Abstract
This work reports the synthesis, radiolabeling and preliminary biodistribution results in tumor-bearing mice of [99mTc(CO)3(PA-TZ-CHC)]+. The novel colchicine (CHC) ligand was successfully synthesized via “click” reaction. Radiolabeling was performed in high yield with [99mTc(CO)3]+ core to get [99mTc(CO)3(PA-TZ-CHC)]+, which was hydrophilic and cationic, and was stable at room temperature. Biodistribution studies in tumor-bearing mice showed that [99mTc(CO)3(PA-TZ-CHC)]+ accumulated in the tumor with good uptake while comparatively low retention. The clearance of the 99mTc-complex from normal organs was fast, resulting in increasing tumor/blood and tumor/muscle ratios. The promising results in preliminary biodistribution studies warrant further research to improve tumor targeting efficacy and pharmacokinetic profile of radiolabeled CHC derivative by structural modification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colchicine (CHC), the major alkaloid of the meadow saffron, is one of the most prominent natural products and, like other tubulin-binding natural products (e.g., taxol and the epothilones), exhibits great pharmaceutical potential [1]. CHC is used for the treatment of acute gout. CHC binds to tubulin, thereby interfering with the polymerization of tubulin, interrupting microtubule dynamics, and arresting the mitosis in the metaphase stage. The cancer cells are dependent on the microtubule dynamics for their uncontrolled growth and division. Highly dynamic mitotic-spindle microtubules are among the most successful targets for anticancer therapy [2]. CHC was, therefore, selected as the biomolecule for preparing tumor-targeted radiopharmaceuticals for imaging or therapy purpose.
CHC, like many other cytotoxic drugs, enters the cell through the lipid bilayer by passive diffusion and binds reversibly to P-glycoprotein (Pgp) [1]. P-gp is a 170-kDa transmembrane drug efflux pump encoded by the MDR-1 gene in humans [3]. Pgp-mediated transport of chemotherapeutic drugs has been studied using single photon emission computed tomography (SPECT) and positron emission tomography (PET). It has been reported the feasibility of imaging Pgp functionality in tumours with [11C] CHC and PET [4, 5]. The potential of CHC for tumor imaging and assessing antiangiogenic effect has been very well documented in the use of 99mTc-ethylenedicysteine colchicine [6]. Trimethylcolchicinic acid, derivatived from CHC, has been labeled by [99mTc(CO)3]+ and [99mTcN]2+ core [7]. The 99mTc-labeled CHC conjugates are used in targeting tumors and reported to exhibit good tumor uptake. CHC has been labeled by 125I directly and found to be suitable for imaging of muscles [8]. Recently, we have reported a radioiodinated pegylated CHC [9] and a 99mTc(CO)3-AOPA CHC conjugate [10]. The former exhibits good clearance from background but poor tumor localization [9]. While the latter exhibits good uptake and retention in tumor with slow clearance from normal organs [10]. CHC has also been labeled by therapeutic radioisotopes, including 90Y and 188Re [11, 12]. Both of the 90Y-DOTA-NCS-CHC and 188Re(CO)3-colchicine complexes exhibit good tumor uptake and retention, which suggest their potential for tumor therapy [11, 12].
99mTc is the most widely used radionuclide for diagnostic imaging with SPECT because of its favorable physical properties (t 1/2 = 6 h, Eγ = 140 keV), low cost, and widespread availability [13]. One-step synthesis of [99mTc(CO)3(H2O)3]+ by direct reduction of 99mTcO4 − with sodium borohydride in aqueous solution was firstly developed by R. Alberto et al. [14]. Since then, a large number of biologically avid molecules have been labelled by [99mTc(CO)3]+ core for the development of site-specific radiopharmaceuticals. The [99mTc(CO)3]+ core possesses many excellent features, such as its small volume, kinetic inertness, and flexibility in the choice of ligands for designing complexes of desirable size, charge, and lipophilicity suitable for the specific study [14–16].
Derivatization of CHC to the suitable precursor was necessary for subsequent 99mTc-labeling. The catalytic “click” reaction of an azide and an alkyne to produce triazole has provided an excellent platform for coupling two molecules for a variety of applications (nanoparticles, polymers, biomolecules) [17, 18]. In this paper, a new CHC derivative was synthesized via “click” reaction to form the “N3” chelator group. We report herein the synthesis, 99mTc(CO)3-labeling and preliminary biodistribution studies of the new CHC ligand in tumor-bearing mice.
