preparation. On the other hand, in serum at 37 °C, the radiochemical purities of the complexes were higher than 90 % even up to 4 h after synthesis, suggesting they possessed a great stability in human serum.
Human serum albumin binding assay
As described in the experiment of human serum albumin binding assay [14], the percentage of human serum protein binding ability for both complexes was measured and calculated. The results showed that the percentage of human serum protein binding of 99mTcO–PHEDTC and 99mTcN–PHEDTC were high, 85.68 ± 0.61 and 86.32 ± 0.19 %, respectively. When compared with 99mTcN–PRODTC (87.42 ± 0.01 %), there was no great difference in the serum protein binding ability among these three complexes.
Partition coefficient (log P)
The log P values of 99mTcO–PHEDTC and 99mTcN–PHEDTC were found to be −1.33 ± 0.07 and −1.52 ± 0.03, respectively, indicating both tracers were hydrophilic. Moreover, 99mTcN–PHEDTC was more hydrophilic than 99mTcO–PHEDTC. As compared to 99mTcN–PRODTC (log P: −2.80 ± 0.11), 99mTcO–PHEDTC and 99mTcN–PHEDTC had a higher lipophilicity due to their structures containing aromatic groups.
Biodistribution study
The results of biodistribution of 99mTcO–PHEDTC and 99mTcN–PHEDTC in mice bearing S180 tumor are shown in Table 1. Results of biodistribution of recently reported 99mTc complexes as tumor imaging agents are shown in Table 2 for comparison.
As shown in Table 1, both 99mTcO–PHEDTC and 99mTcN–PHEDTC have a high uptake and good retention in tumor. The muscle uptakes are low so the T/N ratios are better. Activity accumulation in the kidneys and liver shows that the major route of excretion is renal and hepatobiliary. Low uptake in the stomach is suggestive of in vivo stability of the above 99mTc labeled complexes. As compare to 99mTcN–PHEDTC, 99mTcO–PHEDTC has a higher tumor and liver uptake than 99mTcN–PHEDTC and this possibly be related to its higher lipophilicity. 99mTcO–PHEDTC has a lower muscle uptake and higher tumor uptake, so the T/N ratio of 99mTcO–PHEDTC is much higher than that of 99mTcN–PHEDTC. However, due to the higher blood uptake, the T/B ratio of 99mTcO–PHEDTC is lower than that of 99mTcN–PHEDTC.
From Table 2, it is observed that 99mTcO–PHEDTC and 99mTcN–PRODTC show a much higher tumor uptake when compared to the other three complexes. A decrease in the order is observed: 99mTcO–PHEDTC > 99mTcN–PHEDTC > [99mTc(CO)3(IDA–PEG3–CB)]− > 99mTcN–PRODTC > [99mTc(CO)3(PA–TZ–CHC)]+. In the limits of our study, among 99mTcO–PHEDTC, 99mTcO–PHEDTC and 99mTcN–PRODTC, the results demonstrated that the 99mTc labeled complexes containing aromatic amino acid moieties possibly increased the tumor uptake, confirming our hypothesis that radiolabeled aromatic amino acids are possibly more promising for tumor imaging. Moreover, different 99mTc core for preparing the complexes may exhibit significant impact on the tumor uptake, T/B and T/N ratios.
Conclusion
In present study, a novel ligand PHEDTC was synthesized and its 99mTc-oxo core and 99mTc-nitrido core complexes were successfully prepared in high yields through a ligand-exchange reaction. The preliminary in vivo studies showed both of them had a relative high tumor uptake and good tumor-to-muscle ratios. Especially for 99mTcO–PHEDTC, it would be a promising tumor imaging agent, justifying further investigations.