Methods and materials
Materials
Commercially available reagents and solvents were used without further purification. Compound 1 (deacetylcolchicine), 2-(2-azidoethoxy)ethyl 4-methylbenzenesulfonate and N-(pyridin-2-ylmethyl)prop-2-yn-1-amine were synthesized according to the described methods respectively [19–21]. Column chromatography was carried out on silica gel. 1H NMR spectra were recorded with a 400 MHz spectrometer by using TMS as the internal standard. Mass spectrometer was performed on a Bruker Daltonics esquire6000 mass spectrometer with electrospray-ionisation probe. A 99Mo/99mTc generator was obtained from the China Institute of Atomic Energy (CIAE).
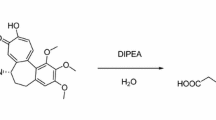
Synthesis of PA-TZ-CHC (Fig. 1)
To 0.5 mL of DMF were added compound 1 (130 mg, 0.364 mmol), 2-(2-azidoethoxy)ethyl 4-methylbenzenesulfonate (124 mg, 0.435 mmol) and K2CO3 (151 mg, 1.093 mmol). The reaction mixture was heated to 90 °C for 5 h. DMF was removed under reduced pressure. The residue was dissolved in 30 mL of EtOAc, and washed by saturated salt water (10 mL × 3). The organic phase was dried with Na2SO4. The solvent was removed under reduced pressure and the residue was purified by silica gel column (EtOAc/MeOH = 30/1) to give compound 2 (70 mg, 0.149 mmol, 40.9 %) as a light-yellow oil. 1H NMR (400 MHz, CDCl3): δ = 7.87 (s, 1 H), 7.21 (d, J = 5.2 Hz, 1 H), 6.79 (d, J = 5.2 Hz, 1 H), 6.54 (s, 1 H), 3.99 (s, 3 H), 3.93 (s, 3 H), 3.91 (s, 3 H), 3.67-3.48 (m, 7 H), 3.36 (m, 3 H), 2.63 (m, 1 H), 2.52-2.15 (m, 5 H), 1.90 (br, 1 H) ppm. ESI–MS: 471.3 (MH+).
To 1 mL of THF were added compound 2 (70 mg, 0.149 mmol), N-(pyridin-2-ylmethyl)prop-2-yn-1-amine (26.1 mg, 0.179 mmol) and CuI (3 mg, 0.015 mmol). The yellow-green mixture was stirred at room temperature for 5 h. The solvent was removed under reduced pressure and the residue was purified by silica gel column (EtOAc/MeOH/Et2NH = 100/10/3) to give PA-TZ-CHC (23 mg, 0.0373 mmol, 25.0 %) as a yellow oil. 1H NMR (400 MHz, CDCl3): δ = 8.52 (d, J = 2.4 Hz, 1 H), 7.82 (s, 1 H), 7.77 (s, 1 H), 7.63 (t, 1 H), 7.35 (d, J = 4.0 Hz, 1 H), 7.22 (d, J = 5.2 Hz, 1 H), 7.15 (t, 1 H), 6.79 (d, J = 5.2 Hz, 1 H), 6.53 (s, 1 H), 4.55 (t, 2 H), 3.98 (m, 7 H), 3.92 (s, 3 H), 3.91 (s, 3 H), 3.87 (m, 2 H), 3.60 (s, 3 H), 3.47 (t, 2 H), 3.34 (m, 1 H), 2.60 (m, 1 H), 2.48-2.00 (m, 7 H) ppm. ESI–MS: 617.5 (MH+), 309.3 (M + 2H+)/2.
Radiosynthesis of [99mTc(CO)3(PA-TZ-CHC)]+ (Fig. 2)
The tricarbonyl technetium precursor was prepared according to the procedure published by Alberto and his coworkers [14, 15]. The pH of the intermediate was adjusted to ~7.0 with 1 N HCl. A 0.5 mL of the freshly prepared [99mTc(CO)3(H2O)3]+ precursor (185 MBq) was added into a 5 mL vial containing PA-TZ-CHC (100 μg) in 0.5 mL 100 mM PBS (pH = 7.4). The reaction mixture was heated at 100 °C for 15 min. After cooling to room temperature, the radiochemical purity (RCP) of the mixture was evaluated by TLC, which was performed on a polyamide strip eluted with dichloromethane/methanol = 1/1 (V/V).
Determination of the partition coefficient
The partition coefficient was determined by mixing the complex with an equal volume of n-octanol and phosphate buffer (25 mM, pH 7.4) in a centrifuge tube. The mixture was vigorously stirred for 10 min at room temperature, and was then transferred to an Eppendorf microcentrifuge tube. The tube was centrifuged at 8,000 rpm for 10 min. Samples in triplets from n-octanol and aqueous layer were obtained, and were counted in a well γ-counter. The partition coefficients were calculated using the following equation: P = (activity concentration in n-octanol)/(activity concentration in aqueous layer). The final partition coefficient value was expressed as log P.
Paper electrophoresis
A sample of the complex (1 μL) was spotted on Whatman 1 chromatography paper (15 × 1 cm), saturated with 0.05 M pH 7.4 phosphate buffer, in an electrophoresis bath. The analyses were carried out using phosphate buffer (0.05 M pH 7.4) at 150 V for 2 h. The strips were dried and the distribution of radioactivity on the strip was determined.
Stability studies
The stability of the complex was determined by measuring the RCP at room temperature (25 °C) at different time points (0, 1, 2, 4, 6 h) after preparation.
Biodistribution studies in tumor-bearing mice
The Kunming mice were provided by Gansu Academy of Medical Sciences. In vivo growth was initiated by hypodermic injection of approximately 106 H22 cells into the left front leg of female Kunming mice. 7–8 days after inoculation, the tumor size was in the range of 0.5–0.8 g, and animals were used for biodistribution studies. A solution of the [99mTc(CO)3(PA-TZ-CHC)]+ (100 μL, 3.7 × 105 Bq) was injected into the tumor-bearing mice via the tail vein. The mice were sacrificed at 5, 30, 60, 120 and 240 min post-injection (p.i.). The organs of interest and blood were collected, weighed and measured for radioactivity. The accumulated radioactivity in the tissue of organs was calculated in terms of percentage of injected dose per gram organ (%ID/g). The biodistribution data and T/NT ratios are reported as an average plus the standard variation. All biodistribution studies were carried out in compliance with the national laws related to the conduct of animal experimentation.
Results and discussion
Synthesis
It’s necessary to derivative CHC to suitable precursor for 99mTc-labeling. In this research, deacetylcolchicine was converted into the azide compound (2), and then the new CHC derivative (PA-TZ-CHC) was synthesized via typical “click” reaction of the azide and the alkyne (Fig. 1). The new ligand contains “N3” chelator group, which is a very attractive tridentate precursor for the introduction of the small [99mTc(CO)3]+ core, with high labeling efficiency and stable labeled complex (Fig. 2). All compounds have been characterized by NMR and ESI–MS.
Radiosynthesis of the [99mTc(CO)3(PA-TZ-CHC)]+
[99mTc(CO)3(H2O)3]+ precursor could be prepared over 95 % yield, and this precursor was used without further purification. The ligand PA-TZ-CHC could be labeled by [99mTc(CO)3]+ very effectively. The suggested structure of [99mTc(CO)3(PA-TZ-CHC)]+ was drawn in Fig. 2 [15, 16]. The RCP of the complex, checked by thin layer chromatography (TLC), was over 95 % after the preparation. When TLC was performed on a polyamide strip developed by dichloromethane/methanol = 1/1 (V/V), [99mTc(CO)3(PA-TZ-CHC)]+ moved to the solvent front (Rf = 0.8–1.0), while 99mTcO2···nH2O, 99mTcO4 − and [99mTc(CO)3(H2O)3]+ remained at the origin.
Partition coefficient (log P)
The partition coefficient (log P) of [99mTc(CO)3(PA-TZ-CHC)]+ was obtained to be −0.051 ± 0.0060, suggesting that it was hydrophilic. The lipophilicity of the complex was mainly because of the tricyclic skeleton, and the 99mTc-chelate contributed to the hydrophilicity.
Paper electrophoresis
The paper electrophoresis pattern of [99mTc(CO)3(PA-TZ-CHC)]+ showed that the complex moved to the point of cathode (percentage of radioactivity: 93.1 %), suggesting that it was a cationic complex.
Stability of the complex
The RCP of the product was nearly constant (>90 %) over the observed period of 6 h, suggesting that the complex possessed a great stability in the reaction mixture at room temperature.
Biodistribution studies in tumor-bearing mice
Biodistribution characteristics of [99mTc(CO)3(PA-TZ-CHC)]+ were evaluated using Kunming mice bearing H22 liver cancer xenografts. The data are summarized in Table 1. [99mTc(CO)3(PA-TZ-CHC)]+ did exhibit good initial tumor uptake (2.46 ± 0.93 ID%/g at 5 min) while comparatively low retention (60 min: 0.37 ± 0.17, 120 min: 0.33 ± 0.08, 240 min: 0.32 ± 0.14 ID%/g). The complex was excreted via hepatobiliary and renal route. The clearance from normal organs was fast, leading to low background at 4 h p.i. (blood: 0.14 ± 0.06, heart: 0.12 ± 0.07, spleen: 0.23 ± 0.09, lung: 0.49 ± 0.12, muscle: 0.05 ± 0.03 ID%/g). Nevertheless, the uptake in liver and kidneys was still considerable at delayed time point. The T/B (tumor/blood) and T/M (tumor/muscle) ratios increased steadily during the observed period (5 min: T/B = 0.40 ± 0.16, T/M = 2.24 ± 0.41 Versus 240 min: T/B = 2.35 ± 0.69, T/M = 4.45 ± 0.69).