References
Delbeke D, Coleman RE, Guiberteau MJ, Brown ML, Royal HD, Siegel BA, Townsend DW, Berland LL, Parker JA, Hubner K, Stabin MG, Zubal G, Kachelriess M, Cronin V, Holbrook S (2006) Procedure guideline for tumor imaging with F-18-FDG PET/CT. J Nucl Med 47:885–895
Strauss LG (1997) Positron emission tomography: current role for diagnosis and therapy monitoring in oncology. Oncologist 2:381–388
Chang JM, Lee HJ, Lee HY, Lee JJ, Chung JK, Im JG (2006) False positive and false negative FDG–PET scans in various thoracic diseases. Korean J Radiol 7:57–69
Rosenbaum SJ, Lind T, Antoch G, Bockisch A (2006) False-positive FDG PET uptake: the role of PET/CT. Eur Radiol 16:1054–1065
Chen W, Silverman DHS, Delaloye S, Czernin J, Kamdar N, Pope W, Satyamurthy N, Schiepers C, Cloughesy T (2006) F-18-FDOPA PET imaging of brain tumors: comparison study with F-18-FDG PET and evaluation of diagnostic accuracy. J Nucl Med 47:904–911
Hoegerle S, Altehoefer C, Ghanem N, Brink I, Moser E, Nitzsche E (2001) F-18-DOPA positron emission tomography for tumour detection in patients with medullary thyroid carcinoma and elevated calcitonin levels. Eur J Nucl Med 28:64–71
Koopmans KP, De Vries EGE, Kerna IP, Elsinga PH, Neels OC, Sluiter WJ, van der Horst-Schrivers ANA, Jager PL (2006) Staging of carcinoid tumours with F-18-DOPA PET: a prospective, diagnostic accuracy study. Lancet Oncol 7:728–734
Schiesser M, Veit-Haibach P, Muller MK, Weber M, Bauerfeind P, Hany T, Clavien PA (2010) Value of combined 6-F-18 fluorodihydroxyphenylalanine PET/CT for imaging of neuroendocrine tumours. Br J Surg 97:691–697
Jager PL, Chirakal R, Marriott CJ, Brouwers AH, Koopmans KP, Gulenchyn KY (2008) 6-L-18F-fluorodihydroxyphenylalanine PET in neuroendocrine tumors: basic aspects and emerging clinical applications. J Nucl Med 49:573–586
de Vries EFJ, Luurtsema G, Brussermann M, Elsinga PH, Vaalburg W (1999) Fully automated synthesis module for the high yield one-pot preparation of 6-F-18 fluoro-L-DOPA. Appl Radiat Isot 51:389–394
Kersemans K, Mertens J, Caveliers V (2010) Radiosynthesis of 4-F-18 fluoromethyl-l-phenylalanine and F-18 FET via a same strategy and automated synthesis module. J Label Compd Radiopharm 53:58–62
Wang LM, Lieberman BP, Plossl K, Qu WC, Kung HF (2011) Synthesis and comparative biological evaluation of l- and d-isomers of F-18-labeled fluoroalkyl phenylalanine derivatives as tumor imaging agents. Nucl Med Biol 38:301–312
Huang CF, Yuan LY, Rich KM, McConathy J (2013) Radiosynthesis and biological evaluation of alpha F-18 fluoromethyl phenylalanine for brain tumor imaging. Nucl Med Biol 40:498–506
Liu M, Lin X, Song XQ, Cui Y, Li PW, Wang XB, Zhang JB (2013) Synthesis and biodistribution of a novel Tc-99m nitrido radiopharmaceutical with proline dithiocarbamate as a potential tumor imaging agent. J Radioanal Nucl Chem 298:1659–1663
Kong FL, Zhang YH, Ali MS, Oh C, Mendez R, Kohanim S, Tsao N, Chanda M, Huang WC, Yang DJ (2010) Synthesis of 99mTc-EC-AMT as an imaging probe for amino acid transporter systems in breast cancer. Nucl Med Commun 31:699–707
Zhang JB, Ren JL, Lin X, Wang XB (2009) Synthesis and biological evaluation of a novel 99mTc nitrido radiopharmaceutical with deoxyglucose dithiocarbamate, showing tumor uptake. Bioorg Med Chem Lett 19:2752–2754
Lin X, Jin ZH, Ren JL, Pang Y, Zhang WF, Huo JF, Wang XB, Zhang JB, Zhang YY (2012) Synthesis and biodistribution of a new 99mTc-oxo complex with deoxyglucose dithiocarbamate for tumor imaging. Chem Biol Drug Des 79:239–245
Baird IR, Cameron BR, Skerlj RT (2003) Unique chemistry of amino acid dithiocarbamates with Ru(III) bis-beta-diketonates. Inorg Chim Acta 353:107–118
Blower PJ, Singh J, Clarke SEM (1991) The chemical identity of pentavalent technetium-99m-dimercaptosuccinic acid. J Nucl Med 32:845–849
Zhang JB, Yu Q, Huo JF, Pang Y, Yang S, He YN, Tang TT, Yang CC, Wang XB (2010) Synthesis and biodistribution of a novel Tc-99m-DMSA-metronidazole ester as a potential tumor hypoxia imaging agent. J Radioanal Nucl Chem 283:481–485
Pasqualini R, Duatti A (1992) Synthesis and characterization of the new neutral myocardial imaging agent [99mTcN(noet)2] (noet = N-ethyl-N-ethoxy dithiocarbamato). J Chem Soc Chem Commun 18:1354–1355
Wang JJ, Duan XJ, Mao HN, Yang J, Tan CM, Tian Y, Wu WS (2013) Synthesis, 99mTc(CO)3-labeling and preliminary biodistribution studies of a novel colchicine complex. J Radioanal Nucl Chem 295:227–231
Wang JJ, Yang J, Yan ZY, Duan XJ, Tan CM, Shen YL, Wu WS (2011) Synthesis and preliminary biological evaluation of [99mTc(CO)3(IDA–PEG3–CB)]− for tumor imaging. J Radioanal Nucl Chem 287:465–469
Acknowledgments
The work was financially supported, in part, by National Natural Science Foundation of China (21171024, 81101069), Beijing Natural Science Foundation (7112035), Fundamental Research Funds for the Central Universities (105566), and Beijing Nova Program (xxhz201306).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, J., Wang, Y., Li, Z. et al. Synthesis and biological evaluation of novel 99mTc-oxo and 99mTc-nitrido complexes with phenylalanine dithiocarbamate for tumor imaging. J Radioanal Nucl Chem 302, 211–216 (2014). https://doi.org/10.1007/s10967-014-3160-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3160-8