Conclusions
A novel CHC derivative was successfully synthesized via “click” reaction. The ligand could be labeled by [99mTc(CO)3]+ core in high yield to get [99mTc(CO)3(PA-TZ-CHC)]+, which was hydrophilic and cationic, and was stable at room temperature. Biodistribution studies in tumor-bearing mice showed that [99mTc(CO)3(PA-TZ-CHC)]+ accumulated in the tumor with good uptake while comparatively low retention. Its clearance from normal organs was fast, resulting in increasing T/B and T/M ratios. Further modification on the linker or/and 99mTc-chelate will be necessary to improve tumor targeting efficacy and pharmacokinetic profile.
References
Graening T, Schmalz HG (2004) Angew Chem Int Ed Engl 43:3230–3256
Jordan MA, Wilson L (2004) Nat Rev Cancer 4:253–265
Gros P, Croop J, Housman D (1986) Cell 47:371–380
Hendrikse NH, Franssen EJF, Van der Graaf WTA, Vaalburg W, de Vries EGE (1999) Eur J Nucl Med 26:283–293
Levchenko A, Mehta BM, Lee JB, Humm JL, Augensen F, Squire O, Kothari PJ, Finn RD, Leonard EF, Larson SM (2000) J Nucl Med 41:493–501
Zareneyrizi F, Yang DJ, Oh CS, Ilgan S, Yu DF, Tansey W, Liu CW, Kim EE, Podoloff DA (1999) Anticancer Drugs 10:685–692
Korde A, Satpati D, Mathur A, Mallia M, Banerjee S, Kothari K, Sarma HD, Choudhari P, Venkatesh M (2006) Bioorg Med Chem 14:793–799
EI-Azony KM, El-Mohty AA, Killa HM, Seddik U, Khater SI (2008) J Label Compd Radiopharm 52:1–5
Zheng X, Dong F, Yang J, Duan X, Niu T, Wu W, Wang J (2011) J Radioanal Nucl Chem 287:113–117
Wang J, Duan X, Mao H, Yang J, Niu T (2011) J Radioanal Nucl Chem 288:635–639
Satpati D, Korde A, Pandey U, Dhami P, Banerjee S, Venkatesh M (2006) J Label Compd Radiopharm 49:951–958
Satpati D, Korde A, Kothari K, Sarma HD, Venkatesh M, Banerjee S (2008) Cancer Biother Radiopharm 23:741–748
Jurisson SS, Lydon JD (1999) Chem Rev 99:2205–2218
Alberto R, Schibli R, Egli A, Schubiger AP (1998) J Am Chem Soc 120:7987–7988
Alberto R, Schibli R, Waibel R, Abram U, Schubiger AP (1999) Coord Chem Rev 190–192:901–919
Schibli R, Bella RL, Alberto R, Garcia-Garayoa E, Ortner K, Abram U, Schubiger AP (2000) Bioconjug Chem 11:345–351
Armstrong AF, Valliant JF (2007) Dalton Trans 38:4240–4251
Wang J, Tian Y, Duan X, Yang J, Mao H, Tan C, Wu W (2012) J Radioanal Nucl Chem 292:177–181
Lagnoux D, Darbre T, Schmitz ML, Reymond JL (2005) Chemistry 11:3941–3950
Gill HS, Tinianow JN, Ogasawara A, Flores JE, Vanderbilt AN, Raab H, Scheer JM, Vandlen R, Williams SP, Marik J (2009) J Med Chem 52:5816–5825
Struthers H, Viertl D, Kosinski M, Spingler B, Buchegger F, Schibli R (2010) Bioconjug Chem 21:622–634
Acknowledgments
The work was financially supported by National Natural Science Foundation of China (21001060, J1030932) and the Fundamental Research Funds for the Central Universities (lzujbky-2012-63).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, J., Duan, X., Mao, H. et al. Synthesis, 99mTc(CO)3-labeling and preliminary biodistribution studies of a novel colchicine complex. J Radioanal Nucl Chem 295, 227–231 (2013). https://doi.org/10.1007/s10967-012-1834-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-012-1834-7